Heart failure (HF) is a clinical syndrome with significant rates of mortality, morbidity and hospital admissions. The rates are higher among older adults, and with an ageing population, the prevalence will continue to rise. Systolic heart failure (SHF) is a subset of HF with the most unfavorable prognosis despite significant improvements in medical therapies. There is a clinical need to develop standardized and accurate diagnostic procedures and therapeutic strategies to achieve better clinical outcomes for SHF patients. Thus, the present review seeks to aggregate research evidence on prognostication, pathophysiology, diagnosis and clinical management of HF. The review also includes meta-analyses of diagnosis of AHF in acute settings and medical management of SHF.
systolic heart failure, left ventricular failure, reduced ejection fraction
Heart failure (HF) represents the end-stage clinical syndrome in a broad variety of cardiovascular conditions with high rates of hospitalization, morbidity and mortality especially among older adults [1-4]. The need to improve clinical outcomes of HF motivated research into its natural course, symptomatology and severity yielding many different classification systems: acute vs. chronic, left-sided vs. right-sided, high-output vs. low output, and systolic vs. diastolic. The present review focuses on systolic vs. diastolic classification, and specifically SHF. Following the publication of the 2013 American College of Cardiology/American Heart Association (ACC/AHA) [5] and the 2016 European Society of Cardiology (ESC) [6] clinical guidelines for the diagnosis and management of HF, newer echocardiographic diagnostic parameters and medical therapies have been proposed. The emerging medical therapies when used in combination with the conventional HF medication may potentially ameliorate unfavorable outcomes associated with HF. Thus, the purpose of the present systematic review and meta-analysis is to aggregate current and emerging published evidence on the diagnosis and clinical management of SHF aimed to broaden the understanding on the clinical status and outcomes of SHF.
Definition, epidemiology and prognosis
Definition
Systolic dysfunction: The cardiac cycle consists of ventricular systole (contraction and ejection) and ventricular diastole (relaxation and filling). It involves pressure, volume and valvular movements occurring during systole and diastole [7]. Ventricular systole occurs during atrial diastole (relaxation) and is divided into two phases. In the initial phase, termed as isovolumetric contraction phase (because all the cardiac valves are closed), ventricular pressure is rapidly elevated. Once it exceeds atrial pressure, ventricular pressure results in the closure of the mitral and tricuspid valves in turn causing the first heart sound. Once the pressure in the LV and RV exceeds the aortic and pulmonary pressure, the aortic and pulmonary valves open and the second (ejection) phase of systole begins [8]. Initially, ejection is rapid and then gradually decreases. When the ventricular pressure falls below the aortic and pulmonary pressure, blood flow reverses briefly causing the aortic and pulmonary valves to close resulting into the second heart sound, at which systole completes [7-9].
With each LV systole, the LV does not eject the total volume of blood. Only a proportion of the intra-ventricular volume is pumped out, termed the ejection fraction (the volume of blood pumped divided by the total volume of blood in the LV). In a normal heart, about 65% of blood of the total ventricular volume is ejected with every beat. Cardiac output is the total volume of blood ejected per cardiac cycle (or stroke volume) over time [8]. Systolic dysfunction refers to impaired ability of the pump faction (reduced ejection fraction) and enlarged end-diastolic chamber. It is distinct from diastolic dysfunction, which is determined by increased resistance to ventricular filling in the presence of elevated filling pressures [10-11]. However, isolated systolic dysfunction is very rare. Most patients with SHF have a predominant systolic dysfunction accompanied with some degree of diastolic impairment. Systolic dysfunction in the absence of diastolic dysfunction is unlikely in clinical practice [12-14].
Systolic heart failure: The definitions of SHF have evolved over time. Early definitions described SHF as a condition in which the heart fails to discharge its contents adequately [15]; a pathophysiological state in which an abnormality of the cardiac function is responsible for the failure of the heart to pump blood at a rate commensurate with the requirements of the metabolizing tissues [16]; or a heart syndrome resulting from an impairment of the contractile or pump function [17]. Although accurate, these early definitions centered on describing the principal pathophysiologic mechanisms of SHF, thus were difficult to deploy in clinical practice. Efforts to overcome this challenge led to definitions that are more specific with a particular focus on etiology, pathophysiology and symptomatology, thus enabling their application in the clinical diagnosis and management of SHF.
