Previously it was shown that wheat germ agglutinin (WGA) and, at a minor extent, phaseolus vulgaris agglutinin (PHA), are able to induce platelets activation. Since the endothelial nitric oxide synthase (eNOS)/nitric oxide (NO)/soluble guanylyl cyclase/cGMP/cGMP-dependent protein kinase (PKG) pathway is one of the major antiaggregating mechanism present in platelets, we tested the WGA or PHA effect on this pathway. It has been shown that platelets treated with WGA did not produce NO, while PHA stimulated NO production in a dose and time dependent manner. It has been found that the increased NO formation induced by PHA was dependent on eNOS phosphorylation/activation. The Ca2+/calmodulin-dependent kinase kinase/AMP activated protein kinase pathway seems to be greatly involved as STO-609 and Compound C, Ca2+/calmodulin protein kinase kinase/AMP kinase inhibitors respectively, cancelled eNOS phosphorylation induced by PHA. One crucial effect of NO and cGMP elevation is the activation of PKG, that can phosphorylate vasodilator-stimulated phosphoprotein (VASP). It was found that NO and cGMP elevation and VASP phosphorylation both on ser239 and thr278 were greatly stimulated by PHA and strongly inhibited by STO-609 and Compound C and by the eNOS inhibitor L-NAME. Thus, the CaMKK/AMPK pathway activated by PHA can regulate platelet activation stimulating the eNOS/NO/cGMP/PKG signalling pathway.
CaMKK/AMPK pathway, eNOS phosphorylation/activation, human platelets, nitric oxide, phaseolus vulgaris agglutinin, wheat germ agglutinin
Abbreviations
ACC: acetylCoA carboxylase; AKT: protein kinase B; AMPK: AMP-activated protein kinase; CaMKK: Ca2+/calmodulin kinase kinase; eNOS: endothelial nitric oxide synthase; LKB1: liver kinase B1; NO: nitric oxide; PHA: phaseolus vulgaris agglutinin; PI3K: phosphatidylinositol 3 kinase; PKA: protein kinase A; PKG: protein kinase G; PLC: phospholipase C; VASP: vasodilator-stimulated phosphoprotein; WGA: wheat germ agglutinin.
Platelet activation is involved in both haemostasis and thrombosis. When platelets encounter matrix proteins exposed by injury to the vessel wall, they stop on the exposed subendothelial surface, become activated showing morphological alterations, secrete the content of their granules and aggregate. Inhibition of platelet aggregation can be produced by the block of membrane receptors interaction with intracellular signalling pathways, by interfering with platelet-activating messengers or by potentiating the action of physiological platelet inhibitors such as endothelium derived PGI2 and nitric oxide (NO). These compounds activate adenylyl and guanylyl cyclases leading to cAMP and cGMP increase, respectively. The elevation of these two platelet cyclic nucleotides interferes with platelet activatory signalling pathways such as the intracellular Ca2+ elevation and the reorganization of the cytoskeleton.
In human platelets NO formation depends on endothelial nitric oxide synthase (eNOS) activation. Platelet eNOS is now largely considered a Ca2+-independent enzyme and the phosphorylation/dephosphorylation of ser1177 and/or thr495 residues plays a vital role in the regulation of its activity. Phosphorylation of ser1177 residue activates eNOS, while phosphorylation of thr495 residue inhibits the activity of the enzyme [1]. Several kinases regulate eNOS phosphorylation including AMP-activated protein kinase (AMPK) [2-4]. AMPK is a metabolic sensor that coordinates metabolism and energy demand [5]. The α isoform of AMPK is activated in platelets upon thrombin or the endocannabinoid 2-arachidonoylglycerol stimulation of human platelets. Activated AMPK phosphorylates and inhibits acetylCoA carboxylase (ACC) that is considered a marker of AMPK activation [6,7]. Recently it has been shown that AMPK/ACC signalling regulates thromboxane A2 and granule release in response to collagen and hence influences thrombus formation [8]. AMPK can phosphorylate eNOS on ser1177 exerting a role in the regulation of eNOS activity in cardiac myocytes under ischemic conditions associated with cellular energy depletion [9]. Moreover, AMPK has a role in the phosphorylation of the neuronal NOS in exercising skeletal muscle and regulates the insulin-induced activation of eNOS in human platelets [10,11]. In addition, AMPK generates oxidative stress through the phosphorylation of neuronal NOS in cardiomyocytes [12].
Lectins are proteins or glycoproteins, usually of plant origin that recognise and bind to carbohydrate moieties of complex glycoconjugates without altering the covalent structure of any of the recognized glycosyl ligands [13]. Wheat germ agglutinin (WGA) is a cereal lectin specific for two types of N-acetylated sugars, N-acetyl-D-glucosamine and N-acetyl-D-neuraminic acid and interacts with sialyted-cell surface receptors [14,15]. Phaseolus vulgaris agglutinin (PHA) is a tetrameric protein with a molecular weight of 120kDa with sugar specificity for glucosaminyl, mannopyranosyl residue [16].
Previously, we have shown that WGA and PHA both are able to stimulate platelet aggregation but with different potency, being WGA more powerful than PHA [17]. Carrying on these studies we have tested the effect of WGA and PHA on NO production. The results obtained have demonstrated that PHA is able to induce NO formation, while WGA has no effect. Moreover the Ca2+/calmodulin-dependent kinase kinase (CaMKK) b/AMPKa pathway appears to be mainly involved in eNOS phosphorylation and in the consequent NO elevation induced by PHA, as STO-609, inhibitor of CaMKK, and Compound C, potent reversible inhibitor of AMPK, significantly reduce eNOS phosphorylation/activation and NO formation.
