Abstract
Eosinophilic cholangitis (EC) arises from eosinophilic infiltration and fibrosis of the biliary tree. Patients present with obstructive jaundice and symptoms such as weight loss raising the alarm for cholangiocarcinoma (CCA). A 44-year-old man presented with abdominal pain, jaundice, and abnormal liver chemistries. Imaging showed intrahepatic ductal dilation and MRCP revealed an abrupt transition and stricture in the common hepatic duct. An ERCP with single-operator per-oral cholangioscopy (SOPOC) demonstrated a frond-like mucosa with increased vascularity concerning for malignancy. Biopsies were unobtainable but SOPOC features seemed definitive leading to resection. Histological examination found eosinophilic cholangitis.
Keywords
eosinophilic infiltration; biopsies; eosinophilic cholangitis
Introduction
Eosinophilic Cholangitis (EC) is a rare and benign fibrostenotic disorder of the biliary system that arises from eosinophilic infiltrates [1]. Studies estimate that it occurs in 2.2% of patients with sclerosing cholangitis [2]. SOPOC has advanced the evaluation of biliary strictures through direct visualization and targeted biopsy. Studies showed SOPOC imaging alone is adequate to distinguish malignant and benign strictures. However, our case showed the SOPOC features of EC can resemble CCA and targeted biopsy is imperative in diagnosis.
Case presentation
A 44-year-old man presented with days of RUQ pain, dark urine, pale stool, and scleral icterus as well as fatigue, anorexia, and 30-lbs of weight loss. Examination found scleral icterus. Laboratory analysis showed total bilirubin 13.2 mg/dL (direct 8.8 mg/dL), alkaline phosphatase 537 U/L, AST 328 U/L, ALT 688 U/L, and CA 19-9 387 U/L. WBC was 8.9 K/mm3 with 20% eosinophils. A CT showed intrahepatic ductal dilation. An MRCP showed intrahepatic biliary ductal dilation with an abrupt transition and stricturing at the junction of the common hepatic duct (CHD) and common bile duct (CBD), Figure 1. Wall thickening was noted with a mass-like enhancement.
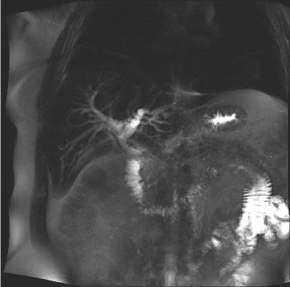
Figure 1. MRCP images showing intrahepatic biliary dilation and abrupt transitioning of the duct.
An ERCP found diffusely dilated ducts. The CBD had a severe stenosis 15 mm length and brushings were obtained. SOPOC revealed frond-like, nodular mucosa with increased vascularity concerning for malignancy, Figure 2. Unfortunately, targeted biopsies were not taken during the procedure due to lack of instruments.
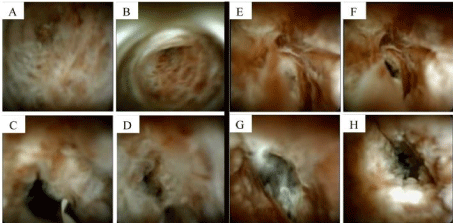
Figure 2. Comparison of CCA (images A-D) and EC (images E-H). Note that they are indistinguishable from each other on SOPOC.
Biopsies were non-diagnostic and brushings showed reactive glandular cells. He underwent excision of bile ducts and Roux-en-Y hepaticojejunostomy and cholecystectomy. Examination of the tissue showed thickened fibrotic bile duct and gallbladder walls exhibiting dense inflammatory infiltrate composed predominantly of eosinophils consistent with eosinophilic cholangitis and eosinophilic cholecystitis, Figure 3.
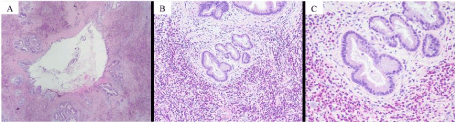
Figure 3. A) Bile duct with transmural inflammation and fibrosis (magnification 1x). B) Bile duct with numerous eosinophils (magnification 10x). C) Bile duct with numerous eosinophils (magnification 20x). All specimens with H&E staining.
Discussion and conclusion
EC is a rare and benign fibrostenotic disorder of the biliary tract from eosinophilic infiltrates [3,4]. A retrospective study found EC occurred in 2.2% of patients with sclerosing cholangitis and 30% of those where no cause was found during work up [2,5]. EC occurs most commonly in the 4th and 5th decade of life [6-8]. Abdominal pain was the most frequent symptom (44.4%) followed by jaundice (29.6%) and fever (18.5%) [7]. While peripheral eosinophilia occurs in 55.5-69.6% of EC cases, the relationship between EC and hypereosinophilic syndrome remains unclear [7]. Unintentional weight loss and an elevated CA 19-9 have been documented in several cases as well which emphasizes its role as a “malignant masquerade” [9-11].
EC presents with dense transmural eosinophilic infiltration to the bile duct causing thickening of the fibromuscular layer and fibrosis of the subserosal layer [3,12]. While no number of eosinophils has been established for diagnosis, > 30 eosinophils/hpf have been noted [2]. Consistent with reports of EC patients displaying high IgE, an IgE level of 749 kU/L was found in our case [12]. This coupled with observations of increased IL-5 raises the possibility that underlying allergic mechanisms may contribute to recruitment of eosinophils to the bile duct [12]. Eosinophils release free radicals and inflammatory proteins which cause fibrosis and lead to strictures [12].
