In type 2 diabetes mellitus (T2DM) the global metabolic disturbances are known to involve all macromolecules. Although the carbohydrates and lipid profiles are routinely tested in T2DM, limited data is available about proteins and amino acids (AAs). In this case-control prospective study the AAs profile in T2DM was investigated and the results were interpreted in a context of metabolic changes in diabetes and associated disorders. Fasting blood samples from 70 Bahraini subjects, 35 with T2DM treated with metformin only and 35 healthy controls, were collected. The plasma levels of 13 AAs were measured by HPLC in addition to the glycemic and lipid profiles parameters. Several statistical models were used to test 21 variables, including the 13 AAs, for prediction of T2DM. The results revealed a marked decrease in median plasma level of Phe in T2DM compared with the control, 12.73, 6.84-94.32 vs. 136.88, 65.15-206.47, p<0.001, and a significant decrease in levels of 4 non-essential AAs (Gly, Gln, Asp, and Tyr). The results were confirmed by multiple logistic regression analysis. The Bivariate and Factor analyses revealed that the AAs panel was comparable to the glycemic and lipid panels in T2DM prediction. The implication of AAs deficiency in protein and AAs derivatives’ synthesis in T2DM and associated disorders e.g. hypothyroidism was discussed. In conclusion, T2DM treated with metformin only was associated with marked depletion of Phe and deficiency of other non-essential AAs. The potential use of AAs as supplements as well as a biomarker in T2DM were suggested.
T2DM, HPLC, amino acids profile, phenylalanine, metformin
AAAs: Aromatic AAs; AAs: Amino acids; ANOVA: Analysis of variance; Asp: Aspartic acid or aspartate; BCAAs: Branched-chain amino acids; BP: Biochemical parameters; DA: Discriminant Analysis; DV: Dependent variables; FA: Factor analysis; FBG: Fasting blood glucose; Gln: Glutamine; Gly: Glycine; HbA1c: Glycated hemoglobin; HDL-C: High-density lipoprotein cholesterol; HPLC: High performance liquid chromatography; IV: Independent variables; LDL-C: Low-density lipoprotein cholesterol; MetS: Metabolic syndrome; MW: Mann whitney rank sum test; Phe: Phenylalanine; SMC: Salmanyia medical complex; T2DM: Type 2 diabetes mellitus; TG: Triglyceride; Tyr: Tyrosine
Type 2 diabetes mellitus (T2DM) is characterized by loss of metabolic homeostasis largely due to insulin resistance [1]. Consequently, the autoregulation of metabolism of macromolecules, carbohydrates, lipids and proteins, is largely disturbed [2]. The proteins including enzymes, neurotransmitters and insulin and counterbalancing hormones, glucagon, growth hormone, thyroxine, catecholamines, and etc. [3], are the actual players in metabolism. However, synthesis of proteins and amino acids (AAs) derivatives, e.g. tyrosine (Tyr) derivatives, is determined by the availability of free AAs. Worth noting, the protein synthesis and degradations is a bidirectional process that link proteins with plasma free AAs pool. Therefore, the metabolic changes in T2DM are expected to be reflected on the free AAs pool. The AAs profiling in T2DM has been studied in different ethnic groups, however, only few AAs profiling studies were conducted in the Arab region [4], although the diabetes mellitus prevalence is high.
Standard AAs are grouped into three categories: essential, nonessential, and semi-essential based on nutritional requirement [5,6]. The body can synthesize nonessential AAs either from the breakdown of proteins or from other essential AAs. Essential AAs must be provided in diet [6]. The essentials and non-essential AAs as defined before [5] and analyzed in this study are listed in (Table 1). The Val, Leu, and Ile are known as branched-chain amino acids (BCAAs), and Phe, Trp, His, and Tyr are known as aromatic AAs (AAAs).