Materials
Anti-p-VASP (thr278), aprotinin, apyrase, Colorburst™ electrophoresis marker, compound C, digitonin, Dowex AG 50W-X8, EGTA, leupeptin, b-mercaptoethanol, L-NAME, PGE1, PMSF, protease inhibitor cocktail (Cat. N° P8340), staurosporine, STO-609, 96-well plate (CostarÒ) and all chemicals were from Sigma-Aldrich, USA. MK2206 was from Selleck Chemicals USA. Anti-p-eNOS (ser1177), anti-p-serine and anti-p-threonine antibodies, lectins (WGA and PHA) and LY294002 were purchased from Merck Millipore, Germany. LY294002, MK2206, Compound C and STO-609 were diluted in saline from a stock DMSO solution immediately before each experiment. OxiselectÔ Nitric oxide assay kit (Cat. N° STA-802) was from Cell Biolabs, Inc. USA. cGMP EIA kits (Cat. N° 900-164) was from Assay Design USA. L-[2,3,4,5-3H] arginine was from PerkinElmer Life and Analytical Sciences, USA. Anti-p-ACC (ser79), anti-p-AMPKa (thr172), anti-p-VASP (ser239), anti-LKB1, horseradish peroxidase-conjugated secondary antibodies and anti-b-actin were purchased from Santa Cruz Biotechnology, USA. ECLÒ system was from GE Healthcare, USA. Nitrocellulose membranes (pore size 0.45 µm) were from Bio-Rad Laboratories, USA.
Blood collection and preparative procedures
Freshly drawn venous blood from healthy volunteers of the “Centro Trasfusionale, Ospedale San Martino” in Genoa was collected into 130 mM aqueous trisodium citrate anticoagulant solution (9:1). The donors claimed to have not taken drugs known to interfere with platelet function during two weeks prior to blood collection and gave their informed consent. Washed platelets were prepared centrifuging whole blood at 100´g for 25 min. To the obtained platelet-rich plasma (PRP) 4 mU/mL apyrase and 4 µM PGE1 were added. PRP was then centrifuged at 1100´g for 15 min. Pellet was washed once with pH 5.2 ACD solution (75 mM trisodium citrate, 42 mM citric acid and 136 mM glucose), centrifuged at 1100´g for 15 min and then resuspended in Ca2+-free HEPES buffer containing 145 mM NaCl, 5 mM KCl, 1 mM MgSO4, 10 mM glucose, 10 mM Hepes (pH 7.4).
Nitrite + nitrate (NOx) measurement
Washed platelets (1.0×109 platelets/mL), preincubated at 37°C with saline, were stimulated, in the presence of 100 µM L-arginine, with lectins as indicated. In other experiments, washed platelets (1.0×109 platelets/mL) were preincubated with saline, 500 µM L-NAME, 10 µM STO-609, 10 µM Compound C, 20 µM LY294002 or 20 µM MK2206 and then treated with 10 µg/mL PHA for 120 sec. Incubation was stopped by putting samples on ice. NOx content was measured at 540 nm in a 96-well plate by spectrophotometry using iMarkÔ microplate reader (Bio Rad Laboratories) following the manufacturer’s instruction of the commercial kit.
cGMP Assay
Washed platelets (1.0×109 platelets/mL) were preincubated at 37°C with saline and then stimulated, in the presence of 100 µM L-arginine, with lectins. In other experiments, washed platelets (1.0×109 platelets/mL) were preincubated with saline, 500 µM L-NAME, 10 µM STO-609, 10 µM Compound C, 20 µM LY294002 or 20 µM MK2206 and then challenged with 10 µg/mL PHA for 120 sec. The reaction was stopped by the addition of cold perchloric acid (2 M). Precipitated proteins were removed by centrifuging at 12000×g for 2 min at 4°C. Obtained supernatants, neutralized with 2 M NaOH, were immediately analysed by a cGMP specific EIA kit according to the manufacturer’s protocol.
Immunoblotting analysis of phosphoproteins
In the experiments in which the dose-dependent effect of PHA was evaluated, washed platelets (1.0´109 platelets/mL), preincubated with saline, were stimulated with increasing concentrations of the lectin for 120 sec. When the time-dependence was assessed, platelets were challenged with 10 µg/mL PHA. In other experiments washed platelets (1.0×109 platelets/mL), preincubated at 37°C with saline, 10 µM STO-609, 20 µM LY294002, 20 µM MK2206 or 10 µM Compound C, were stimulated with 10 µg/mL PHA for 120 sec. Incubation was stopped by adding 2×Laemmli-SDS reducing sample buffer. Samples, heated for 5 min at 100°C, were separated by 5-10% SDS-PAGE and transferred to nitrocellulose membranes. Running was performed in the presence of Colorburst™ Electrophoresis weight markers. Blots were blocked in 5% BSA dissolved in TBST (Tris buffer saline, pH 7.6, containing 10 mM Tris, 150 mM NaCl, and 0.1% Tween 20) at 37°C for 30 min, and then incubated overnight at 4°C with anti-p-ACC (ser79), anti-p-AMPKa (thr172), anti-p-eNOS (ser1177), anti-p-VASP (ser239) or anti-p-VASP (thr278) (1:500 dilutions) antibodies. Membranes were extensively washed and incubated for 60 min at room temperature with horseradish peroxidase-conjugated secondary antibody. After further washings, blots were developed using the ECLÒ system. Nitrocellulose membranes were then stripped by incubation with 62.5 mM Tris/HCl (pH 6.7), 2% SDS, 100 µM β-mercaptoethanol for 30 min at 50°C and reprobed with anti-b-actin. Band density, reported as fold-change relative to control and normalized to b-actin, was directly quantified by the Bio-Rad Chemi-Doc software package.
