Aim: To determine whether obesity has an impact on the short-term efficacy of inter sphincteric resection (ISR) for patients with ultra-low rectal or anal canal cancer.
Methods: This retrospective study includes 276 patients with rectal or anal canal cancer who received treatment from the Rectal Surgery Group of the Gastrointestinal Surgery Center of West China Hospital. According to the WHO, the overweight has a BMI greater than 25. We compare the intraoperative related indicators, postoperative recovery indicators and the rate of occurrence of complications between Group A and Group B.
Results: The time of operation in Group B is apparently longer than that in Group A (143.41 min VS. 130.91 min P < 0.05), the intraoperative blood loss, the anastomotic patterns and the reconstruction pattern are not statistically different. The rate of perianal infection of Group B is significantly higher than that of Group A (6.5% VS. 1.5% P < 0.05), and the rate of incision infection of Group B is significantly higher than that of Group A (5.6% VS. 0.6% P < 0.05). The rate of occurrence of other complications between two groups is not statistically different.
Conclusion: Obesity increases the difficulty of performing ISR for ultra-low rectal or anal canal cancers, extends the time of operation, and increases the incidence rate of perianal infection post-operatively. There is no significant difference between the indications of postoperative recovery, and the incidence rate of complications in obese patients and that in normal weight patients. In terms of the short-term effects, the operations for obese patients are safe and effective.
anal canal cancer, inter sphincteric resection, obesity, short-term efficacy, ultra-low rectal cancer
Although multi-disciplinary treatment has made great progress in rectal cancer treatment, surgical operation is still the key link in the effective treatment of rectal cancer. Traditionally, for patients with ultra-low rectal or anal canal cancer defined as tumor height less than 5 cm away from the anal verge or less than 2 cm away from the dentate line, abdominoperineal resection (APR) is the standard operation. APR greatly affects the patient’s quality of life because of permanent stoma. However, doctors not only are required to remove the tumor completely, but also they should avoid permanent stoma, so that the postoperative quality of life can be maximized. In recent years, doctors are in resection partial or whole of the internal sphincter to get sufficient distal margin, and the left of external sphincter is preserved to maintain the continuity of the intestinal tract and the bowel controlling ability, so the ISR can improve postoperative quality of life [1-6]. Colorectal surgeons have demonstrated the future curative effect of ISR for the patients with ultra-low rectal or anal canal cancer, and, thus, ISR has been used as an alternative surgical technique for APR in many medical institutions.
Obesity has been a severe health problem, especially in developed countries. Le Marchard [7] found that the rate of rectal cancer diagnosis of men with high body mass index (BMI) is 2.9 times higher than that of men with low BMI. A meta-analysis authored by Yan Lei Ma [8] shows that obesity can increase the rate of rectal cancer. Thus, with the number of obese patients with rectal cancer increasing, more attention is paid to the safety and effectiveness of operative treatments for obese patients with rectal cancer. On account of the narrow and deep pelvic, the operative field exposure is ineffective in obese patients. There are fairly high technical difficulties in radical resection of rectal cancer, and these difficulties increase the difficulty in operations in obese patients.
When carrying out ISR for ultra-low rectal or anal canal cancer, because very low tumor, the indication for intraoperative exposure is limited, and thus obese increases the operative difficulty. However, few literature reports on the impact of obesity on the short-term efficacy of inter sphincteric resection of patients with ultra-low rectal or anal canal cancer. The purpose of this article is to evaluate whether obesity will delay the postoperative recovery effect and improve the complications.
Methods
This retrospective study includes, from January to December, 2017, 311 patients with rectal or anal canal cancer who received treatment from the Rectal Surgery Group of the Gastrointestinal Surgery Center of West China Hospital, and 276 examples are included according to the research conditions, as showed in table 1. The inclusion criteria are the following:
- Patients who are diagnosed with rectal or anal canal cancer after the colonoscopy or postoperative pathology,
- Illness with primary rectal or anal canal cancer and performed ISR,
- the distance between the tumor and the anus is ≤ 5 cm.