Table 1. The analyzed amino acids: Abbreviations, description, response factor (RF), correlation coefficient (R2) and average retention time and the internal standard (IS-Norvaline)
Amino Acid (AA)
|
Abbrev. |
Description |
RF |
R2 |
Average retention time,
minutes
|
Alanine |
Ala |
Glucogenic |
0.6977 |
0.9899 |
15.684 |
Aspartate |
Asp |
Glucogenic |
4.1901 |
0.9870 |
6.790 |
Glutamate |
Glu |
Glucogenic |
0.9137 |
0.9930 |
7.329 |
Glutamine |
Gln |
Glucogenic |
0.5127 |
0.9918 |
11.053 |
Glycine |
Gly |
Glucogenic |
2.116 |
0.9986 |
13.074 |
Serine |
Ser |
Glucogenic |
0.0469 |
0.9769 |
9.469 |
Tyrosine |
Tyr |
Glucogenic/Ketogenic AAA |
1.46 |
0.9966 |
15.922 |
Isoleucine |
Ile |
Glucogenic/Ketogenic BCAA |
1.1994 |
0.9943 |
27.853 |
Leucine |
Leu |
Ketogenic
BCAA |
0.9316 |
0.9998 |
29.536 |
Lysine |
Lys |
Ketogenic
|
5.64 |
0.9607 |
24.745 |
Phenyalanine |
Phe |
Glucogenic/Ketogenic AAA |
6.718 |
0.9181 |
27.089 |
Threonine |
Thr |
Glucogenic/Ketogenic |
0.9249 |
0.9600 |
12.832 |
Valine |
Val |
Glucogenic BCAA |
1.1854 |
0.9999 |
22.935 |
IS – 250 µl |
|
|
|
|
26.5 |
|
Average |
|
2.0412 |
0.9820 |
|
AAA: Aromatic amino acid; BCAA: Branched chain amino acid.
Note: AAs are categorized into "ketogenic", "glucogenic" or "both" based on the type of intermediates that are formed during their catabolism.
The increased plasma levels of BCAAs was noticed in T2DM [7], metabolic syndrome [8] and metabolic dyslipidemia [9]. Also, the levels of BCAAs and AAAs were found to be associated with visceral obesity, insulin resistance, and T2DM [10,11]. Furthermore, it was found that the plasma levels of the AAs are altered during acute diabetic ketoacidosis; while the Asp, Glu, and BCAAs are increased, the Asn and Gln are reduced [12]. A close correlation of the BCAAs and AAAs levels with insulin resistance was also found in Chinese population [13]. Finally, it was found that abnormal AA levels might contribute to elevated liver enzymes in T2DM [14].
In this study, we aimed to measure the fasting plasma levels of free AAs in metformin-treated T2DM Bharani subjects and correlate the levels with glycemic and lipid profile parameters. Genetic, and environmental (cultural, nutritional habits, co-morbidities and etc.) factors are expected to have an influence in the AAs pool in different regions. The results will be discussed in relation to the altered proteins metabolism in T2DM noticed in this setting [15].
Study subjects
Blood samples were collected from 70 Bharani subjects; 35 patients with T2DM treated with metformin only (15 males) and 35 non-diabetic controls (9 males).
The T2DM inclusion criteria included Bharani subject with T2DM, under treatment with metformin only, aged between 40 and 60 years, and willing to be involved in the study. The exclusion criteria were: non-Bahraini nationality, T1DM, use of other anti-diabetic treatment, other chronic diseases. The patients were recruited from Salmanyia Medical Complex (SMC) and Health Centers in the Kingdom of Bahrain. The healthy controls were recruited from the College of Health Sciences, Bahrain University, in Bahrain. Verbal informed consent was obtained from each study subject before blood collection. The study design followed the Helsinki declaration terms. Finally, the study was approved by the ethical committee in the Arabian Gulf University (AGU) and the Research Committee in SMC.
Sample collection
Approximately 10 ml of venous blood samples were collected from each study subject after 10-12 hours of overnight fasting, into heparinized collection tube (Thermo Fisher Scientific, Massachusetts, U.S.A.) for plasma and into separation gel tubes (Thermo Fisher Scientific, Massachusetts, U.S.A.) for serum collection. Serum was used for lipid analysis. Plasma was used for fasting glucose and AAs measurements.
Blood glucose, HbA1c and serum lipid profile determination
The biochemical tests were done in SMC. Clinical analyzer ROCHE COBAS INTEGRA 800 (Rotkreuz, Switzerland), was used for measurement of fasting blood glucose (FBG) and lipid profile parameters (total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and triglyceride (TG). The glycated hemoglobin (HbA1c%) was measured by an automated analyzer (Beckman Coulter, Inc., 250 S. Kraemer Blvd. Brea, CA 92821, USA).
Measurement of plasma amino acids (AAs) concentrations by reverse phase high-pressure liquid chromatography (RP-HPLC)
The plasma levels of the test AAs were measured by RP-HPLC, Alliance 2695 separation module, from Waters (Massachusetts, U.S.A.), as originally mentioned [16]. In brief, the samples were hydrolyzed, pre-column derivatized with O- phthalaldehyde (OPA) and in-line analyzed by RP-HPLC with fluorescence detection. A linear relation between peak response and concentration from 5 to 800 mol/L for all AAs (correlation coefficients (R2)>0.999) was obtained. In this study, the method was slightly modified where the mobile phase NaH2PO4 was used instead of KH2PO4. The total analysis time was also increased to 35 minutes instead of 17 minutes to improve peak separation. The excitation wavelength was changed from 230 to 330 nm and the emission wavelength from 389 to 450 nm.