At present, the ACC/AHA and ESC provide the most cited definitions of SHF. The ACC/AHA defines SHF as a complex clinical syndrome resulting from any structural or functional cardiac abnormality that impairs the ability of the ventricle to eject blood with manifestations of dyspnea, fatigue, exercise intolerance and fluid retention leading to splanchnic congestion and/or peripheral edema [5]. Similarly, the ESC defines SHF as a clinical symptom characterized by typical symptoms of breathlessness, ankle swelling and fatigue that may be accompanied by signs of elevated jugular pressure, pulmonary crackles and peripheral edema caused by structural and/or functional cardiac abnormality resulting in reduced cardiac output [6]. The two definitions further distinguish SHF from diastolic heart failure (DHF), the former resulting from impaired contractile function (defined by depressed ventricular ejection < 40%) while the latter results from impaired ventricular filling, compliance or relaxation (defined by a mildly reduced or preserved ejection fraction > 50%).
Whereas the definitions of both the ACC/AHA and ESC have been widely accepted, the definitions confine themselves to stages of the disease at which clinical symptoms become apparent. However, prior to the onset of clinical symptoms, SHF may be present with asymptomatic structural or functional systolic LV dysfunction, which are precursors to SHF. Recognition of these precursors is important because asymptomatic LV dysfunction is as prevalent as symptomatic SHF in the general population and it is associated with poor prognosis and outcomes [17,18]. In addition, commencing treatment at the asymptomatic stage significantly improves therapeutic efficacy and clinical outcomes as well as improves hospital-free survival in SHF patients [6]. Since a significant proportion of SHF patients maybe asymptomatic, their inclusion in the definition of SHF will have a positive implication of promoting research into approaches to improve their diagnosis.
Epidemiology: The prevalence of HF varies between studies depending on the definition applied, demographics of the included population (mainly age and sex), clinical setting (primary care, hospital clinic, hospital admission) and region [18-25]. Despite the differences, the prevalence is approximately 1% to 2% of the adult population in developed countries, raising to ≥ 10% for older adults (> 70 years) [26-29]. It is primarily a disease of the elderly affecting about 6.6% males and 4.8% females aged 70 to 79 years with females accounting for a larger proportion in the > 80 years age group (13.5%) compared to males (10.6%) [30]. Data on temporal trends based on patients hospitalized with HF suggests decreasing incidence of SHF relative to diastolic HF [31,32]. Among all individuals with HF (asymptomatic or validated clinical HF), the prevalence of LV systolic dysfunction is 6%, which includes patients suffering from SHF and reduced LEVF [27]. With the lack of epidemiological studies focused on SHF, large clinical registries providing the prevalence of SHF relative to diastolic HF report decreasing incidence of SHF due to lower severity and better treatment of acute HF [33]. However, prevalence is increasing due to an ageing population and increased survival due to significant improvement in diagnostic technology and treatment [34,35]. The Get With The Guidelines-HF (GWTG-HF) registry enrolling 99,825 reported SHF accounted for 49% [36]. In the European Society of Cardiology Heart Failure Long-Term (ESC-HF-LT) Registry recruiting 9,134 HF patients, SHF accounted for 60% [37] while in the Swedish HF Registry recruiting 40,230 HF patients, SHF accounted for 57% of all hospitalized HF patients [38].
Prognosis
Severity of heart failure: The severity of HF could be described using the New York Heart Association (NYHA) functional class [39] or the four stages (A to D) of HF developed by the ACC/AHA [40] (Table 1). The NYHA functional class describes the severity of HF based on the presence or absence of symptoms either at rest or during physical activity, together with the extent of physical (or exertional) limitation [39]. Although NYHA has validity and reproducibility challenges, it is an important marker for mortality [41,42]. On the other hand, the stages of HF describe the severity of HF based on disease progression defined by the presence or absence of HF symptoms and/or structural heart disease and has been used to describe both individuals and populations [40]. The two systems used to measure the severity of HF show a direct correlation between worsening symptoms and death in SHF patients [43].