eNOS activity assay
eNOS activity was measured by evaluating the conversion of L-[3H]arginine to L-[3H]citrulline, according to the method adapted to human platelets [18]. Briefly, aliquots of washed platelets (1.0´109 platelets/mL), prewarmed at 37°C with saline, were incubated with PHA as indicated in the presence of 1 µCi L-[3H] arginine. In the experiments in which the inhibitors effect was determined, washed platelets (1.0×109 platelets/mL), preincubated at 37°C with saline, 500 µM L-NAME, 10 µM STO-609, 10 µM Compound C, 20 µM LY294002 or 20 µM MK2206, were treated with 10 µg/mL PHA for 120 sec. Incubation was stopped by putting samples on ice. Platelets were then pelleted by centrifugation at 2000xg for 4 min. After sonication, platelet lysates were mixed with Dowex AG 50W-X8 (Na+-form) to absorb L-arginine and L-[3H] citrulline was measured in supernatants by liquid scintillation counting (Packard Instruments).
Immunoprecipitation
Washed platelets (1.0×109 platelets/mL) were prewarmed at 37°C with saline and then incubated with 10 µg/mL PHA. Incubation was stopped by adding an equal volume of lysis mixture (0.5% SDS, 1% Triton X-100, 0.75% sodium deoxycholate, 10 mM EDTA, 1 mM PMSF, 50 mM NaF, 200 µM Na3VO4, 100 µM leupeptin, 100 µg/mL aprotinin, 10 µM staurosporine and 10 mL/mL protease inhibitor cocktail). Lysates, after a brief centrifugation, were treated with 1.0 µg of anti-LKB1 antibody for 2 h at 4°C. The immunocomplexes were precipitated with protein G-sepharose fast-flow. After 60 min on ice, samples were washed with 1.0 ml of IP-wash 1 (10 mM pH 7.4 Tris/HCl, 150 mM NaCl, 0.5% Triton X-100), followed by IP-wash 2 (10 mM pH 7.Tris/HCl, 750 mM NaCl, 0.5% Triton X-100) and finally again with IP-wash 1. Samples were extracted with 100 µL of 2×Laemmli-SDS reducing sample buffer, heated at 80°C for 10 min and resolved on 5-10% SDS-PAGE. Proteins were transferred to nitrocellulose membranes, blots blocked in 5% BSA dissolved in TBST at 37°C for 30 min, incubated overnight at 4°C with anti-p-serine or anti-p-threonine antibodies (1:500 dilutions), were treated as above detailed. Finally, blots were stripped as above described and reprobed with anti-LKB1.
Statistical analysis
Data are mean ± SD of at least four independent experiments, each performed in duplicate. Statistical comparisons between two groups were made through the unpaired Student’s t-test. To compare multiple groups two-way ANOVA followed by Tukey’s post hoc test was used. Statistical significance was defined as p<0.05.
Effect of PHA and WGA on NOx and cGMP formation
Treatment of platelets with PHA leads to a significant NO production. The lectin effect is dose- and time-dependent. PHA peaks at 10 mg/mL (Figure 1A), reaches the maximum after 120 sec and maintained for 5 min (Figure 1B). On the contrary WGA does not change basal NO levels (Figures 1A & 1B). NO through the guanylyl cyclase activation stimulates cGMP formation. Thus, we have measured the lectins effect on cGMP production. cGMP level is significantly increased in platelets treated with PHA, but not with WGA (Figures 1C & 1D). The PHA effect is dose- (Figure 1C) and time-dependent (Figure 1D). The cGMP and NO elevation induced by PHA are strictly correlated (R2=0,9971, P>0,0001).
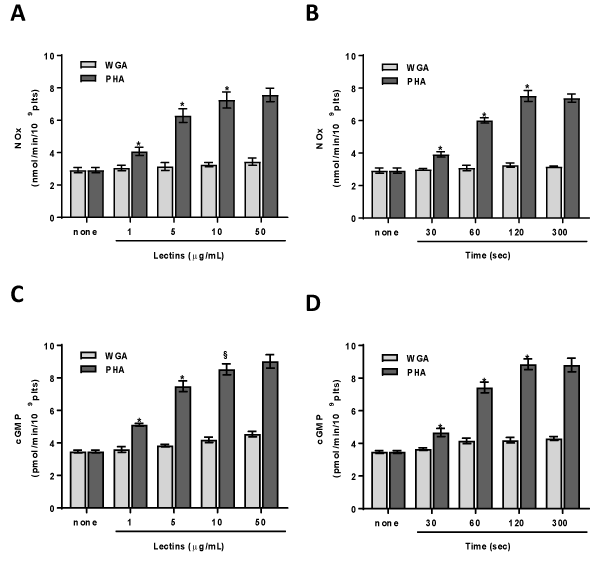
Figure 1. PHA and WGA effect on NOx and cGMP concentration. Washed platelets (1.0×109 platelets/mL) were incubated, in the presence of 100 µM L-arginine, for 120 sec with varying concentrations (panels A, C) or with 10 µg/mL lectins (panels B, D) as indicated. NOx (panels A, B) and cGMP (panels C, D) were assayed as detailed in Methods. Each bar represents the mean ± SD of four independent experiments carried out in duplicate. Two-way ANOVA-Tukey’s post hoc test: *P<0,0001, §P<0,001
Effect of PHA on eNOS phosphorylation and activation
Since WGA does not induce platelet NO elevation, the studies were going on PHA. Results obtained put in evidence that PHA stimulates eNOS phosphorylation at ser1177 residue. The effect is dose- and time- dependent peaking at 10 mg/mL (Figure 2A) and 120 sec (Figure 2B), respectively. In order to determine whether platelet treatment with PHA leads to the activation of eNOS, we have measured the lectin effect on the conversion of L-[3H] arginine to L-[3H] citrulline. We have found that PHA activates eNOS and in particular 10 mg/mL PHA causes about three-fold increase in citrulline formation (Figures 2C & 2D). The eNOS activity is in strict correlation with eNOS phosphorylation (R2=0,9928, P=0,0003) and NO production (R2=0,9991, P>0,0001).