Waters HPLC system (Waters 2695 Separations Module) operated by the Empower Photodiode Array (PDA) software (Waters, Massachusetts, U.S.A.) was used to create AAs project.
Chromatographic conditions for AAs profiling: Separations of the AAs were performed by gradient elution. Symmetry Column 18 from Waters (100Å, 5 µm, 4.6 mm X 250 mm, RP) was used. The sample injection volume was 10 µL. Fourteen AAs were tested in this study including Norvaline as internal standard (IS) (Table 2), the remaining AAs were not analyzed because their analysis requires special preparation. The pooled working standard solutions (10 ml) were prepared by mixing the 14 AAs, and were stored as aliquots at -20°C.
Table 2. The fasting plasma levels of 13 amino acids in T2DM and healthy control subjects
Amino Acid |
T2DM
(arbitrary units) |
Healthy controls
(arbitrary units) |
P value |
Ala |
2308.73 ± 915.49 |
1918.00 ± 1037.19 |
0.099* |
Ser |
3331.01, 2408.28 - 4212.36 |
3503.73, 2546.15 - 4378.43 |
0.664 |
Glu |
170.74, 93.65 - 204.97 |
134.13, 97.68 - 215.98 |
0.622 |
Gly |
11.83, 10.78 - 33.10 |
22.08, 14.28 - 63.78 |
0.018 |
Gln |
208.18, 114.05 - 258.84 |
245.65, 183.17 - 389.24 |
0.010 |
Asp |
1.64, 1.12 - 1.90 |
2.31, 1.35 - 3.04 |
0.012 |
Tyr |
211.65, 161.20 - 250.22 |
260.31, 230.16 - 402.87 |
<0.001 |
Phe |
12.73, 6.84 - 94.32 |
136.88, 65.15 - 206.47 |
<0.001 |
Val |
1267.54 ± 410.48 |
1042.74 ± 538.39 |
0.054* |
Thr |
2139.52, 1573.36 - 2517.41 |
2208.25, 1341.33 - 3019.20 |
0.672 |
Lys |
333.81, 303.36 - 396.69 |
384.56, 181.01 - 521.55 |
0.124 |
Ile |
413.81 ± 125.36 |
375.63 ± 161.94 |
0.274* |
Leu |
926.10, 673.36 - 1008.87 |
760.99, 659.46 ± 903.86 |
0.133 |
Note: Grey rows indicates the non-essential amino acids.
*T-test was used for comparison analysis (normally distributed data), the values are the mean ± standard deviation (SD). The remaining analysis done by Mann Whitney Rand Sum test, the values are (median, 25% - 75% percentile).
Amino acid peak identification: The AAs were detected based on the retention time established for the individual AA standard (250 µM) under the defined experimental conditions. After the run was completed, the acquired data were exported from Empower to Microsoft Excel for calculating the AA concentration in the samples. The mean and standard deviation of the AA area for 3 injections were calculated. The RP-HPLC Chromatogram detailing analyte retention time, in minutes, for the pooled standard AAs, control and T2DM samples is shown in Figure 1.
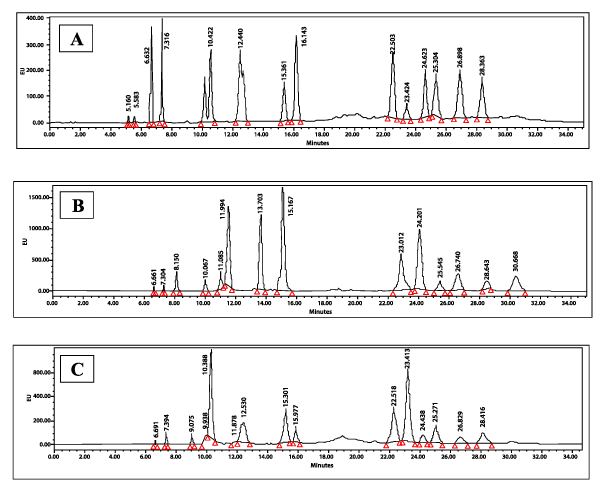
Figure 1. Representative reverse phase high-pressure liquid chromatography (RP-HPLC) chromatogram detailing analyte retention time (minutes): (A) Pooled Standard AAs (50 µM), (B) non-diabetic sample and (C) T2DM sample under specified conditions
Statistical analysis
Sigma Stat software was used for data analysis. Simple comparisons of normally distributed data between T2DM and control subjects were analyzed by the T-test. Mann-Whitney Rank Sum Test was used for analysis of skewed data. Correlations were tested by Pearson Product Moment Correlation.