The exclusion criteria are the following:
- Permanent colostomy surgery
- Tumor height is > 5 cm from the anus
- Patients with missing data
The research data is collected, sorted out and extracted by a data-processing group in colorectal surgery. The information of each group should be completed and should not be missing any data. The data is recorded on an Excel spreadsheet (Office 2007, Microsoft co. Seattle, USA). The baseline index such as sex, age, BMI, diameter of tumor, differentiation of tumor, histological type of tumor, operative duration, postoperative hospitalization duration and intraoperative blood loss can be obtained from direct measurements or pathological examination. The time of first flatulence, defecating, feeding and moving out of the bed is settled. The first time of water intake is regarded as the start of eating time. The recorded postoperative complications include anastomotic leakage, anastomotic bleeding, near-term intestinal obstruction, gastric retention and stress ulcer. The patients are followed at regular intervals by outpatient service, phoned or sent the mail after discharge.
Statistics
Using the statistical software SPSS 17.0, the measurement data are expressed by (x ± s), the enumeration data are expressed by n (%). T-tests were used in normal distributions. Rank sum tests were used in other distributions. Chi-square test is used in the comparison of the enumeration data. The inspection level is α = 0.05.
Clinical data
There are 175 men and 101 women included in this study. The ages of these included patients vary from 22 to 78 and the average value is 55.8 ± 11.30. The BMI varies from 19.0 to 37.5, and the average value is 23.6 ± 3.44. According to the definition made by the World Health Organization, one who has the normal body weight has a BMI between 18.5 to 24.9, and one who is overweight has a BMI bigger than 25.
This study defines Group A as the patients who have a BMI between 18.5 and 24.9, and Group B as the patients who have a BMI greater than 25. The information is summarized in table 1.
Table 1. Baseline characteristic of patients among subgroup
|
Group A
(168 cases) |
Group B
(108 cases) |
For
chi-square |
P |
Sex [n(%)] |
Male |
103(61.3%) |
72(66.7%) |
0.813 |
0.367 |
Female |
65(38.7%) |
36(33.3%) |
Age (year, x ± s) |
55.71 ± 11.187 |
55.96 ± 11.677 |
1.109 |
0.294 |
BMI (kg/m2, x ± s) |
21.765 ± 1.559 |
27.841 ± 2.760 |
18.123 |
0.000 |
Diameter of tumor
[cm, x ± s] |
4.012 ± 1.805 |
4.094 ± 1.707 |
0.128 |
0.721 |
Differentiation [n(%)] |
High differentiation |
11 (6.5%) |
5 (4.6%) |
0.479 |
0.787 |
Moderately differentiation |
108 (64.3%) |
72 (66.5%) |
Poorly differentiation |
49 (29.2%) |
31 (28.7%) |
Histological type [n(%)] |
Adenocarcinoma |
139 (82.7%) |
91 (84.3%) |
0.127 |
0.939 |
Mucinous adenocarcinoma |
21 (12.5%) |
12 (11.1%) |
Else |
8 (4.8%) |
5 (4.6%) |
Distance from anal [cm,x ± s] |
2.332 ± 2.108 |
2.598 ± 1.789 |
0.235 |
0.628 |
Preoperative chemoradiotherapy [n(%)] |
Yes |
157 (93.5%) |
99 (91.7%) |
0.312 |
0.577 |
No |
11 (6.5%) |
9 (8.3%) |
The difference between baseline and intraoperative indicators of the subgroups
The gender composition, age distribution, tumor histologic types, differentiation, tumor size, distance from the anal and preoperative treatment between these two groups are not statistically different (p > 0.05). Their baselines are consistent and comparable; when it comes to intraoperative indicators, the operative time in Group B is apparently longer than that in Group A (p = 0.023). The intraoperative blood loss, the anastomotic patterns and the reconstruction pattern are not statistically different, which are shown in table 1 and 2.
