Abstract
It is well-known that Native Americans (NA) clinically present with a very high rate of alcoholism and other drugs of abuse. It is also known that NA also display a very high rate of suicide compared to other ethnic groups. Furthermore, individuals with various psychiatric disorders (e.g., depression) also have higher rates of suicide that are frequently alcohol related. Males are as much as four times more likely to die from suicide than females. Studies comparing Native to other populations within the same geographic regions in North America divulged, almost universally, that alcohol involvement is higher among Native suicides than among the local, non-Native people. Unfortunately, suicide is the eighth leading cause of death in the U.S. and is the third cause of death in those ages 15-24. With these disappointing statistics, we are hereby proposing that because of such a high genetic risk as supported by the work of Barr and Kidd showing that NA carriers the DRD2 A1 allele at the rate of 86%, compared to a highly screened reward deficiency free control of only 3%. It seems reasonable that early identification, especially in children, be tested with the Genetic Addiction Risk Score (GARS®) and concomitantly be offered the precision pro-dopamine regulator (KB220PAM), one that matches their unique brain polymorphisms involving serotonergic, endorphinergic, glutaminergic, gabaergic and dopaminergic pathways among others. We believe that using the Precision Addiction Management (PAM®) platform at an early age may be prophylactic, while in adults PAM may reduce substance craving affecting tertiary treatment and even relapse and mortality prevention.
Key words
Alcoholism, American Indians, Brain Reward Circuitry, Dopamine, Genetic Addiction Risk Score (GARS), Pro-dopamine regulation
Introduction
Heavy Drinking Linked to Suicide in Native –Americans (NA)
The consensus of the scientific literature is that Native –Americans (NA) have a unique romance with alcohol and conclude that as high as 95 % drink alcohol with a very high rate linked to suicide ideation [1,2]. Here are highlights of the facts:
- Alcohol is often a precipitating factor in many cases of suicide.
- Research has revealed a significantly higher annual and even lifetime rates of suicide and attempts than in the general population.
- Those with various psychiatric disorders (e.g., depression) also have higher rates of suicide that are frequently alcohol related.
- Suicide is the eighth most significant cause of death in the US and is the third leading cause of demise for those between the ages of 15-24.
- Suicide is four times more likely to result in death for males.
- Firearms are associated with 60% of suicide deaths in males, as well as all others.
- Suicide rates are highest among Caucasian males and second highest among Native American/Alaskan men.
- Suicide rates in the Native American/Alaskan population are approximately 72% higher than in the total U.S. population (19.3 per 100k compared to 11.2%; IHS, 1998-1999).
- Among Native Americans and Alaska Natives, a more frequent relationship between alcohol use and suicidal death has been documented.
- In Alaska, a study reported that more alcohol-related suicides occurred among Natives than non-Natives (79% vs. 48%).
- Also, of those that completed suicide, blood alcohol concentrations above intoxication (0.1) were seen with higher frequency among Natives (54%) than others (20%).
- Alcohol involvement mean was 69% with a range from 30%-100% in at least 29 other studies of completed suicide among Natives and is significantly higher than in non-natives.
- Therefore, studies of Native suicide indicate a more frequent relationship between alcohol and suicidal death than published articles concerning non-Native populations.
- Seventy-one percent of Native male suicides were alcohol-related compared to 50% for females.
- It is well established that one issue involved in NA heavy drinking could be genetic especially regarding an ALDH genotype preventing the normal metabolism of ethanol (see Figure 1).
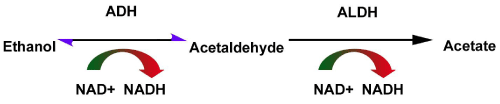
Figure 1. Acetaldehyde conversion of ethanol in normal metabolism
In spite of flushing and other uncomfortable side effects, NA's continue to drink heavily. While there are approved Medication Assisted Treatments for alcoholism such as Naltrexone (including long-acting depot); disulfiram (inhibits ADHD mechanism) and Acamprosate (blocks NMDA receptors reducing glutaminergic drive with reduced Dopamine release at reward site leading to reduced euphoria). Recent reports from the Directors of both NIAAA and NIDA while approving the short-term use of these FDA approved MATS strongly suggest that newer options including stabilizing brain dopamine may be another alternative with even better outcomes [3].