Multiple regression analysis
The multiple regression analysis was preformed to model the relationships between the plasma AAs profiles, biomedical variables and T2DM. In order to develop the model, multiple statistical tests were performed including bivariate analysis, Test of variance (ANOVA), factor analysis, discriminant function analysis and finally logistic regression analysis. In the multiple regression analyses the dependent variables (DVs) are T2DM and healthy controls, and the independent variables (IVs) included 21 variables; 13 AAs, 6 biochemical parameters (BP) - glycemic and lipid profile parameters (FBG, HbA1c, total cholesterol, LDL-C, HDLC and TGs), and 2 demographic factors (age and sex).
Discriminant analysis (DA): It was performed to determine whether the IVs e.g. AAs, could distinguish between controls and cases (DVs). The DA was performed in 2 stages using 2 settings; the first setting included 20 variables (FBG excluded as it parallels HbA1c) named (20 IVs) and second setting included only the 9 good predictors selected by logistic regression (9 IVs)..
Fasting plasma levels of AAs in T2DM and non-diabetic healthy subjects
Compared with the healthy controls, the T2DM subjects showed significantly lower levels of 5 out of 13 tested AAs, 4 of the 5 AAs are non-essential. In more detail, the level of Gly in T2DM vs. controls was significantly lower (median, 25 -75% percentile, 11.83, 10.78 - 33.10 vs. 22.08, 14.28 – 63.78, P=0.018, Mann Whitney Rank Sum test - MW). Similarly was the Asp level in T2DM vs. controls (1.64, 1.12 - 1.90 vs. 2.31, 1.35 - 3.04, P=0.012, MW) as well as Tyr (211.65, 161.20 - 250.22 vs. 260.31, 230.16 - 402.87, P<0.001, MW) and Gln (208.18, 114.05 - 258.84 vs. 245.65, 183.17 - 389.24, P=0.010, MW). In contrast, only one essential AA, Phe, out of six tested, was significantly markedly lower in T2DM compared to the controls, 12.73, 6.84 - 94.32 vs. 136.88, 65.15 - 206.47, P<0.001. Of the above AAs the Phe and Tyr are aromatic amino acids (AAAs), both were significantly reduced in T2DM as mentioned above. In contrast, the BCAAs (Val, Leu, and Ile) were comparable between T2DM and control subjects. For levels of the remaining AAs see (Table 2).
The correlations between the AAs levels and the glycemic and lipid parameters in T2DM
The correlations of the glycemic parameters, FBG and HbA1c, with the fasting plasma levels of the 5 AAs (Gly, Gln, Asp, Tyr, Phe) which were significantly decreased in T2DM, were tested. The fasting plasma levels of Gln were found to be positively correlated with FBG and HbA1c levels (CC 0.520, P=0.001; CC 0.456, P=0.006, respectively), similarly the fasting levels of Tyr (CC 0.505, P=0.002 and CC 0.455, P=0.006, respectively). However, the fasting plasma levels of Gly, Asp and Phe were not correlated with the levels of FBG (P=0.116, 0.122 and 0.285, respectively) or HbA1c (P=0.251, 0.971, and 0.285, respectively), (data not shown).
In contrast, the levels of the lipid profile parameters, HDL-C and TG, none of which was correlated with the levels of any of the 5 AAs. Age as co-variate was not correlated with any of the test parameters, including glycemic and lipid profiles and the 5 AAs levels. The FBG, HA1c, and TG levels were strongly positively correlated with each other (data not shown)
Multiple logistic regression analysis
The multiple logistic regression analysis included 21 IVs, of which the FBG, HbA1c, TAG and HDL-C are variables well known to be associated with T2DM, while some others are not known to be associated with T2DM e.g. Total and LDL cholesterols and sex. These variables were used for validation of the data and models. In addition 13 AAs were included in the models
Bivariate correlations of the plasma levels of the AAs and other IVs with T2DM: As seen in (Table 3), the bivariate correlation between DVs and IVs revealed that out of the 21 IVs, only 9 showed clear correlation with the DVs (health vs. disease): 5 were positively correlated with health: Phe, Asp, Tyr, GLN, and HDL-C, and 4 negatively correlated with health (i.e. increased in T2DM); FBG, TG, HbA1c and age.