Table 1. Severity of heart failure based on NYHA and stages of heart failure
|
NYHA Class |
I |
II |
III |
IV |
|
Description |
No physical limitation |
Slight limitation of physical activity |
Marked limitation of physical activity |
Symptoms present even at rest |
|
Stages of HF |
A |
B |
C |
D |
|
Description |
At risk of HF without structural heart disease |
Structural heart disease present with no HF symptoms |
Structural heart disease with HF symptoms |
End-stage heart failure |
HF: Heart Failure; NYHA: New York Heart Association
Prognosis markers: Systolic HF has been associated with worse prognosis compared to diastolic HF [44]. Thus, knowledge of prognosis markers for morbidity, hospitalization and mortality of SHF is important to assist care givers make informed decision on the most appropriate time and type of therapy, as well as in planning resource allocation in healthcare facilities [6]. Prognosis markers have been traditionally associated with worsening symptoms, severity of diastolic dysfunction and presence of cardiovascular and non-cardiovascular comorbidities [13]. Javaheri et al. [45] reported central sleep apnea, severe right ventricular systolic dysfunction and low diastolic pressure have an adverse effect on the survival of SHF patients despite medical therapy. The 2016 ESC HF guidelines identified many different markers for a worse prognosis in SHF patients (Table 2).
Table 2. Predictors of worse prognosis in systolic heart failure patients
|
Prognosis Markers |
Description |
|
Patient characteristics |
Older age, male sex and low socio-economic status |
|
Worsening symptoms |
High resting heart rate, pulmonary congestion, peripheral edema, , jugular venous dilatation, and hepatomegaly |
|
Severity of LV dysfunction |
Depressed LVEG, LV dilatation, severe LV dysfunction, mitral regurgitation, aortic stenosis and left atrial dilatation. |
|
Increased biomarker levels |
Increased natriuretic peptides (B-type natriuretic peptide [BNP] and/or N-terminal proBNP [NT-proBNP]). |
|
Cardiovascular comorbidities |
Atrial fibrillation (AF), ventricular arrhythmia, coronary artery disease, previous stroke or transient ischemic attack, myocardial infarction and arterial hypotension. |
|
Others |
Non-adherence with recommended treatment and clinical events such as HF hospitalization, aborted cardiac arrest and ICD shocks. |
Systolic heart failure has been associated with epidemiological profiles mostly older age, male sex and low socio-economic status, in which prevalence rates are higher suggesting they are important risk factors for the development or exacerbation of SHF [5,6]. Worsening symptoms, documented by transition into a higher NYHA functional class or the ACC/AHA HF stages, has a direct correlation with the progression of SHF, and considered prognostic markers for worsening SHF. Other important prognostic markers for death or hospitalization listed by the 2016 ESC HF guidelines include the severity of LV dysfunction, marked elevation in the levels of cardiac biomarkers (mainly natriuretic peptides), the presence of cardiovascular comorbidities, non-adherence to recommended medication and clinical events such as repeat hospitalization, aborted cardiac death and implantable cardioverter defibrillator [ICD] shocks [6].
Mortality rate: Increased evidence establishes that patients with SHF have a higher mortality rate compared to those with diastolic HF [46]. Five studies [46-50] comparing mortality rates between systolic and diastolic HF in older patients reveal higher rates for SHF patients in short- and long-term ranging between 19% and 47% compared to diastolic HF ranging between 9% and 28% (Table 3). In addition, Aronow et al. [49] studied mortality rates between one and five years in HF patients after prior myocardial infarction (MI) and found systolic dysfunction was a significant independent risk factor for mortality with a risk ratio of 2.2. In a related study, systolic dysfunction and atrial fibrillation significantly increased the risk of death with risk ratios of 2.2 and 1.5 respectively [51].
Table 3. Reported mortality rates between systolic and diastolic heart failure
|
Study [Ref #] |
Systolic Heart Failure |
Diastolic Heart Failure |
Period (yr.) |
|
|
No. of Patients |
Mortality Rate |
No. of Patients |
Mortality Rate (%) |
|
|
Aronow et al. [46] |
98 |
47 |
68 |
22 |
1.0 |
|
Pernenkil et al. [47] |
228 |
38 |
171 |
28 |
1.0 |
|
Vasan et al. [48] |
36 |
19 |
37 |
9 |
6.2 |
|
Aronow et al. [49] |
340 |
41 |
226 |
19 |
1.0 |
|
Gustafsson et al. [50] |
3274 |
24 |
2218 |
19 |
1.0 |
Etiology and pathophysiology
Etiology: Heart failure is a clinical syndrome with heterogeneity in both etiology and pathophysiology. Etiology of HF varies in different world regions [52]. However, coronary artery disease (CAD) has been singled out as the leading etiology of SHF, approximated to cause two-thirds of cases of SHF, with hypertension and diabetes cited as probable contributing factors. Besides CAD, other underlying causes of SHF include cardiomyopathy, aortic stenosis, mitral regurgitation, viral myocarditis and arrhythmia (Table 4).