A brief synopsis of neurotransmission and alcoholism in Native Americans (NA)
Generally, there are a plethora of studies related to the important role of neurotransmitters and alcoholism globally whereby hypodopaminergia is indeed a major culprit [4]. The understanding of the role of catecholamines in the Brain Reward Cascade (BRC) first reported by Blum et al. [5] acted as a blueprint for the seminal finding of the first association of a genetic variant in the dopamine D2 receptor gene (DRD2A1) and severe alcoholism in the general population [6]. This matters, because Blum and Noble subsequently determined it and their team in 1991, that carries of this variant (allele) at birth confers a 30-40% reduce D2 receptors in the reward site of the brain [7]. What is even more meaningful is that Blum and his associates using a mathematical model developed by 16 century Monks called Bayesian Theorem found that at birth carriers of the DRD2 A1 variant will have a 74% chance of becoming addicted to a number of related drug and non-drug behaviors [8]. These findings led to an understanding of what constitutes a “Happy” or “Unhappy brain [see Figure 2]. These behaviors have a common rubric based on low brain dopamine function due to both genetics and environmental (epigenetic) factors coined by Kenneth Blum in 1995 – “Reward Deficiency Syndrome (RDS)” [Figure 3]. There is a remarkable list of addictive-impulsive –compulsive behaviors including drugs, alcohol, smoking, obesity, sexual addiction, and gambling. Importantly, all of these behaviors are connected to dopamine [9].
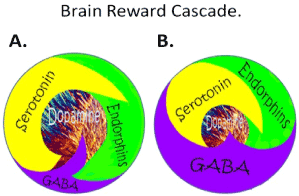
Figure 2. Schematic of the brain reward cascade: normal and abnormal representation.AThe normal physiologic homeostatic state of the neurotransmitter interaction at the mesolimbic region of the brain. In essence, serotonin in the hypothalamus stimulates neuronal projections of methionine enkephalin in the hypothalamus that, in turn, inhibits the release of GABA in the substantia nigra. This allows for the typical amount of dopamine to be released at the nucleus accumbens (NAc) reward site of the brain.B Hypodopaminergic function of the mesolimbic region of the brain. The hypodopaminergic state is due to gene polymorphisms as well as environmental elements (epigenetics), including both stress and neurotoxicity from aberrant abuse of psychoactive drugs (i.e., alcohol, heroin, cocaine, etc.) and genetic variables [with Permission from Blum K]
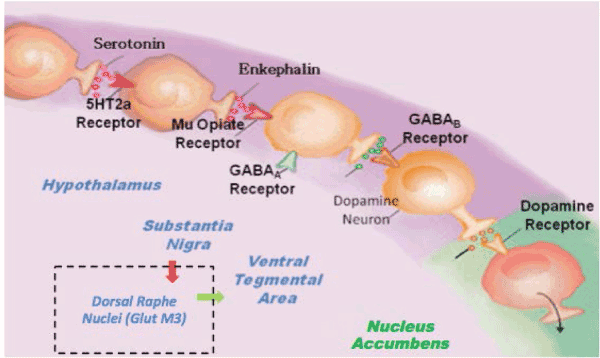
Figure 3. Brain Reward Cascade [with permission from Blum K]
Neurogenetics and the Native American population
Many studies show an active link between a number of gene variations across the BRC and net dopamine release in the brain. Work by Long et al. [10] in NA show evidence of linkage between alcohol dependence and chromosome 4 and 11. This is supportive of dopaminergic involvement since the DRD2 gene and other dopaminergic related genes (tyrosine –hydroxylase the rate-limiting step in the synthesis of brain dopamine) including the alcohol dehydrogenase gene cluster. There are studies by Ehlers and Wilhelmsen [11] indicating that Body Mass Index (BMI) is linked with substance abuse in Mission Indian population and, furthermore, provide preliminary data suggesting that 'consumption phenotypes' may share some genetic determinants [12]. This takes on even more critical when one coupled this with the work of Tataranni et al.13; specifically, Tataranni et al. [13] found a Ser311Cys mutation of the human DRD2 produces a marked functional impairment