Table 3. Bivariate analysis results, showing the association between health status (healthy control vs. T2DM) as dependent variables (DVs) and 21 independent variables (IVs). Nine IVs showed significant association with the DVs
|
Variables |
Pearson Correlation |
Sig. (2-tailed) |
Nature of the IV |
|
health |
1 |
|
|
-
|
PHE |
0.415** |
0.000 |
Amino acids
|
-
|
ASP |
0.370** |
0.002 |
-
|
TYR |
0.292* |
0.014 |
-
|
GLN |
0.276* |
0.021 |
-
|
VAL |
-0.232 |
0.054 |
-
|
ALA |
-0.199 |
0.099 |
-
|
GLY |
0.184 |
0.126 |
-
|
LYS |
0.168 |
0.165 |
-
|
ILE |
-0.133 |
0.274 |
-
|
GLU |
0.123 |
0.309 |
-
|
THR |
0.114 |
0.349 |
-
|
SER |
-0.059 |
0.630 |
-
|
LEU |
0.012 |
0.919 |
-
|
Glucose(mM) |
-0.590** |
0.000 |
Glycemic parameter |
-
|
TG |
-0.446** |
0.000 |
Lipid parameter |
-
|
HDL |
0.442** |
0.000 |
-
|
HbA1c |
-.377** |
0.001 |
Glycemic parameter |
-
|
CHOL |
0.132 |
0.278 |
Lipid parameter |
-
|
LDL |
0.105 |
0.388 |
-
|
sex |
-0.213 |
0.077 |
Demographic parameter |
-
|
Age |
-0.395** |
0.001 |
Note: The first 13 IVs are amino acids. The bold text indicates significant association with the DV (health status).
**indicates stronger association.
Factor analysis (FA): The FA limited the IVs that influence the health into 11 variables out of the 21 analyzed. In addition it confirmed that the AAs, Phe, Asp, Tyr, Gln and Leu were the most correlated with health i.e. they are depleted in T2DM, while Gly was not identified as IV (Table 4).
Table 4. Factor analysis (FA) for the association of all studied variables with health status (healthy vs, T2DM): Correlation coefficient and P values
|
|
|
Communalities |
|
Correlation coefficient |
Sign (p) |
Initial |
Extraction |
Correlation |
health |
1.000 |
0.000 |
1.000 |
0.769 |
Phe |
0.415 |
0.001 |
1.000 |
0.841 |
Asp |
0.370 |
0.007 |
1.000 |
0.601 |
Tyr |
0.292 |
0.010 |
1.000 |
0.830 |
Gln |
0.276 |
0.027 |
1.000 |
0.813 |
Val |
-0.232 |
0.050 |
1.000 |
0.736 |
Ala |
-0.199 |
0.063 |
1.000 |
0.774 |
Gly |
0.184 |
0.082 |
1.000 |
0.752 |
Lys |
0.168 |
0.137 |
1.000 |
0.853 |
Ile |
-0.133 |
0.154 |
1.000 |
0.852 |
Glu |
0.123 |
0.175 |
1.000 |
0.747 |
Thr |
0.114 |
0.315 |
1.000 |
0.759 |
Ser |
-0.059 |
0.460 |
1.000 |
0.566 |
Leu |
0.012 |
0.000 |
1.000 |
0.880 |
Glucose |
-0.590 |
0.000 |
1.000 |
0.789 |
TG |
-0.446 |
0.000 |
1.000 |
0.482 |
HDL-C |
0.442 |
0.001 |
1.000 |
0.441 |
HbA1c |
-0.377 |
0.139 |
1.000 |
0.736 |
Total cholesterol |
0.132 |
0.194 |
1.000 |
0.940 |
LDL-C |
0.105 |
0.038 |
1.000 |
0.856 |
Sex |
-0.213 |
0.000 |
1.000 |
0.688 |
Age |
-0.395 |
0.000 |
1.000 |
0.444 |
|
|
|
|
|
16.149 |
Wald test: Finally, in Binary Logistic Regression analysis, the Wald test is used for testing the IVs used in the model. Result revealed that, only Phe (odds ratio 0.978, S.E. 0.012, P=0.051) and Asp (odd ratio .018, S.E. 1.572, P=0.010) were associated with T2DM in addition to age (odds ratio 1.577, S.E. 0.195, P=0.019) and HDL-C (odds ratio 0.000, S.E. 2.909, P=0.007). For interpretation of the Exp(B), e.g. age has an Exp(B) of 1.577 (or e0.456), hence, when the age increases one unit, the odds that the DV =1 (T2DM) increase by a factor of 1.577, (Table 5).