Table 2. The comparison of intra-operation index
|
Group A
(168 cases) |
Group B
(108 cases) |
For
chi-square |
P |
Intraoperative blood loss (ml,x ± s) |
29.90 ± 18.014 |
40.09 ± 37.449 |
5.463 |
0.018 |
Time of operation (min,x ± s) |
130.91 ± 30.281 |
143.41 ± 34.331 |
4.832 |
0.023 |
Anastomotic patterns [n(%)] |
Handwork |
143 (85.1%) |
90 (83.3%) |
0.159 |
0.690 |
Anastomat |
25 (14.9%) |
18 (16.7%) |
Reconstruction way [n(%)] |
|
|
|
|
End-to-end-intestinal anastomosis |
151 (89.9%) |
91 (84.3%) |
2.112 |
0.348 |
J -Pouch |
13 (7.7%) |
14 (13.0%) |
W -Pouch |
4 (2.4%) |
3 (2.8%) |
The difference between postoperative indicators of subgroups
In terms of the postoperative recovery index, the length of hospital stay, time of first flatulence, defecating time, feeding time and the time of moving out of the bed between the two groups are not statistically different (P > 0.05). Also, the duration of day using drainage tube, urine tube and stomach tube are not statistically different (P > 0.05).
For complications of the two groups, the rate of perianal infection and the infection of incision of Group B is significantly higher than that of Group A, and the difference is statistically significant (P < 0.05). However, the rate of occurrence of other complications between the subgroups is not statistically different, which is showed in table 3. Among the three cases of anastomotic fistula, two cases who received surgical treatment, one case who received conservative treatment, four cases with intestinal obstruction patients who improved after conservative treatment. Among the ten cases of perianal infection, three cases who received surgery, seven cases who improved after conservative treatment. All the seven cases of infection of incision debridement improved after debridement.
Table 3. The comparison of postoperative index
|
Group A |
Group B |
Chi-square |
P |
Headcount |
168 |
108 |
|
|
First farting time (d x ± s) |
3.98 ± 1.087 |
3.92 ± 1.547 |
2.657 |
0.105 |
First eating time (d x ± s) |
1.55 ± 2.026 |
2.25 ± 2.327 |
0.178 |
0.673 |
First defecating time (d x ± s) |
5.42 ± 1.916 |
5.12 ± 1.693 |
0.280 |
0.597 |
Duration of day using drainage tube (d x ± s) |
2.01 ± 2.294 |
1.67 ± 1.506 |
2.859 |
0.093 |
Duration of day using urine tube (d x ± s) |
5.62 ± 2.347 |
5.49 ± 1.713 |
0.464 |
0.497 |
Duration of day using stomach tube
(d x ± s) |
1.02 ± 0.186 |
1.04 ± 0.196 |
1.814 |
0.180 |
Time of moving out of bed (dx ± s) |
2.22 ± 1.531 |
2.45 ± 1.677 |
0.539 |
0.464 |
Length of stay (d x ± s) |
7.40 ± 2.572 |
8.1 ± 2.943 |
1.481 |
0.225 |
Complications [n(%)] |
Intestinal obstruction |
2 (1.2%) |
1 (0.9%) |
0.043 |
0.836 |
Perianal infection |
3 (1.8%) |
7 (6.5%) |
4.151 |
0.042 |
Anastomotic fistula |
2 (1.2%) |
2 (1.9%) |
0.201 |
0.654 |
Infection of incisional wound |
1 (0.6%) |
6 (5.6%) |
6.544 |
0.011 |
For complications of the two groups, the LARS scores of 3 months, 6 months and 1 year after operation are not statistically different (P = 0.307, 0.119, 0.672). However, the LARS scores are improved with the prolongation of time in both groups A and B, and the difference is statistically significant (P = 0.01, 0.03), which are shown in figure 1.
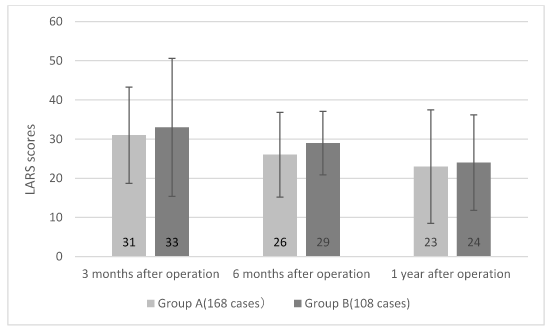
Figure 1. The comparison of postoperative LARS scores
The short-term effect of ISR for obese patients with ultra-rectal or anal canal cancer
Three hundred and eleven (311) patients with ultra-rectal or anal canal cancer are included in the study, and 276 patients are included into the analysis. The average intraoperative blood loss is 35.12 ± 13.112 ml, the operative time is 137.92 ± 25.101 min, the rate of complications is 10.1%, and the length of hospital stay is 7 days. Table 4 shows the comparison to similar studies.