of the receptor and is associated with higher BMI in Pima Indians. The total energy expenditure (doubly labeled water) measured in 89 non-diabetic Pima Indians was 244 kcal/ day lower in homozygotes for the Cys311-encoding allele when compared with those heterozygous and homozygous for the Ser311-encoding allele (normal) (p = 0.056). This becomes more critical when one considers the work of Blum et al. [14] showing that linear trend analyses revealed the increasing use of drugs was positively and significantly associated with an A1 variant of the DRD2 gene classification (p < 0.00001). These strongly suggests that the DRD2 A1 allele is associated with increased risk not only for obesity but also for other related addictive behaviors (previously referred to as the Reward Deficiency Syndrome) and that a BMI over 25 by itself (without characterization of macro selection or the comorbid substance use disorders) is not sufficient to suggest an association with the DRD2 A1 allele. In fact, other related work confirmed that the DRD2 A1 variant increases fat cell production in humans [15].
Many other neurogenetic informative research involving many reward genes and NA’s have been published including associations with alcohol dependence and DRD2/ANKK1 A1 allele; serotonin (HTR3B); mu opioid receptor (OPRM1); GABA (5q34 {gamma}-aminobutyric acid type A gene cluster); genetic linkage to 'craving for alcohol' on chromosome 5; CRN1, COMT, and MAOA [16-20].
Generational impact on Native American children
It is now known that one's environment can impact up to at least two generations. This insult is called epigenetics a mechanism by which the environment either adds methyl groups to the DNA chromatin structure on histones (reducing messenger RNA expression) or adds acetyl groups by inhibiting deacetylation (increasing messenger RNA expression) [21]. In various animal models, it has been shown that changes to mRNA can still be present up to two generations. In the world of addiction now many studies have pinpointed a number of reward-based genes that increase lifetime use of alcohol [22]. In humans, for example, DNA generational findings mainly related to dopamine function has been observed in many classic studies including five-generational research on the DRD2 gene and RDS as a phenotype [23].
While not a biological mirror to the brain, like gene testing, a significantly positive family history of alcoholism, tends to be one of the most consistent and powerful predictors of a person's risk for developing this disorder. This finding has stimulated much research on etiological vulnerability factors and mechanisms by which children of alcoholic parents are at high risk for developing alcohol-related problems. In primarily Euro-American samples, parental alcoholism has been associated with a variety of adverse outcomes for children and adolescents, including problematic behavior. Native-American Indians in the US, in addition to high rates of alcoholism and alcohol-related mortality, have the highest prevalence of positive family history for alcoholism [24].
The role of dopamine, especially about high risk for all RDS behaviors, is highlighted in an earlier study providing population-based evidence for the frequency of the DRD2 A1 allele in many ethnic groups (see Figure 4) [25]. Given that the A1 allele of the DRD2 varies significantly in frequency from one population to another, i.e., 0.09 in Yemenite Jews (known to have meager rates of alcoholism) to 0.74 in Cheyenne American Indians (known to have very high rates of alcoholism) [25].