SOPOC allows direct visualization and improvement in image quality, single-operator use, and the capability to perform targeted biopsies allowed SOPOC to become a powerful tool in diagnostic challenges of biliary strictures. A meta-analysis showed pooled sensitivity of 66.2% and specificity of 97.0% in diagnosis of malignant stricture [13]. Another randomized trial demonstrated the overall accuracy of targeted biopsy of SOPOC was superior (87.1%) compared to standard brushing or biopsy sampling (65.5%) [14]. The visual inspection alone from SOPOC can differentiate malignant from benign stricture with reasonable accuracy (97.2%) [15]. However, the SOPOC images of our patient illustrates EC can have features to suggest malignant stricture such as frond-like nodular mucosa, luminal narrowing, and increased vascularity. Comparison of our patient with EC to another patient with cholangiocarcinoma are shown in Figure 2. Their images are indistinguishable.
Symptoms may resolve spontaneously without intervention or after administration of steroids [1,16]. Budesonide and Prednisone work well and should be attempted [4,12]. In 23 cases of EC, 8 patients (34.8%) had complete resolution of symptoms with surgery alone and 7 patients (30.4%) improved with steroids [4]. This case illustrates EC can be indistinguishable from malignancy by SOPOC images and targeted biopsy must be taken. Although preoperative diagnosis of EC may have avoided surgery, given his young age and degree of stricture, we believe surgical resection was acceptable treatment.
References
- Matsumoto N, Yokoyama K, Nakai K, Yamamoto T, Otani T, et al. (2007) A case of eosinophilic cholangitis: imaging findings of contrast-enhanced ultrasonography, cholangioscopy, and intraductal ultrasonography. World J Gastroenterol 13: 1995-1997. [Crossref]
- Walter D, Hartmann S, Herrmann E, Peveling-Oberhag J, Bechstein WO, et al. (2017) Eosinophilic cholangitis is a potentially underdiagnosed etiology in indeterminate biliary stricture. World J Gastroenterol 23: 1044-1050. [Crossref]
- Akolkar S, Patel J, Abdu B, Sorser S (2019) The Cloaked Intruder: An Unexpected Case of Eosinophilic Cholangitis. ACG Case Rep J 6: e00235. [Crossref]
- De Roza MA, Lim CH (2017) Eosinophilic cholangitis treatment with budesonide. World J Hepatol 9: 1385-1388. [Crossref]
- Walter D, Hartmann S, Albert JG (2017) Indeterminate biliary stricture with suspicion for malignancy unmasked as eosinophilic cholangitis by cholangioscopy. Gastrointest Endosc 85: 265-266. [Crossref]
- Hoilat JN, Hoilat GJ, AlQahtani S, Alhussaini HF, Alabbad SI (2018) Atypical Presentation of a Rare Disease: Eosinophilic Cholangitis Posing as a Cancer. Am J Case Rep 19: 76-81. [Crossref]
- Tanaka S, Sakai A, Masuda A, Ashina S, Yamakawa K, et al. (2019) Eosinophilic Cholangitis Without Biliary Stricture After the Treatment of Eosinophilic Esophagitis. ACG Case Rep J 6: e00099. [Crossref]
- Oh SR, Kim D, Kim TH (2012) Eosinophilic cholangiopathy. Gastrointest Endosc 75: 669; discussion 669-670. [Crossref]
- Tai J, Perini MV, Azlanudin A, Muralidharan V, Christophi C (2019) Bile duct resection for eosinophilic cholangitis. ANZ J Surg 89: 1508-1509. [Crossref]
- Dubay D, Jhala N, Eloubeidi M (2010) Eosinophilic cholangitis. Clin Gastroenterol Hepatol 8: A22.
- Rodgers MS, Allen JP, Koea JB, McCall JL (2001) Eosinophilic cholangitis: a case of 'malignant masquerade'. HPB (Oxford) 3: 235-239. [Crossref]
- Fragulidis GP, Vezakis AI, Kontis EA, Pantiora EV, Stefanidis GG, et al. (2016) Eosinophilic Cholangitis--A Challenging Diagnosis of Benign Biliary Stricture: A Case Report. Medicine (Baltimore) 95: e2394. [Crossref]
- Navaneethan U, Hasan MK, Lourdusamy V, Njei B, Varadarajulu S, et al. (2015) Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc 82: 608-614.e602. [Crossref]
- Gerges C, Beyna T, Tang RSY, Bahin F, Lau JYW, et al. (2020) Digital single-operator peroral cholangioscopy-guided biopsy sampling versus ERCP-guided brushing for indeterminate biliary strictures: a prospective, randomized, multicenter trial (with video). Gastrointest Endosc 91: 1105-1113. [Crossref]
- Tieu AH, Kumbhari V, Jakhete N, Onyimba F, Patel Y, et al. (2015) Diagnostic and therapeutic utility of SpyGlass(®) peroral cholangioscopy in intraductal biliary disease: single-center, retrospective, cohort study. Dig Endosc 27: 479-485. [Crossref]
- Butler TW, Feintuch TA, Caine WP (1985) Eosinophilic cholangitis, lymphadenopathy, and peripheral eosinophilia: a case report. Am J Gastroenterol 80: 572-574. [Crossref]