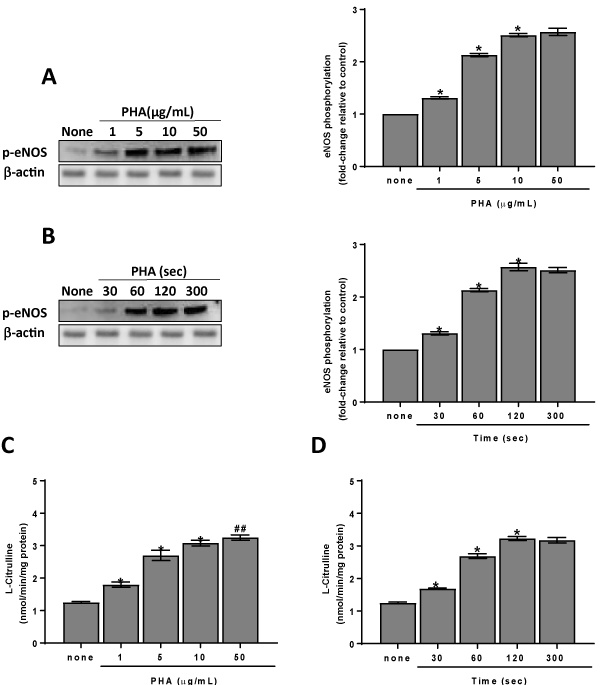
Figure 2. PHA effect on eNOS phosphorylation/activation. Panels A, B: washed platelets (1.0×109 platelets/mL) were incubated for 120 sec with varying concentrations (panel A) or with 10 µg/mL PHA (panel B) as indicated. Suitable aliquots were immunoblotted with anti-p-eNOS (ser1177) as detailed in Methods. Blots are representative of four independent experiments. In the right panels fold-change relative to control of densitometric scanning ± SD of eNOS phosphorylation measured in four experiments is reported. Panels C, D: washed platelets (1.0×109 platelets/mL) were incubated, in the presence of 100 µM L-arginine, for 120 sec with varying concentrations (panels C) or with 10 µg/mL PHA (panels D) as indicated. eNOS activity was assayed as described in Methods. Each bar represents the mean ± SD of four independent experiments carried out in duplicate. Two-way ANOVA-Tukey’s post hoc test: *P<0,0001, ##P<0,05
The involvement of AMPK in eNOS phosphorylation/activation
The eNOS enzyme is modulated by different regulatory mechanisms including phosphorylation by specific protein kinases such as protein kinase B (AKT) and AMPK [9,10,19]. Thus, we have tested the effect of specific inhibitors of such kinases on eNOS phosphorylation stimulated by PHA. It was found that LY294002 and MK2206, inhibitors of phosphatidylinositol 3 kinase (PI3K) and AKT respectively, were ineffective on this parameter (Figure 3A & 3B), while the AMPK inhibitor Compound C had a great inhibiting effect. One of the main upstream kinases involved in AMPK activation is CaMKKb. Hence, to verify the influence of the CaMKKb/AMPKα pathway on eNOS phosphorylation we have tested the effect of the specific CaMKKb inhibitor, STO-609 on eNOS phosphorylation induced by PHA. We have shown that STO-609 has a great inhibiting effect (Figures 3A & 3B). In the same way the eNOS inhibitor L-NAME, STO-609 and Compound C significantly (p<0,0001) reduce eNOS activity, NO and cGMP elevation while LY294002 and MK2206 do not change these parameters in platelets treated with PHA (Figures 3C & 3D).
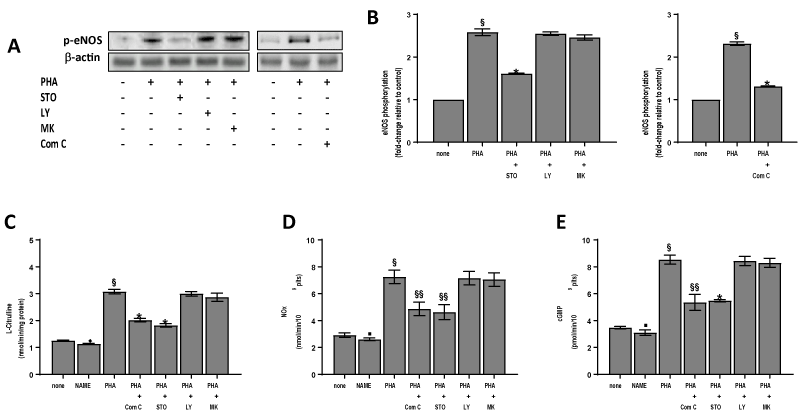
Figure 3. Effect of inhibitors on lectin-induced eNOS phosphorylation, eNOS activity, NOx and cGMP concentration. Washed platelets (1.0×109 platelets/mL), prewarmed at 37°C with saline, 500 µM L-NAME, 10 µM Compound C (Com C), 10 µM STO-609 (STO), 20 µM LY294002 (LY) or 20 µM MK2206 (MK) were incubated for 120 sec with 10 µg/mL PHA (PHA) in the presence of 100 µM L-arginine when required. Suitable aliquots were immunoblotted with anti-p-eNOS (ser1177) (panels A, B). In the right panels (B) fold-change relative to control of densitometric scanning ± SD of eNOS phosphorylation of four experiments is reported. The eNOS activity (panel C), NOx (panel D) and cGMP (panel E) content were assayed as detailed in Methods. Each bar of panels B, C, D, E, represents the mean ± SD of four independent experiments carried out in duplicate. Student’s t-test: §P<0,0001, ♦P<0,005, ▪P<0,05 vs none; *P<0,0001, §§P<0,005, vs PHA
The effect of PHA on AMPK phosphorylation/activation
Data reported in Figure 3 indicate that the CaMKKb/AMPKα pathway is greatly involved in eNOS phosphorylation/activation induced by PHA. In fact, PHA stimulates phosphorylation of AMPK at thr172 in a dose- and time-dependent manner (Figures 4A & 4B). Once activated AMPK can phosphorylate many targets, including ACC. The phosphorylation of this enzyme at ser39 is typically used as a marker of AMPK activation in cells and tissues including platelets [6,7]. ACC results phosphorylated after platelet treatment with PHA and its phosphorylation pattern is superimposable to that of AMPK (Figures 4A & 4B).