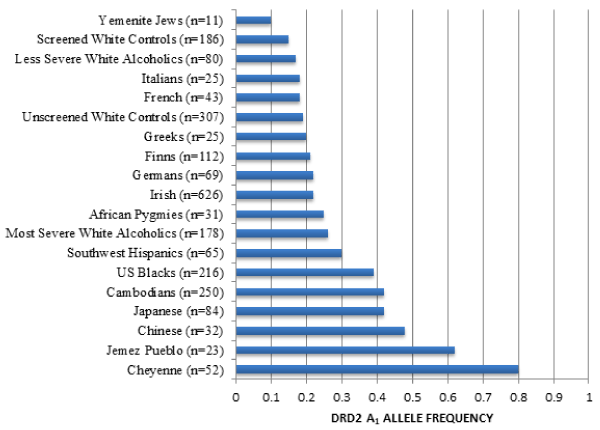
Figure 4: DRD2 frequency as a function of ethnicity. The DRD2 Taq 1 allele frequency as a function of ethnicity was derived from many independent investigations. The number in parentheses denotes the proband size. Modified from Barr & Kidd [25]
Risk of alcoholism and other addictive behaviors in Native –American children & adolescents
Addiction professionals are becoming increasingly concerned about preteenagers and young adults' involvement with substance abuse as a way of relieving stress and anger. The turbulent underdeveloped central nervous system, especially in the prefrontal cortex (PFC), provides impetus to not only continue essential neuroimaging studies in both human and animal models but also to encourage preventive measures and cautions embraced by government and various social media outlets. It is common knowledge that before teenagers reach their 20s, PFC development is undergoing significant changes and, as such, hijacks appropriate decision making in this population. Understandably, family history, parenting styles, and attachment may be modified by various reward genes, including the known bonding substances oxytocin/vasopressin, which effect dopaminergic function [26]. In one study, Friese et al [27]. suggest those sons of alcoholics of Mission Indian heritage experience more problems than sons of nonalcoholic, but also indicate that Mission Indian children of alcoholics are not more vulnerable to behavioral problems than children of alcoholic parents of other ethnic backgrounds.
Based on these and other biological measures conducted by many neuroscientists across the globe showing that electrophysiological "markers" as well as genetics load onto higher risks for all RDS behaviors especially alcoholism in Native –Americans. Ehlers et al. [28] discovered that the P3 component of the Event-Related Potential (ERP) is sensitive to the effects of alcohol. A reduction in the P3a component across the scalp was found in Native American men following alcohol when compared with placebo ingestion. This effect was significantly influenced by the presence of a polymorphism in the alcohol metabolizing enzyme alcohol dehydrogenase (ADH). Men with an ADH2 x 3 allele had substantially higher amplitude P3 components at placebo and also demonstrated more alcohol-induced reductions in P3 amplitude than men with ADH2 x 1 alleles only. Interestingly, less intense response to alcohol has been associated with higher risk of the development of alcohol-related problems. This suggests the presence of specific biological variables within this Native American population may confer both risk and protection for the future development of alcohol dependence.
It is imperative that for children of Native –Americans of both alcoholic and non-alcoholic parents early genetic testing for addiction risk alleles will offer valuable information that could potentially be utilized by their parents and caregivers before use of psychoactive drugs by these youths. Understandably, various factors including family history, attachment and parenting styles, appear to be modified by the various reward genes. These include oxytocin/vasopressin associated with bonding, which effects dopaminergic function via innervation. Neuroimaging studies have continued to reflect region-specific differential responses to drugs and other addictions including food (and other non-substance-addictive behaviors) via either "surfeit" or "deficit." Keeping this in mind, we hereby propose a "reward deficiency solution system" or what we now term “Precision Addiction Management (PAM®)” that combines early genetic risk diagnosis, medical monitoring, and nutrigenomic dopamine agonist modalities to combat this significant global dilemma that is preventing our youth from leading healthy productive lives, which will in turn make them happier [29].