Table 4. Comparison of the short-term efficacy in our and other studies
|
Sample capacity |
Intraoperative blood loss (ml) |
Time of operation (min) |
Hospital stay (d) |
Rate of complication (%) |
This study |
276 |
35 |
138 |
7 |
10.1 |
Shoichi Fujii [19] |
77 |
100 |
345 |
13 |
22.1 |
Rullier [20] |
32 |
ND |
420 |
9 |
31.3 |
Fujimoto [21] |
35 |
40 |
293 |
17 |
8.6 |
Yamamoto [22] |
29 |
109 |
335 |
8 |
24.1 |
Lim [23] |
111 |
299 |
215 |
11 |
21.6 |
Hamada [24] |
15 |
108 |
386 |
18 |
20 |
Laurent [25] |
110 |
ND |
390 |
9 |
40.9 |
The location of tumor is low, and it is difficult to get safe distal cut edge, so the ultra-low rectal or anal canal cancer is often considered the forbidden zone of anus preservation operation in the past. However, since inter sphincteric resection (ISR) was come up, ultra-low rectal or anal canal cancer patients can receive anus preservation operations. By removing one or the whole part of the internal sphincter, ISR can get a larger -than -2 cm cut edge, which most colorectal cancer surgeons consider safe in order to decrease the rate of postoperative local recurrence. The previous study shows that the five-year accumulated rate of the local recurrence is between 2% and 10.6% [9-11].
ISR is very difficult as obesity influences the exposure of pelvic surgical field. Le Marchard [7] and Yanlei Ma [8] shows that obesity can increase the rate of having colorectal cancer. Therefore, more attention is paid to the safety and effectiveness of operation for obese patients with rectal cancer. This study discusses whether obesity can influence the short-term effect of ISR for the patients with ultra-rectal or anal canal cancer by using a retrospective case-control design, so that we can find out whether doctors can finish the ISR successfully for obese patients with ultra-rectal or anal canal cancer and preserve the anus as well.
For rectal cancer, especially ultra-low rectal or anal canal cancer, radical operation needs to be done in narrow pelvis which causes the exposure of surgical field is often insufficient, a high surgical technique is required as well, so the difficulty of the operation is high. Daniel Leonard [9] holds that it is more difficult to do a sufficient TME for the obese patients than for the normal patients. However, some studies [10-13] demonstrated that obesity increases the difficulty of the surgical treatment for the rectal cancer. Our study shows that the time of operation of the obese group is greater than that of normal group and the difference is statistically significant (p < 0.05). Also, a study by Saito N [2] shows that obesity increases the operative difficulty and the time of operation for the patients with ultra-low rectal or anal canal cancer who receive ISR. However, it is worth mentioning that obesity does not increase the intraoperative blood loss according to our research (p > 0.05), and perhaps it relates to the degree of specialization of our medical group.