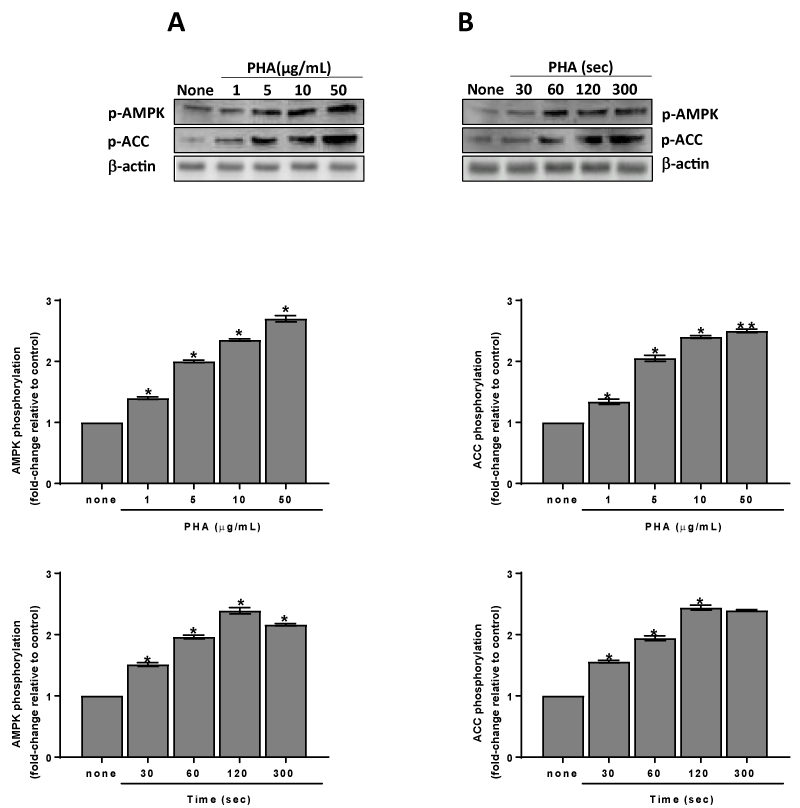
Figure 4. Phosphorylation and activation of AMPKa induced by PHA. Washed platelets (1.0×109 platelets/mL) were incubated for 120 sec with varying concentrations (panels A) or with 10 µg/mL (panels B) PHA as indicated. Suitable aliquots were immunoblotted with anti-p-AMPKa (thr172) or anti-p-ACC (ser79) as detailed in Methods. Blots are representative of four independent experiments. In the bottom panels fold-change relative to control of densitometric scanning ± SD of AMPKa and ACC phosphorylation measured in four experiments is reported. Two-way ANOVA-Tukey’s post hoc test: *P<0,0001, **P<0,0005
In addition to CaMKKb, liver kinase B1 (LKB1) can be also the upstream kinase involved in the AMPK activation in human platelets [1]. Thus, we have measured LKB1 phosphorylation after immunoprecipitation of platelet extracts. No phosphorylation on LKB1 serine or threonine residues was observed after platelet treatment with PHA (data not shown). Therefore CaMKKb is the main kinase involved in AMPK phosphorylation/activation induced by PHA. This finding is supported by data of Figure 5 showing that both AMPK and ACC phosphorylation challenged by PHA are significantly reduced (p<0,0001) by STO-609.