Precision addiction management (PAM) to combat addiction
The global opioid epidemic resultant in deaths have soared for men and women of all social, economic status and age from heroin and fentanyl overdoses. Specifically, in the United States, deaths from narcotic overdoses have reached alarming metrics since 2010. The Fentanyl rise is driven by drug dealers who sell it as heroin or who use it to lace cocaine or to make illegal counterfeit prescription opioids. The President's Commission on the crisis has linked the death toll as equivalent to "September 11th every three weeks." The Center for Disease Control (CDC) in the US has released data revealing opioid-related deaths had increased by 15% within the first three quarters of 2016 (in contrast to 2015). Blum et al. [29] have argued that unless the scientific community embraces genetic addiction risk coupled with possible precision or personalized neuro-nutrients (to induce a "dopamine homeostasis"), real change will not happen.
We now have significant evidence that a ten-gene [and eleven single nucleotide polymorphism (SNP)] panel predicts Addiction Severity Index (ASI) for both alcohol and drugs of abuse (e.g., Opioids). In a large multi-addiction center study, the genetic addiction risk score (GARS®) was shown to have a predictive relationship with ASI-MV derived alcohol (≥ seven alleles), and other drugs (≥ four alleles) severity risk scores. In some neuroimaging studies, on a Pro-dopamine regulator (KB220PAM), we have shown in both animal (bench) and abstinent Chinese severe heroin-dependent patients (bedside), BOLD dopamine activation across the brain reward circuitry revealed increases in resting state functional connectivity as well volume connectivity. It is also known that published nutrigenomic (coupling gene polymorphisms with altered KB220PAM) studies reveal improved clinical outcomes related to obesity. This becomes more critical when it refers to the Native-American population because it is well known that this population has a higher risk for many reward deficiency behaviors including increased BMI, diabetes and even gambling in alcohol dependence [11,30,31].
Policy perspectives
Recent reports from national studies have presented extremely high rates for many personality disorders in American Indian communities including violence [32]. In this regard, Jervis et al. [32] published that men generally presented as perpetrators and women as victims. Men often described themselves as ready participants in a violent world, while women were quite clear that aggression for them was often merely required as they tried to defend themselves from male violence.
2021 Copyright OAT. All rights reserv
According to May who evaluated Alcohol policy for Indian reservations suggested many solutions, but the strongest was a comprehensive and positive alcohol policy that has been ignored for too long in Indian country, and the resultant toll in morbidity, mortality, and suffering is too high [33]. While some suggest that the high risk for alcoholism in Native- Americans may be due to culture in the opinion of key neuroscientists, it is indeed the combination of both genetics and epigenetics that predisposes this population especially with the co-morbidity of a TRIAD known as alcoholism, diabetes, and depression [34,35].
Summary
Blum and Kozlowski have published on the “Brain Reward Cascade” (BRC) [36]. This concept has served as a new blueprint for the interaction of neurotransmitters within the brain reward system. Also, it has been well established that genes regulating chemical messengers within the reward system directly influences dopamine levels released into the reward circuit and in other regions of the brain.
Furthermore, it is well established that resting-state functional connectivity integrity is essential for healthy homeostatic functioning. Understanding the common mechanisms between alcohol and opioids may help understand why not only Native Americans especially adolescents may have an equal predisposition for both of these addictive agents. Zhang et al. [37] recently revealed that in heroin addicts there was a significant reduction of connectivity between the rostral anterior cingulate (rACC) and the dorsal anterior cingulate cortex (dACC), as well as reduced connectivity between the dACC and subcallosal (sACC). These findings of variations in the functional connectivity within three subregions of the ACC within heroin addicts has implied that these subregions, together with crucial other brain areas (such as the putamen, dorsal striatum, orbital frontal cortex, dorsal striatum, cerebellum, amygdala, etc.) may play essential aspects in heroin and even alcohol addiction.