Table 5. The Wald test showing the statistical significance for each of the 9-independent variables (IVs) used in the model for prediction of T2DM
Variables in the Equation |
|
B |
S.E. |
Wald |
df |
Sig. |
Exp(B) |
Step 1a |
Age |
0.456 |
0.195 |
5.479 |
1 |
0.019 |
1.577 |
PHE |
-0.023 |
0.012 |
3.794 |
1 |
0.051 |
0.978 |
ASP |
-4.039 |
1.572 |
6.604 |
1 |
0.010 |
0.018 |
TYR |
0.002 |
0.005 |
0.103 |
1 |
0.748 |
1.002 |
GLN |
-0.002 |
0.002 |
0.894 |
1 |
0.344 |
0.998 |
VAL |
0.003 |
0.002 |
2.337 |
1 |
0.126 |
1.003 |
TG |
1.627 |
1.594 |
1.042 |
1 |
0.307 |
5.089 |
HDL-C |
-7.835 |
2.909 |
7.256 |
1 |
0.007 |
0.000 |
HbA1c |
0.024 |
0.040 |
0.364 |
1 |
0.547 |
1.024 |
Constant |
-9.894 |
7.145 |
1.918 |
1 |
0.166 |
0.000 |
In this study, we found that T2DM was associated with low levels of 5 out of 13 AAs (6 essential AAs), been tested compared with healthy controls, of the 5 AAs only the Phe is essential AA. The Phe and Tyr were the only AAAs in this study, and both were found to be significantly reduced in T2DM. The median levels of Phe were more than 10 times lower in T2DM compared to the controls. In previous studies, increased levels of Phe were found to be associated with T2DM [17], and to predict T2DM development [18]. Also, increased Phe was found to be associated with the development of metabolic syndrome (MetS) [19,20]. A systemic review and meta-analysis of 46 studies of different metabolites in diabetes and prediabetes, revealed that AAs were the most consistently associated with the risk of developing T2DM and that higher Phe levels were associated with diabetes [21]. In other studies, Phe levels were found to be correlated positively with plasma glucose and inversely with insulin sensitivity [22,23]. The contradiction between this study and others could be explained by i. Ethnic difference, ii. Disease stage, e.g. pre-diabetic in one study [18] or targeting MetS [19,20]. iii. The metformin only as treatment.
Tyr, a non-essential and AAA, was also significantly decreased in T2DM in this study as expected since Phe, the precursor of Tyr, was markedly low. Tyr was reported to be the strongest among BCAA and AAAs for prediction of future development of T2DM [24]. To interpret the current result in the context of T2DM, it is important to recall the physiological roles of Tyr; it is the precursor for melanin, the skin pigment, and other physiological molecules e.g. thyroxine, adrenaline, noradrenaline, and Dopa, which are largely antagonizing insulin in metabolism [3]. The low levels of Tyr could be due to decreased de novo synthesis secondary to the low Phe levels or due to over conversion to the specialized molecules e.g. thyroxine. However, in an observational study included more than 5000 patients, it was found that the rate of primary hypothyroidism in T2DM is greater than in non-diabetic population [25]. Can hypothyroidism in T2DM explained by Tyr deficiency?
The remaining 3 AAs, Gly, Gln, and Asp, are non-essential AAs, and all were significantly decreased in T2DM. The decreased levels of Gly in T2DM is in line with previous studies [23,26]. Dietary Gly was reported to increase insulin secretion, improve glucose tolerance and reduces systemic inflammation [27]. The Gly is part of the glutathione, a molecule involved in detoxification of free radicals, the latter play an important pathological role in T2DM [28].
The Gln, was also decreased in T2DM in this study, although, it was found to be raised in T2DM and insulin resistance in some studies [17,23]. In a large longitudinal study of the Finish population, decreased levels of Gln were found to be associated with hyperglycemia and insulin resistance [22]. Finally, with reference to a large meta-analysis study included more than 45 studies [21], the depletion of Gly and Gln in T2DM was concluded, which is supporting to our findings.
Although, the Asp was found to be reduced in T2DM and this association was confirmed in multiple logistic regression analyses in this study, in another study it was found to be raised in children with diabetic ketoacidosis [12]. Worth-noting the association of Asp with T2DM was the least to be reported in the literate between the 5 AAs.
Controversial to what has been reported before, the BCAAs were comparable between the T2DM and control subjects in this study, in other studies levels of BCAAs were increased in T2DM [17,26]. On the other hand, the AAAs e.g. Phe and Tyr, consistently and across several studies in different nations were shown to be increased in T2DM [11,13,19], however, they were markedly decreased in this study. The deficiency of the 5 AAs in T2DM in this study, can be explained by the utilization of carbon skeletons of these glucogenic AAs for glucose production (gluconeogenesis) [29]. Furthermore, we recently reported marked upregulation of protein synthesis in T2DM [15], a process that consume AAs. The predominant deficiency of the non-essential AAs, is suggestive of non-nutritional cause/s.