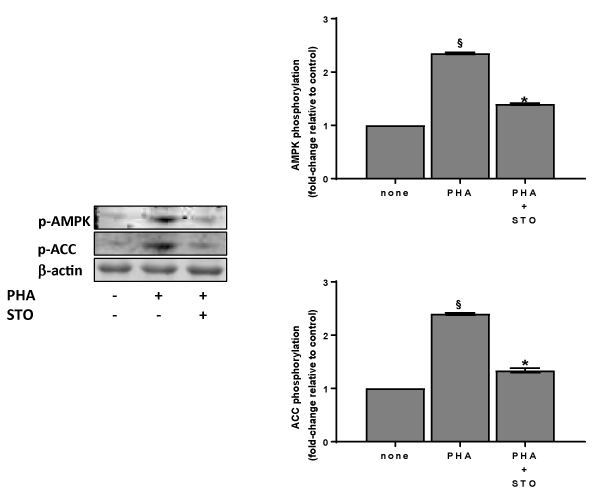
Figure 5. Effect of STO-609 on lectin-induced AMPK phosphorylation/activation. Washed platelets (1.0×109 platelets/mL), prewarmed at 37°C with saline or 10 µM STO-609 (STO) were incubated for 120 sec with 10 µg/mL PHA. Suitable aliquots were then immunoblotted with anti-p-AMPK (thr172) or anti-p-ACC (ser79) as detailed in Methods. Blots are representative of four independent experiments. In the right panels fold-change relative to control of densitometric scanning ± SD of AMPKa and ACC phosphorylation of four experiments is reported. Student’s t-test: §P<0,0001, vs none; *P<0,0001 vs PHA
Effect of PHA on vasodilator-stimulated phosphoprotein (VASP) phosphorylation
The cGMP elevation, through the protein kinase G (PKG) activation, induces VASP phosphorylation [20]. Thus, we have evaluated the PHA effect on ser239 and thr278 residues phosphorylation of VASP. The effect of PHA is dose- and time-dependent (Figures 6A & 6B). The involvement of the CaMKKb/AMPKα pathway on VASP phosphorylation is supported by the results obtained in platelets pre-treated with STO-609 or with Compound C. Both inhibitors cancel PHA induced VASP phosphorylation on ser239 and thr278 (Figure 7).
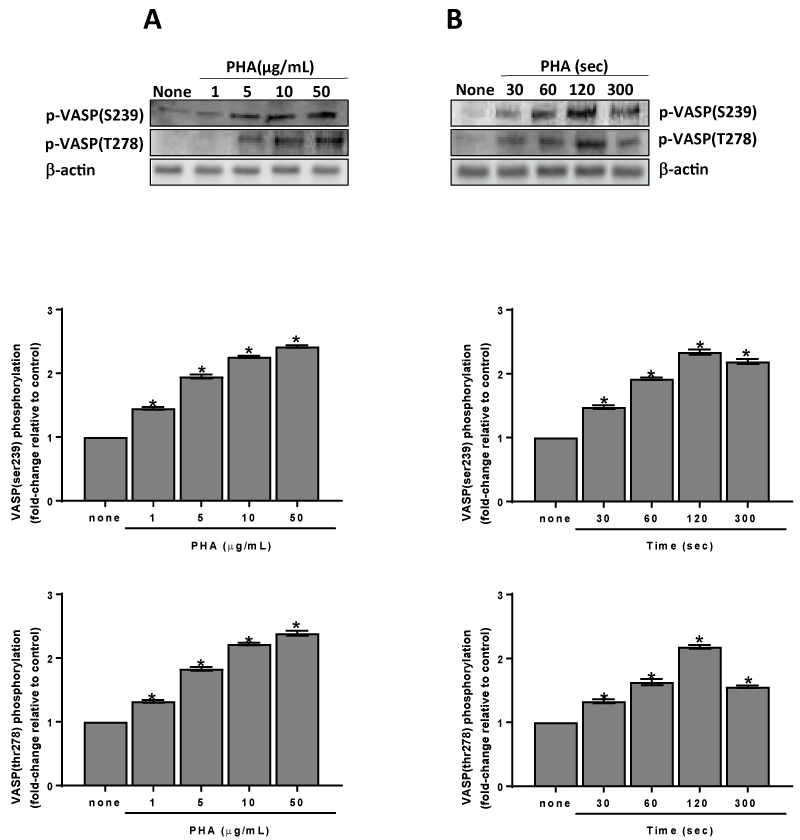
Figure 6. Phosphorylation of VASP induced by PHA. Washed platelets (1.0×109 platelets/mL) were incubated for 120 sec with varying concentrations (panel A) or with 10 µg/mL (panel B) PHA as indicated. Suitable aliquots were immunoblotted with anti-p-VASP (thr278) or anti-p-VASP (ser239) as detailed in Methods. Blots are representative of four independent experiments. In the bottom panels fold-change relative to control of densitometric scanning ± SD of VASPser239 and thr278 phosphorylation measured in four experiments is reported. Two-way ANOVA-Tukey’s post hoc test: *P<0,0001
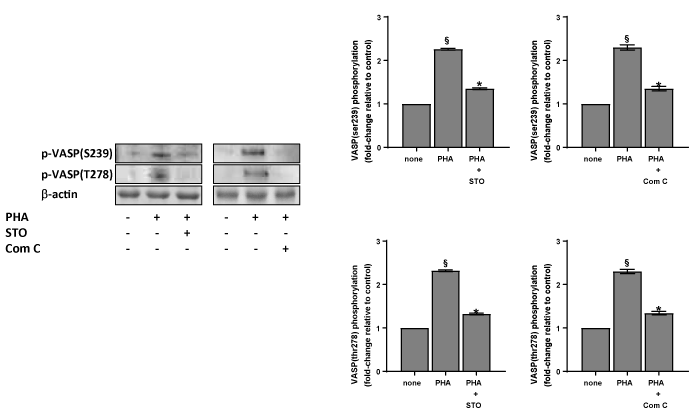
Figure 7. Effect of inhibitors on the phosphorylation of VASP induced by PHA. Washed platelets (1.0×109 platelets/mL), prewarmed at 37°C with saline, 10 µM STO-609 (STO) or 10 µM Compound C (Com C), were incubated for 120 sec with 10 µg/mL PHA. Suitable aliquots were then immunoblotted with anti-p-VASP (ser239) or anti-p-VASP (thr278) as detailed in Methods. Blots are representative of four independent experiments. In the right panels fold-change relative to control of densitometric scanning ± SD of VASPthr278 and ser239 phosphorylation of four experiments is reported. Student’s t-test: §P<0,0001 vs none; *P<0,0001 vs PHA
Lectins are proteins or glycoproteins of plant origin. PHA is a tetrameric protein of 120kDa with sugar specificity for glucosaminyl (1-2) mannopyranosyl residue. WGA, which is a cereal lectin of molecular weight of 120kDa, have sugar specificity for b-D-glucosaminyl residue. Several studies on the WGA effect in cells has been carried out, while only few data on PHA are described. It was shown that WGA activates macrophages and NADPH oxidase activity in human neutrophils, induces rapid protein tyrosine phosphorylation and phospholipase C (PLC) activation in human platelets [21-24]. Recently the Src/Syk pathway, the adapter protein SLP-72 and the exchange protein Vav have been found involved in PLCg2 activation induced by lectins, being WGA more potent than PHA in stimulating these mechanisms [17]. Studies on the role of varying lectins among which PHA and WGA in the activation of murine macrophages with reference to production and regulation of NO have demonstrated that PHA stimulates NO production, while WGA has not effect [25]. The importance of different key signalling pathways in the regulation of NO production on the macrophages stimulated by PHA such as PI3K, protein kinase C, Ca2+, p42-44MAPK, JNK and Jak/STAT pathways has been shown [25]. Several protein kinases can be implicated in eNOS phosphorylation such as AKT, protein kinase A (PKA), protein kinase C, AMPK and Ca2+/calmodulin-dependent protein kinase II [3,4,19,26-29]. In endothelial cells and vessels, leptin is able to stimulate NO release as a consequence of eNOS phosphorylation/activation through the PI3K-independent AKT activation, while bradykinin activates eNOS through AKT or Ca2+/calmodulin-dependent protein kinase II [30,31]. On the other hand, adiponectin stimulates production of NO in vascular endothelial cells through a PI3K-dependent and AKT-independent mechanism involving the phosphorylation of eNOS by AMPK [32]. Other authors put in evidence that AKT is implicated in eNOS phosphorylation stimulated by adiponectin, demonstrating a crosstalk between AMPK and AKT in endothelial cells [33]. Finally, thrombin and histamine stimulate eNOS phosphorylation through an AMPK mediated pathway independent of PI3K/AKT [34]. For the first time in this study we have demonstrated that PHA is able to activate eNOS through a CaMKKb/AMPKα dependent eNOS phosphorylation/activation pathway as STO-609 or Compound C greatly inhibit these enzymes. On the contrary LY294002, inhibitor of PI3K, and MK2206, inhibitor of AKT, are poorly effective (Figure 3). Thus, in human platelets eNOS phosphorylation/activation stimulated by PHA appears to be an AMPK-mediated mechanism, independent of PI3K/AKT pathway. Previously we have shown that WGA behaves as a potent platelet agonist, while PHA appears to be less potent [17]. On the contrary PHA is a powerful lectin in the stimulation of eNOS phosphorylation/activation and NO elevation. NO activates adenylyl and guanylyl cyclase by increasing intraplatelet cAMP and cGMP. These cyclic nucleotides directly activate PKG and indirectly activate PKA. The NO/cGMP/PKG/PKA pathway targets several proteins involved in the inhibition of platelet activation cascades including VASP and myosin light chain kinase [20,35]. VASP is a substrate of PKA, PKG and AMPK that phosphorylate the sites ser157, ser239 and thr278, respectively. The phosphorylation at ser157 influences VASP localization but had a minor impact on F-actin assembly, whereas VASP phosphorylation at ser239 or thr278 impairs VASP-driven actin filament formation [36]. PHA stimulates VASP phosphorylation at ser239 and thr278 residues (Figure 6). Thus, we can suppose that PKG and AMPK-mediated phosphorylation of VASP at these residues interferes with F-actin accumulation and has a minor impact of VASP localization focal adhesion as cells spread.
In conclusion, this study has shown that in human platelets PHA stimulates eNOS phosphorylation/activation through AMPK activation. This mechanism is overall mediated by CaMKKb, while LKB1 is poorly involved. Thus, the phosphorylation/activation of eNOS and the consequent elevation of NO and cGMP intracellular concentrations could exert a regulatory effect on the activation of signalling pathways involved in platelet function.
This work was supported by the Genoa University (Fondo di Ricerca di Ateneo 2017), Italy.
- Randriamboavonjy V and Fleming I (2005) Endothelial nitric oxide synthase (eNOS) in platelets: How is it regulated and what is it doing there? Pharmacological Reports? Pharmacol Rep 57: Suppl 59-65.
- Mount PF, Kemp BE, Power DA (2007) Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 42: 271-279.
- Thors B, Halldórsson H, Jónsdóttir G and Thorgeirsson G (2008) Mechanism of thrombin mediated eNOS phosphorylation in endothelial cells is dependent on ATP levels after stimulation. Biochimica et Biophysica Acta 1783: 1893-1902.
- Liu Y, Oh SJ, Chang KH, Kim YG, L M (2013) Antiplatelet effect of AMP-activated protein kinase activator and its potentiation by the phosphodiesterase inhibitor dipyridamole. Biochem Pharmacol 86: 914-925.
- Hardie DG, Salt IP, Hawley SA, Davies SP (1999) AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. The Biochem J 338: 717-722.