Table 4. Major etiology of systolic heart failure
|
Etiology |
Description |
Effect on the Heart |
Ischemic (coronary artery disease or heart attack) |
Blocks coronary artery limiting blood flow to the myocardium |
Weakens and damages the myocardium impairing its ability to eject blood |
Cardiomyopathy |
A disorders of the myocardium |
Weakens heart muscle reducing the ability to pump blood effectively. |
Hypertension |
Causes blood pressure in the artery to rise. |
Increases cardiac workload to pump blood against increased pressure weakening the myocardium. |
Aortic stenosis |
Opening of aortic valve narrows impairing blood flow |
Cardiac workload increases to pump blood through the narrowed valve, which weakens the myocardium. |
Mitral regurgitation |
Improper closure of the mitral valve causing leakage on the LV |
Increased blood volume stretching and weakening the heart |
Viral myocarditis |
Viral infection of the myocardium |
Inflammation of the myocardium affecting its ability to pump blood effectively. |
Arrhythmia |
Irregular heart beat |
Irregular cardiac rhythm reduces pumping effectiveness |
The 2016 ESC guidelines subsumes the many cited causes of SHF under three major categories:
- Conditions causing myocardial injury: coronary artery disease, cardiomyopathy, viral myocarditis and toxins (anti-cancer medication).
- Conditions leading to abnormal loading: arterial hypertension, aortic stenosis and mitral regurgitation; and
- Arrhythmias: tachyarrhythmias and bradyarrhythmias.
Pathophysiology
The two major pathophysiologic mechanisms, which mainly occur post-infarction and conspire to precipitate or exacerbate the progression of SHF, are (a) the activation of neurohormonal systems (the sympathetic nervous system [SNS] and the Renin Angiotensin Aldosterone System [RAAS]); and (b) myocardial remodeling [53].
Neurohormonal processes: The activation of neurohormonal processes initiated by an insult to the heart, such as myocardial infarction, has been repeatedly cited as the principle contributor to the progressive development of SHF. In the short-term, adaptive changes occur in the myocardium but in the long-term, the changes become maladaptive [6]. Post-myocardial infarction (the initiating insult to the myocardium) causes an increase in sympathetic tone (activation of the sympathetic nervous system [SNS]), which is an adaptive response to increase cardiac output from the weakening heart. Increased activation of the SNS causes increased secretion of the hormone and neurotransmitter norepinephrine, which exerts an inotropic effect leading to increased myocardial contractility (pumping function of the heart) as well as increasing blood pressure through vasoconstriction [53]. Vasoconstriction on the renal efferent arterioles result in increased renal re-absorption of sodium to maintain blood pressure. However, over time, these compensatory mechanisms become maladaptive. Increased sodium handling leads to excessive water retention, which is a characteristic of decompensated HF (deterioration of cardiac function presenting as an acute episode of pulmonary edema, increased exercise intolerance and breathlessness on exertion). Marked reduction in cardiac output, which is a cardinal feature of SHF, results from hypoperfusion of kidneys and the consequent activation of the Renin Angiotensin Aldosterone System (RAAS) and the associated increased in renin production and angiotensin II levels. Higher circulating levels of angiotensin II exerts cardiac effect similar to that of norepinephrine hormone – stabilizes cardiac function and circulation in the short-term through increased vasoconstriction and increased renal sodium re-absorption mediated by aldosterone production stimulated by elevated circulating levels of angiotensin II [54].
Myocardial remodeling: Following an insult to the heart, such as myocardial infarction (MI), macrophages, monocytes and neutrophils migrate into the infarct zone initiating intracellular signaling and neurohormonal activation [55]. Subsequent post-infarction cardiomyocyte apoptosis leads to a reduction in cardiac output and an abrupt increase in loading conditions, which contribute to reparative myocardial remodeling including dilatation, hypertrophy and formation of a discrete collagen scar [56]. Initially, myocardial remodeling involves the expansion of infarct from the degradation of inter-myocyte collagen struts and the activation of the matrix metalloproteinases (MMPs) secreted by the neutrophils. Infarct expansion occurs hours following cardiomyocyte injury leading to wall thinning and ventricular dilatation, which increase systolic wall stress. Increased wall stress stimulates hypertrophy mediated by mechanoreceptors and partly through angiotensin II release leading to contractility. Increased wall stress has also been shown to be a major determinant of ventricular performance [57].