In the multiple logistic regression analysis, the different statistical tests confirmed the correct-fullness of the data and models as well as the depletion of the 5 AAs in T2DM, after correction for all possible co-variants. Furthermore, the AAs profile was found to be compared to the glycemic profile (RBG and HbA1c) in prediction of T2DM, and can distinguish between the patients and controls. The logistic regression model, explained 94% of the variance in T2DM and correctly classified 96% of the cases. Using the Wald test, the contribution of each of variables, namely the AAs, in development of diabetes was estimated (Table 5).
Of the implications of the AAs depletion in T2DM, is the direct effect in synthesis of proteins and specialized products derived from AAs e.g. thyroid hormones, catecholamines, melanin and etc., as these AAs derivatives are largely linked to T2DM pathology, clinical features and complications [25]. Thus, it worth to study the replacement of the deficient AAs in T2DM and to observe the clinical consequences with special emphasis on the complications as mentioned elsewhere [30]. Other potential applications for the AAs profile includes its utilization in diagnosis/prognosis of T2DM patients under treatment when the glycemic profile appears normal.
In conclusion, fasting plasma levels of 5 AAs, (Phe, Tyr, Gly, Gln, and Asp) were found to be reduced in metformin-treated T2DM patients. This finding was reproducible in the different statistical tests and models of the multiple logistic regression analysis. The Phe remained the most markedly altered AAs in all models. Furthermore, the AAs profile was found to be a predictor for T2DM comparable to FBG and HBA1c, probably the metformin treatment imposes more effect on the glycemic than the AA profile. Finally, can AAs replacement contributes to the wellbeing of T2DM patients?
The contribution of the study subjects and the clinicians in Salmanyia Medical Complex (Manama, Bahrain), and in Arad, Al-Dair and Sheikh Salman Health Centers (Muharraq, Bahrain), is highly appreciated. Mr. Diab Eltayeb, at Princess Al Jawhara Centre for Molecular Medicine and Inherited Disorders, Arabian Gulf University (AGU), Manama, Kingdom of Bahrain, is thanked for assistance in HPLC analysis.
Conflict of interest: For all authors, there is no conflict of interest to declare.
Ethical approval: The study received ethical approval from the Ministry of Health, Kingdom of Bahrain (9.7.2012) and from the College of Medicine and Medical Sciences (CMMS), Arabian Gulf University (AGU), reference number 62-PI-4-16. All performed procedures involving human participants were conducted in accordance with the ethical standards of the relevant institutional and national research committees and with the 1964 Helsinki Declaration and its later amendments.
Informed consent: Verbal informed consent was obtained from all study subjects.
The authors: Rabab Asghar Abdulwahab (RAA), Jaffar Abbas (JA), Abdul Ameer A Allaith (AAA), Hayder A. Giha (HAG).
Authors’ contributions: RAA, and HAG developed the research idea and study design; RAA and JA collected the samples, RAA, did the lab work, RAA, AAA, and HAG carried out the statistical analysis, RAA, AAA, JA and HAG drafted the manuscript and all authors were contributed to the final version of the manuscript.
Data Availability: The data are available from the corresponding author upon request.
Funding: This study received no financial support.
- Nolan CJ, Prentki M (2019) Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diab Vasc Dis Res 16: 118-127.
- Yang Q, Vijayakumar A, Kahn BB (2018) Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cell Biol 19: 654-672.
- Rizza RA, Cryer PE, Gerich JE (29179) Role of glucagon, catecholamines, and growth hormone in human glucose counter regulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest 64: 62-71.
- Al-Abbasi F (2013) The effect of glycemic status on the serum amino acid profile of diabetic Saudi patients. J Drug Metab Toxicol 4.
- Litwack G, Fisher H (1957) Role of essential amino acids in the early formation of avian liver xanthine dehydrogenase activity. Am J Physiology 191: 355-358.
- Chen H, Zhou X, Ou-Yang ZC (2002) Classification of amino acids based on statistical results of known structures and cooperativity of protein folding. Phys Rev E 65: 061907.
- Nagao K, Yamakado M (2016) The role of amino acid profiles in diabetes risk assessment. Curr Opin Clin Nutr Metab Care.
- Serralde-Zúñiga A, Guevara-Cruz M, Tovar R (2014) Omental adipose tissue gene expression, gene variants, branched-chain amino acids, and their relationship with metabolic syndrome and insulin resistance in humans. Genes Nutr 9: 1-10.