More recently, in Blum's Geneus Genetic Testing Center, and along with Zhang's group in abstinent heroin addicts showed that KB220Z™ (Pro-Dopamine Regulator) a complex dopamine agonist, one-hour post-acute administration, induced a significant increase in fMRI BOLD activation specifically within the caudate-accumbens-dopaminergic pathways in contrast to placebo [38]. Additionally, KB220Z™ also decreased resting-state activity in the cerebellum of heroin addicts maintaining abstinence in recovery. Within the second tier of this pilot study, all ten-abstinent heroin-dependent subjects, three brain regions of interest (ROIs) were significantly activated in resting state by KB220Z™ contrasted with placebo (p < 0.05). This increased functional connectivity was found in a presumed network which encompassed the dACC, posterior cingulate, nucleus accumbens, medial frontal gyrus, occipital cortical areas, and cerebellum.
The development of a polymorphic gene panel has enabled customized (personalized) anti-obesity compounds and now could provide customized induction of "dopamine homeostasis” across many RDS behaviors [39]. This serves as the basis of personalized addiction management utilizing the patented Genetic Addiction Risk Score (GARS®) along with a matched neuro-nutrient known as KB220PAM. While many more critical scientifically sound studies continue along these lines seeking even better precision- having this disruptive technology available now should be seriously considered by the National Indian Health Service.
Authorship
All authors provided a substantial contribution to the conception, assisted with drafting the article, revising it critically, final approval of the version to be published, and agreement to act as a guarantor of the work.
Acknowledgments
The authors would like to thank Danielle Jean Kradin for formatting the references in the reference section. We appreciate the expert edits of Margaret A. Madigan.
Funding information
KB along with Marjorie Gondre-Lewis from Howard University are recipients of grant 1R41MD012318-01. RDB is partially supported by the National Institutes of Health grants 1R01NS073884 and 1R21MH073624; and VA Merit Review Awards CX000479 and CX000780.
Competing interest
Kenneth Blum, Ph.D., is the holder of many U.S. and foreign patents issued and pending related to Nutrigenomics and Nutraceuticals. Dr. Blum is the Co-founder of Geneus Health LLC. Through Geneus Health, LLC, the Genetic Addiction Risk Score (GARS®) has licensed Dominion Diagnostics, LLC as a sales organization in the addiction space. Through his company Synaptamine, Inc., Dr. Blum licensed a number of retail outlets. He is a paid consultant of Dominion Diagnostics, LLC and The Florida House Experience. Dr. Blum is a member of the scientific advisory board of Dominion Diagnostics, LLC, and is Chief Scientific Advisor of Dominion Diagnostics, LLC. He currently serves as Chairman of the Board and Chief Scientific Officer, of Geneus Health, LLC. Victory Nutrition International, Inc., and Nupathways, Inc. are licensees of the KB220Z/ZBR technology. Dr. Siwicki is co –founder of Geneus Health. Drs. Modestino, Baron and Badgaiyan are members of Geneus Health Scientific Advisory Board. There are no other author conflicts of interest.
References
- Manzo K, Tiesman H, Stewart J, Hobbs GR, Knox SS (2015) A comparison of risk factors associated with suicide ideation/attempts in American Indian and White youth in Montana. Arch Suicide Res 19: 89-102. [Crossref]
- Blum RW, Harmon B, Harris L, Bergeisen L, Resnick MD (1992) American Indian--Alaska Native youth health. JAMA 267: 1637-1644. [Crossref]
- Volkow ND, Koob G (2015) Brain disease model of addiction: why is it so controversial? Lancet Psychiatry 2: 677-679. [Crossref]
- Feltmann K, Borroto-Escuela DO, Ruegg J, Pinton L, De Oliveira Sergio T, et al. (2018). Effects of Long-Term Alcohol Drinking on the Dopamine D2 Receptor: Gene Expression and Heteroreceptor Complexes in the Striatum in Rats. Alcohol Clin Exp Res 42: 338-351.