- Onselaer MB, Oury C, Hunter RW, Eeckhoudt S, Barile N, et al. (2014) The Ca(2+) /calmodulin-dependent kinase kinase ß-AMP-activated protein kinase-a1 pathway regulates phosphorylation of cytoskeletal targets in thrombin-stimulated human platelets. J Thromb Haemost 12: 973-986. [Crossref]
- Signorello MG, Leoncini G (2018) Activation of CaMKKß/AMPKa pathway by 2-AG in human platelets. Journal of Cellular Biochemistry, 119: 876-884. [Crossref]
- Lepropre S, Kautbally S, Octave M, Ginion A, Onselaer MB, et al. (2018) AMPK-ACC signaling modulates platelet phospholipids and potentiates thrombus formation. Blood 132: 1180-1192. [Crossref]
- Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, et al. (1999) AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Letters 443: 285-289.
- Chen ZP, McConell GK, Michell BJ, Snow RJ, Canny BJ, et al. (2000) AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Am J Physiol Endocrinol Metab 279: E1202-E1206. [Crossref]
- Fleming I, Schulz C, Fichtlscherer B, Kemp BE, Fisslthaler B, et al. (2003) AMP-activated protein kinase (AMPK) regulates the insulin-induced activation of the nitric oxide synthase in human platelets. Thromb Haemost 90: 863-871.
- Kar R, Kellogg DL, Roman LJ (2015) Oxidative stress induces phosphorylation of neuronal NOS in cardiomyocytes through AMP-activated protein kinase (AMPK). Biochem Biophys Res Commun 459: 393-397. [Crossref]
- Van Damme EJM (2014) History of Plant Lectin Research. Methods Mol Biol 1200: 3-13. [Crossref]
- Kumar Kk, Reddy Gs, Reddy B, Sheka Pc, Sumanthi J, et al. (2012) Biological role of lectins: A review. J Orofacial Sci 4: 20.
- Lovrien RE, Anderson RA (1980) Stoichiometry of wheat germ agglutinin as a morphology controlling agent and as a morphology controlling agent and as a morphology protective agent for the human erythrocyte. J Cell Biol 85: 534-548. [Crossref]
- Liener IE, Sharon N, Goldstein IJ (1986) The Lectins? Properties, functions, and applications in biology and medicine. Academic Press.
- Signorello MG, Leoncini G (2017) The molecular mechanisms involved in lectin-induced human platelet aggregation. Biological Chemistry 398: 1335-1346.
- Russo I, Doronzo G, Mattiello L, De Salve A, Trovati M, et al. (2004) The activity of constitutive nitric oxide synthase is increased by the pathway cAMP/cAMP-activated protein kinase in human platelets. New insights into the antiaggregating effects of cAMP-elevating agents. Thromb Res 114: 265-273. [Crossref]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, et al. (1999) Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601-605.
- Blume C, Benz PM, Walter U, Ha J, Kemp BE, et al. (2007). AMP-activated protein kinase impairs endothelial actin cytoskeleton assembly by phosphorylating vasodilator-stimulated phosphoprotein. J Bio Chem 282: 4601-4612.
- Tomioka H, Saito H (1987) Comparison of wheat germ agglutinin- and phorbol myristate acetate-mediated triggering for macrophage H2O2 release: susceptibilities to various macrophage inhibitors. Microbiol Immunol 31: 211-221.
- Karlsson A (1999) Wheat germ agglutinin induces NADPH-oxidase activity in human neutrophils by interaction with mobilizable receptors. Infection and Immunity 67: 3461-3468.
- Inazu T, Taniguchi T, Ohta S, Miyabo S, Yamamura H (1991) The lectin wheat germ agglutinin induces rapid protein-tyrosine phosphorylation in human platelets. Biochem Biophys Res Commun 174: 1154-1158.
- Ohmori T, Yatomi Y, Wu Y, Osada M, Satoh K, et al. (2001) Wheat germ agglutinin-induced platelet activation via platelet endothelial cell adhesion molecule-1: involvement of rapid phospholipase C gamma 2 activation by Src family kinases. Biochemistry 40: 12992-3001.
- Kesherwani V, Sodhi A (2007) Differential activation of macrophages in vitro by lectin Concanavalin A, Phytohemagglutinin and Wheat germ agglutinin: production and regulation of nitric oxide. Nitric Oxide 16: 294-305.
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, et al. (1999) Erratum: Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597-601.
- Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, et al. (2002) Shear stress stimulates phosphorylation of eNOS at Ser (635) by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol 283: H1819-H1828.
- Michell BJ, Chen Z, Tiganis T, Stapleton D, Katsis F, et al. (2001) Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase c and the cAMP-dependent protein kinase. J Biol Chem 276: 17625-17628.
- Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R (2001) Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circulation Research 88: E68-75.
- Vecchione C, Maffei A, Colella S, Aretini A, Poulet R, et al. (2002) Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes 51: 168-173.
- Harris MB, Ju H, Venema VJ, Liang H, Zou R, et al. (2001) Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem 276: 16587-16591.
- Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ (2003) Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem 278: 45021-45026.
- Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, et al. (2004) Adiponectin stimulates angiogenesis by promoting crosstalk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem 279: 1304-1309.
- Thors B, Halldórsson H, Thorgeirsson G (2004) Thrombin and histamine stimulate endothelial nitric-oxide synthase phosphorylation at Ser1177 via an AMPK mediated pathway independent of PI3K-Akt. FEBS Letters 573: 175-180.
- Nishikawa M, de Lanerolle P, Lincoln TM, Adelstein RS (1984) Phosphorylation of mammalian myosin light chain kinases by the catalytic subunit of cyclic AMP-dependent protein kinase and by cyclic GMP-dependent protein kinase. J Biol Chem 259: 8429-8436.
- Benz PM, Blume C, Seifert S, Wilhelm S, Waschke J, et al. (2009) Differential VASP phosphorylation controls remodeling of the actin cytoskeleton. J Cell Sci 122: 3954-3965.