Because obesity increases the time of ISR operation for the patients with ultra-low rectal or anal canal cancer, it causes more damage to the patients and has negative effect to the postoperative recovery. In the meantime, there is often internal medicine disease such as diabetes, hypertension and cardiovascular disease in the obese patients, so most colorectal surgeons hold that the rate of complications such as infection of incisional wound or cardiopulmonary disease in obese patients is higher than that of normal patients. The study by Aytac E [14] shows that the incidence rate of postoperative anastomosis fistula increases significantly for obese patients with rectal cancer (p = 0.0003). However, this study indicates that the incidence rate of postoperative anastomosis fistula of the two groups is low and not statistically different (1.2% vs. 1.9% p > 0.05). Previous reports [1,2,15,16] show that in experienced medical institutions, the probability of ISR postoperative anastomosis fistula is between 5% and 16%. This may benefit by all patients in this study are operated by the same professional doctors in colorectal surgery who have rich experience in ISR, and this study adopts eversion removal technology, doing anal anastomosis and manual stitching reinforcement under direct view. Our study also shows that the incidence rate of complications of postoperative incision infection, intestinal obstruction, perianal infection, and pelvic infection and the difference of the overall incidence of complications are not statistically different (P > 0.05). The study by Mrak K [17] also shows that there is no evidence to support that obesity may increase the incidence rate of postoperative complications of the patients with rectal cancer. Therefore, doing ISR surgery on obese patients with ultra-low rectal or anal canal cancer is safe and effective judging from the recent curative effect. However, it is worthwhile to mention that, due to the ultra- low anastomotic location, the care of crissum is extremely important for the obese patients with ultra-low rectal or canal cancer as well as receiving ISR, otherwise it may affect patients with postoperative anastomotic healing. The standard practice of our professional group is to wipe zinc oxide or tannin ointment for protection to the crissal skin after surgery. The incidence rate of the perianal infection in this study is 3.6%, and the incidence rate of perianal infection in Group B is apparently higher than that in Group A, and the variance is statistically different (P < 0.05). Zhiming Gan [18] and other scholars say that the patients with rectal or anal canal cancer are vulnerable to perianal infection after anal anastomosis, while fat of the body is likely to increase the susceptibility of patients. In addition, obese patients are prone to fat liquefaction, which have been problematics for surgeons to assess. This study indicates that the rate of incision infection in Group A is higher than Group B and the result is statistically different (0.6% vs. 5.6% P > 0.05).
Furthermore, this research finds that the length of hospital stay, time of first flatulence, feeding time, defecating time and time of moving out of bed between two groups are not statistically different (p > 0.05), and the duration of day using drainage tube, urine tube and stomach tube are not statistically different (p > 0.05). The results support the notion that obesity does not have a negative effect on ISR for the patients with ultra-low rectal or anal canal cancer. This information is important to the fast track programs carried out regularly in colorectal surgery which provides more safety for the postoperative recovery of the patients with rectal cancer.
Obesity increases the difficulty of performing ISR for ultra-low rectal or anal canal cancers, extends the time of operation, and increases the incidence rate of perianal infection post-operatively. However, there is no significant difference between the indications of postoperative recovery, and the incidence rate of complications in obese patients and that in normal weight patients. In terms of the short-term effects, ISR for obese patients is safe and effective. Nonetheless, this research is a retrospective case-control study and the sample capacity is relatively small, so a multi-center randomized controlled study with a large sample capacity is required to verify the conclusions.
- Chamlou R, Parc Y, Simon T, Bennis M, Dehni N, et al. (2007) Long-term results of intersphincteric resection for low rectal cancer. Ann Surg 246: 916-921. [Crossref]