Myocardial remodeling occurs in two phases: early and late remodeling. Early remodeling preserves stroke volume through adaptive responses, which involve the non-infarcted remote myocardium. Infarct expansion causes deformation of the border zone and remote myocardium, which alters Frank/Starling relations and increases shortening [58]. Perturbations in circulation hemodynamics activates the RAAS system, which stimulates the release of serum natriuretic peptides (atrial and BNP). Increased shortening and heart rate due to SNS stimulation causes hyperkinesia of the non-infarcted myocardium and temporary circulatory compensation. Furthermore, increased levels of serum natriuretic peptides decreases intravascular volume and systemic vascular resistance, normalize ventricular filling, and improve pump function [53]. Late remodeling involves alterations in LV architecture (dilatation and distortion of LV share and mural hypertrophy) to distribute the increased wall stress as the extracellular matrix forms a collagen scar to stabilize distending forces and prevent further myocardial deformation. Continued wall stress leads to progressive dilatation, expansion of infarct zone and increased contractile function [53,54]. Based on Starling’s law, increased ventricular filling during diastole causes an increase in end –diastolic volume, ventricular wall stretch and relative increase in cardiac output during systole. Due to remodeling in SHF stimulated by neurohormonal activation, the heart operates at a decreased cardia output, meaning the pump is failing. Continues neurohormonal activation results in worsening HF [54].
Clinical presentation and evaluation
Clinical presentation
Patients with SHF often presents with a combination of typical signs and symptoms of HF. However, these symptoms are heterogeneous and vary widely among patients [59,60]. Despite the heterogeneity, specific symptoms of HF include dyspnea (shortness of breath), orthopnea, paroxysmal nocturnal dyspnea, fatigue (due to HF-induced circulation abnormalities in skeletal muscles), edema and abdominal distension. However, these symptoms are non-sensitive and may not be very reliable in excluding the diagnosis of SHF [61-63] (Table 5).
Table 5. Specificity and sensitivity of typical symptoms of heart failure
|
Specific Symptoms |
Sensitivity (%) |
Specificity (%) |
|
Dyspnea |
84-100 |
17-34 |
|
Orthopnea |
22-50 |
74-77 |
|
Paroxysmal nocturnal dyspnea |
39-41 |
80-84 |
|
Fatigue/edema |
23 |
80 |
While symptoms might be specific, the onset or the early stages of SHF lacks specific signs due to compensatory mechanisms. However, in the later stages of SHF when compensatory mechanisms become maladaptive, typical signs may include tachycardia, pedal edema, increased hepatojugular pressure, pulmonary crackles, gallop rhythm, hepatojugular reflux and ascites. These signs have varying levels of specificity and sensitivity [64,65] (Table 6).
Table 6. Specificity and sensitivity of typical signs of heart failure
|
Typical Signs |
Sensitivity (%) |
Specificity (%) |
|
Tachycardia |
7 |
99 |
|
Increased hepatojugular venous pressure |
39 |
92 |
|
Pulmonary crackles |
60 |
78 |
|
Gallop rhythm (third heart sound) |
13 |
99 |
|
Hepatojugular reflux |
24 |
96 |
|
Ascites |
2 |
97 |
In obese patients and older adults, caution should be taken because they usually present with atypical symptoms [59]. Patients may also present with diverse underlying disease processes such as coronary artery disease (CAD), valvular heart disease, hypertension, cardiomyopathies and congenital abnormalities, which are the major etiologies of SHF [6]. Clinically, SHF may be classified into NYHA functional class or ACC/AHA four stages of HF, which represent the progression of the disease based on symptoms and the presence of structural heart disease [39,40].
Clinical evaluation
The diagnosis of SHF lacks any specific test. It is largely a clinical diagnosis based on a careful clinical history, physical examination, assessment of serum natriuretic peptides and cardiac imaging. Diagnosis algorithm is divided into acute and non-acute settings (Figure 1).