- Blum K, Merritt JH, Wallace JE, Owen R, Hahn JW, et al. (1972) Effects of catecholamine synthesis inhibition on ethanol narcosis in mice. Curr Ther Res Clin Exp 14: 324-329. [Crossref]
- Blum K, Noble EP, Sheridan PJ, Montgomery A, Ritchie T, et al. (1990). Allelic Association of human dopamine D2 receptor gene in alcoholism. JAMA 18: 263: 205560.
- Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ (1991) Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry 48: 648-654. [Crossref]
- Blum K, Wood RC, Braverman ER, Chen TJ, Sheridan PJ (1995) The D2 dopamine receptor gene as a predictor of compulsive disease: Bayes' theorem. Funct Neurol 10: 37-44. [Crossref]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, et al. (1996) The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med 89: 396-400. [Crossref]
- Long JC, Knowler WC, Hanson RL, Robin RW, Urbanek M, et al. (1998) Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet 81: 216-221. [Crossref]
- Ehlers CL, Wilhelmsen KC (2007) Genomic screen for substance dependence and body mass index in southwest California Indians. Genes Brain Behav 6: 184-191. [Crossref]
- Jenkinson CP, Hanson R, Cray K, Wiedrich C, Knowler WC, et al. (2000) Association of dopamine D2 receptor polymorphisms Ser311Cys and TaqIA with obesity or type 2 diabetes mellitus in Pima Indians. Int J Obes Relat Metab Disord 24: 1233-1238. [Crossref]
- Tataranni PA, Baier L, Jenkinson C, Harper I, Del Parigi A, et al. (2001) A Ser311Cys mutation in the human dopamine receptor D2 gene is associated with reduced energy expenditure. Diabetes 50: 901-904. [Crossref]
- Blum K, Braverman ER, Wood RC, Gill J, Li C, et al. (1996) Increased prevalence of the Taq I A1 allele of the dopamine receptor gene (DRD2) in obesity with comorbid substance use disorder: A preliminary report. Pharmacogenetics 6: 297-305. [Crossref]
- Chen AL, Blum K, Chen TJ, Giordano J, Downs BW, et al. (2012) Correlation of the Taq1 dopamine D2 receptor gene and percent body fat in obese and screened control subjects: a preliminary report. Food Funct 3: 40-48. [Crossref]
- Panduro A, Ramos-Lopez O, Campollo O, Zepeda-Carrillo EA, Gonzalez-Aldaco K, et al. (2017) High frequency of the DRD2/ANKK1 A1 allele in Mexican Native Amerindians and Mestizos and its association with alcohol consumption. Drug Alcohol Depend 172: 66-72. [Crossref]
- Ducci F, Enoch MA, Yuan Q, Shen PH, White KV, et al. (2009) HTR3B is associated with alcoholism with antisocial behavior and alpha EEG power--an intermediate phenotype for alcoholism and co-morbid behaviors. Alcohol 43: 73-84. [Crossref]
- Ehlers CL, Lind PA, Wilhelmsen KC (2008) Association between single nucleotide polymorphisms in the mu opioid receptor gene (OPRM1) and self-reported responses to alcohol in American Indians. BMC Med Genet 9: 35. [Crossref]
- Radel M, Vallejo RL, Iwata N, Aragon R, Long JC, et al. (2005) Haplotype-based localization of an alcohol dependence gene to the 5q34 {gamma}-aminobutyric acid type A gene cluster. Arch Gen Psychiatry 62: 47-55. [Crossref]
- Ehlers CL, Wilhelmsen KC (2005) Genomic scan for alcohol craving in Mission Indians. Psychiatr Genet 15: 71-75. [Crossref]
- Ehlers CL, Gizer IR (2013) Evidence for a genetic component for substance dependence in Native Americans. Am J Psychiatry 170: 154-164. [Crossref]
- You C, Vandegrift B, Brodie MS, et al. (2018) Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons. Psychopharmacology (Berl) 235: 1711-1726. [Crossref]