- Saito N, Moriya Y, Shirouzu K, Maeda K, Mochizuki H, et al. (2006) Intersphincteric resection in patients with very low rectal cancer: a review of the Japanese experience. Dis Colon Rectum 49: S13-22. [Crossref]
- Schiessel R, Novi G, Holzer B, Rosen HR, Renner K, et al. (2005) Technique and long-term results of intersphincteric resection for low rectal cancer. Dis Colon Rectum 48: 1858-1865. [Crossref]
- Yamada K, Ogata S, Saiki Y, Fukunaga M, Tsuji Y, et al. (2007) Functional results of intersphincteric resection for low rectal cancer. Br J Surg 94: 1272-1277. [Crossref]
- Rullier E, Goffre B, Bonnel C, Zerbib F, Caudry M, et al. (2001) Preoperative radiochemotherapy and sphincter-saving resection for T3 carcinomas of the lower third of the rectum. Ann Surg 234: 633-640. [Crossref]
- Tiret E, Poupardin B, McNamara D, Dehni N, Parc R (2003) Ultralow anterior resection with intersphincteric dissection--what is the limit of safe sphincter preservation? Colorectal Dis 5: 454-457. [Crossref]
- Le Marchand L, Wilkens LR, Mi MP (1992) Obesity in youth and middle age and risk of colorectal cancer in men. Cancer Causes Control 3: 349-354. [Crossref]
- Ma Y, Yang Y, Wang F, Zhang P, Shi C, et al. (2013) Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One 8: e53916. [Crossref]
- Leonard D, Penninckx F, Fieuws S, Jouret-Mourin A, Sempoux C, et al. (2010) Factors predicting the quality of total mesorectal excision for rectal cancer. Ann Surg 252: 982-988. [Crossref]
- Schwandner O, Farke S, Schiedeck TH, Bruch HP (2004) Laparoscopic colorectal surgery in obese and nonobese patients: do differences in body mass indices lead to different outcomes? Surg Endosc 18: 1452-1456. [Crossref]
- Leroy J, Ananian P, Rubino F, Claudon B, Mutter D, et al. (2005) The impact of obesity on technical feasibility and postoperative outcomes of laparoscopic left colectomy. Ann Surg 241: 69-76. [Crossref]
- Dostalik J, Martinek L, Vavra P, Andel P, Gunka I, et al. (2013) Laparoscopic colorectal surgery in obese patients. Obes Surg 15: 1328-1331. [Crossref]
- Delaney CP, Pokala N, Senagore AJ, Casillas S, Kiran RP, et al. (2005) Is laparoscopic colectomy applicable to patients with body mass index >30? A case-matched comparative study with open colectomy. Dis Colon Rectum 48: 975-981. [Crossref]
- Aytac E, Lavery IC, Kalady MF, Kiran RP (2013) Impact of obesity on operation performed, complications, and long-term outcomes in terms of restoration of intestinal continuity for patients with mid and low rectal cancer. Dis Colon Rectum 56: 689-697. [Crossref]
- Akasu T, Takawa M, Yamamoto S, Fujita S, et al. (2007) Incidence and patterns of recurrence after intersphincteric resection for very low rectal adenocarcinoma. J Am Coll Surg 205: 642-647. [Crossref]
- Rullier E, Laurent C, Bretagnol F, Rullier A, Vendrely V, et al. (2005) Sphincter-saving resection for all rectal carcinomas: the end of the 2-cm distal rule. Ann Surg 241: 465-469. [Crossref]
- Mrak K, Eberl T, Fritz J, Tschmelitsch J (2012) Influence of body mass index on postoperative complications after rectal resection for carcinoma. South Med J 105: 493-499. [Crossref]
- Z G, C M, X W, L L (2013) The quality of the intersphincteric resection for ultra-low rectal cancer and anal cancer. Sichuan Medical Journal 09: 1303-1305.
- Fujii S, Yamamoto S, Ito M, Yamaguchi S, Sakamoto K, et al. (2012) Short-term outcomes of laparoscopic intersphincteric resection from a phase II trial to evaluate laparoscopic surgery for stage 0/I rectal cancer: Japan Society of Laparoscopic Colorectal Surgery Lap RC. Surg Endosc 26: 3067-3076. [Crossref]
- Rullier E, Sa Cunha A, Couderc P, Rullier A, Gontier R, et al. (2003) Laparoscopic intersphincteric resection with coloplasty and coloanal anastomosis for mid and low rectal cancer. Br J Surg 90: 445-451. [Crossref]
- Fujimoto Y, Akiyoshi T, Kuroyanagi H, Konishi T, Ueno M, et al. (2017) Safety and feasibility of laparoscopic intersphincteric resection for very low rectal cancer. J Gastrointest Surg 14: 645-650. [Crossref]
- Yamamoto S, Fujita S, Akasu T, Inada R, Takawa M, et al. (2011) Short-term outcomes of laparoscopic intersphincteric resection for lower rectal cancer and comparison with open approach. Dig Surg 28: 404-409. [Crossref]
- Lim SW, Huh JW, Kim YJ, Kim HR. Laparoscopic intersphincteric resection for low rectal cancer. World J Surg 35: 2811-2817. [Crossref]
- Hamada M, Matsumura T, Matsumoto T, Teraishi F, Ozaki K, et al. (2011) Video. Advantages of the laparoscopic approach for intersphincteric resection. Surg Endosc 25: 1661-1663. [Crossref]
- Laurent C, Paumet T, Leblanc F, Denost Q, Rullier E (2012) Intersphincteric resection for low rectal cancer: laparoscopic vs open surgery approach. Colorectal Dis 14: 35-43. [Crossref]