- Yang P, Hu W, Fu Z, Sun L, Zhou Y, et al. (2016) The positive association of branched-chain amino acids and metabolic dyslipidemia in Chinese Han population. Lipids Health Dis 15.
- Yamakado M, Tanaka T, Nagao K, Ishizaka Y, Mitushima T, et al. (2012) Plasma amino acid profile is associated with visceral fat accumulation in obese Japanese subjects. Clin Obes 2: 29-40.
- Würtz P, Soininen P, Kangas AJ, Rönnemaa T, Lehtimäki T, et al. (2013) Branched chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 36: 648-655.
- Szabó A, Kenesei E, Körner A, Miltényi M, Szücs L, et al. (1991) Changes in plasma and urinary amino acid levels during diabetic ketoacidosis in children. Diabetes Res Clin Pract 12: 91-97.
- Chen T, Ni Y, Ma X, Bao Y, Liu J, et al. (2016) Branched chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci Rep 6: 20594.
- Chen T, Zhang X, Long Y, Yu H, Ran X, et al. (2012) The association of plasma free amino acids with liver enzymes in Type 2 diabetic patients. J Endocrinol Invest 35: 772-775.
- Abdulwahab RA, Alaiya A, Shinwari Z, Allaith AAA, Giha HA (2019) LC‑MS/MS proteomic analysis revealed novel associations of 37 proteins with T2DM and notable upregulation of immunoglobulins. Int J Mol Med 43: 2118-2132.
- Terrlink T, van Leeuwn PA, Houdijk A (1994) Plasma amino acids determined by liquid chromatography within 17 minutes. Clin Chem 40: 245-249.
- Xu F, Tavintharan S, Sum CF, Woon K, Lim SC, et al. (2013) Metabolic signature shift in type 2 diabetes mellitus revealed by mass spectrometry-based metabolomics. J Clin Endocrinol Metab 98: E1060-E1065.
- Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, et al. (2011) Metabolite profiles and the risk of developing diabetes. Nat Med 17: 448-453.
- Weng L, Quinlivan E, Gong Y, Beitelshees AL, Shahin MH, et al. (2015) Association of branched and aromatic amino acids levels with metabolic syndrome and impaired fasting glucose in hypertensive patients. Metab Syndr Relat Disord 13: 195-202.
- Siomkajło M, Rybka J, Mierzchała-Pasierb M, Gamian A, Stankiewicz-Olczyk J, et al. (2017) Specific plasma amino acid disturbances associated with metabolic syndrome. Endocrine 58: 553-562.
- Guasch-Ferré M, Hruby A, Toledo E, Clish CB, Martínez-González MA, et al. (2016) Metabolomics in prediabetes and diabetes: A systematic review and meta-analysis. Diabetes Care 39: 833-846.
- Stancáková A, Civelek M, Saleem NK, Soininen P, Kangas AJ, et al. (2012) Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9,369 Finnish men. Diabetes 61: 1895-1902.
- Palmer ND, Stevens RD, Antinozzi PA, Anderson A, Bergman RN, et al. (2015) Metabolomic profile associated with insulin resistance and conversion to diabetes in the Insulin Resistance Atherosclerosis Study. J Clin Endocrinol Metab 100: E463-E468.
- Tillin T, Hughes AD, Wang Q, Würtz P, Ala-Korpela M, et al. (2015) Diabetes risk and amino acid profiles: cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia 58: 968-979.
- Tamez-Pérez HE, Martínez E, Quintanilla-Flores DL, Tamez-Peña AL, Gutiérrez-Hermosillo H, et al. (2012) The rate of primary hypothyroidism in diabetic patients is greater than in the non-diabetic population. An observational study. Med Clin (Barc) 138: 475-457.
- Drábková P, Šanderová J, Kovařík J, kanďár R (2015) An assay of selected serum amino acids in patients with type 2 diabetes mellitus. Adv Clin Exp Med 24: 447-451.
- Yan-Do R, MacDonald PE (2017) Impaired "Glycine"-mia in type 2 diabetes and potential mechanisms contributing to glucose homeostasis. Endocrinology 158: 1064-1073.
- Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI (2007) The central role of glutathione in the pathophysiology of human diseases. Arch Physiol Biochem 113: 234-258.
- Petersen M, Vatner D, Shulman G (2017) Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol 13: 572-587.
- Scognamiglio R, Negut C, Palisi M, Dioguardi FS, Coccato M, et al. (2008) Effects of oral amino acid supplements on cardiac function and remodeling in patients with type 2diabetes with mild-to-moderate left ventricular dysfunction. Am J Cardiol 101: 111E-115E.