- Blum K, Chen AL, Oscar-Berman M, Chen TJ, Lubar J, et al. (2011) Generational association studies of dopaminergic genes in reward deficiency syndrome (RDS) subjects: selecting appropriate phenotypes for reward dependence behaviors. Int J Environ Res Public Health 8: 4425-4459. [Crossref]
- Wall TL, Garcia-Andrade C, Wong V, Lau P, Ehlers CL (2000) Parental history of alcoholism and problem behaviors in Native-American children and adolescents. Alcohol Clin Exp Res 24: 30-34. [Crossref]
- Barr CL, Kidd KK (1993) Population frequencies of the A1 allele at the dopamine D2 receptor locus. Biol Psychiatry 34: 204-209. [Crossref]
- Blum K, Febo M, Smith DE, Roy AK 3rd, Demetrovics Z, et al. (2015) Neurogenetic and epigenetic correlates of adolescent predisposition to and risk for addictive behaviors as a function of prefrontal cortex dysregulation. J Child Adolesc Psychopharmacol 25: 286-292. [Crossref]
- Friese B, Grube JW, Seninger S, Paschall MJ, Moore RS (2011) Drinking behavior and sources of alcohol: differences between Native American and White youths. J Stud Alcohol Drugs 72: 53-60. [Crossref]
- Ehlers CL, Garcia-Andrade C, Wall TL, Sobel DF, Phillips E (1998) Determinants of P3 amplitude and response to alcohol in Native American Mission Indians. Neuropsychopharmacology 18: 282-292. [Crossref]
- Blum K (2017) Global opioid epidemic: doomed to fail without genetically based precision addiction medicine (PAMTM): Lessons learned from america. Precis Med (Bangalore) 2: 17-22. [Crossref]
- Marley TL, Metzger MW (2015) A longitudinal study of structural risk factors for obesity and diabetes among American Indian young adults, 1994-2008. Prev Chronic Dis 12: E69. [Crossref]
- Elia C, Jacobs DF (1993) The incidence of pathological gambling among Native Americans treated for alcohol dependence. Int J Addict 28: 659-666. [Crossref]
- Jervis LL, Spicer P, Belcourt A, Sarche M, Novins DK, et al. (2014) The social construction of violence among Northern Plains tribal members with antisocial personality disorder and alcohol use disorder. Transcult Psychiatry 51: 23-46. [Crossref]
- May PA (1992) Alcohol policy considerations for Indian reservations and bordertown communities. Am Indian Alsk Native Ment Health Res 4: 5-59. [Crossref]
- Beauvais F (1998) American Indians and alcohol. Alcohol Health Res World 22: 253-259. [Crossref]
- Tann SS, Yabiku ST, Okamoto SK, Yanow J (2007) triADD: the risk for alcohol abuse, depression, and diabetes multimorbidity in the American Indian and Alaska Native populations. Am Indian Alsk Native Ment Health Res 14: 1-23. [Crossref]
- Blum K, Kozlowski GP. (1990) Ethanol and Neuromodulator influences. A cascade model of reward. In Ollat H, Parvez H, Parvez S, editors. Alcohol and Behavior: Basic and Clinical Aspects Progress in Alcohol Research. VSP International Science Publishers 131-150.
- Zhang Y, Gong J, Xie C, Ye EM, Jin X, et al. (2015) Alterations in brain connectivity in three sub-regions of the anterior cingulate cortex in heroin-dependent individuals: Evidence from resting state fMRI. Neuroscience 284: 998-1010. [Crossref]
- Blum K, Liu Y, Wang W, Wang Y, Zhang Y, et al. (2015) rsfMRI effects of KB220ZTM on neural pathways in reward circuitry of abstinent genotyped heroin addicts. Postgrad Med 127: 232-241. [Crossref]
- Blum K, Downs BW, Dushaj K, Li M, Braverman ER, et al. (2016) The benefits of customized DNA directed nutrition to balance the brain reward circuitry and reduce addictive behaviors. Precis Med (Bangalore) 1: 18-33. [Crossref]