Figure 1. The ESC diagnostic workup of systolic heart failure
Diagnosis of SHF is based on acute or non-acute onset. ECG and chest x-ray is the recommended initial tests for both acute and non-acute onset of HF to determine signs and symptoms. Serum NP assessment and echocardiography should be considered for confirmatory diagnosis. Adapted from McMurray, et al. 2012, p. 1799 [52]
Non-acute setting: In patients presenting with typical signs and symptoms of HF in non-acute settings (primary care or hospital outpatient clinic), the probability of HF should be evaluated using prior clinical history, presenting symptoms, physical examination and electrocardiograph assessment (Table 7).
Table 7. Initial clinical evaluation for systolic heart failure in non-acute setting
|
Clinical Evaluation |
Purpose of clinical evaluation |
|
Prior clinical history |
Determination for known cardiac and extra cardiac etiology of heart failure such as coronary artery disease (CAD), arterial hypertension and diabetes, and diuretic use. |
|
Presenting symptoms |
The presence of typical symptoms of heart failure such as orthopnea, exercise intolerance and fatigue. |
|
Physical examination |
Determination of known typical signs of heart failure such as bilateral edema, increased jugular venous pressure, displaced apical beat |
|
Electrocardiography |
Determination of known ECG abnormalities associated with systolic heart failure. |
The purpose of the initial clinical evaluation for SHF in non-acute setting is to determine the presence of known signs and symptoms, and potential underlying causes of SHF. If all the four tests show normal findings, SHF is unlikely and other diagnosis may be considered. However, if at least one of the initial tests show an abnormal finding, evaluation of serum natriuretic peptides usually BNP or NT-proBNP should be considered [60]. Although the assessment of natriuretic peptides is not common in routine clinical practice, their evaluation is highly recommended when echocardiography is not immediately available [5,6,59]. The cut-off values for natriuretic peptides in non-acute settings are BNP ≥ 35 pg/mL or NT-proBNP ≥ 125 pg/mL. The cut-off points were selected to reduce the high rates of false negative. Natriuretic peptides levels above the cut-off points and abnormal ECG findings have high negative predictive values and recommended for the exclusion of SHF rather than confirming diagnosis. Thus, in non-acute settings, findings of natriuretic peptides levels and ECG are important for selecting appropriate patients for cardiac examination [6].
However, in evaluating natriuretic peptides, other causes of elevated levels of BNP and NT-proBNP such as old age (> 75 years), atrial arrhythmias, LV hypertrophy, chronic obstructive pulmonary disease (COPD), and chronic kidney disease (CKD) should be considered. Natriuretic peptides not only provide valuable information on pathophysiology of SHF but also provide valuable information on the severity of SHF. The National Academy of Biochemistry report the assessment of natriuretic peptides in HF patients enables clinicians to (a) identify possible underlying and reversible causes of SHF; (b) raise clinical suspicion of SHF; (c) estimate the severity of SHF and disease progression [66]. Serum natriuretic peptides also provide important prognostic information for mortality – BNP levels < 200 pg/mL at admission are associated with 2% mortality rate compared to 9% mortality with BNP > 200 pg/mL [67]. On the other hand, NT-proBNP > 5,000 pg/mL at admission are associated with in-hospital mortality rate of 22.5% and a longer hospital stay in surviving patients [68].
Echocardiography is the most widespread non-invasive imaging modality for the assessment of systolic dysfunction in patients suspected with SHF because of wide availability, inexpensive, ease of use and reproducibility [5]. It is recommended for patients with abnormal ECG findings or higher levels of serum natriuretic peptide beyond the cut-off values [52]. Echocardiography is useful in providing immediate information on cardiac chamber volumes, systolic function, wall thickness, valve function and pulmonary hypertension [69-76]. The information is critical in establishing diagnosis and determining the most appropriate treatment [6]. Common echocardiography abnormalities assessed in SHF patients include LV abnormalities – reduced ejection fraction, fractional shortening, regional function, end-diastolic size, end systolic size, and LV outflow tract velocity (Table 8).
Table 8. Common echocardiography abnormalities in systolic heart failure
|
Measurement |
Abnormality |
Clinical Implication |
|
LV ejection fraction |
Depressed (< 40%) |
LV global systolic dysfunction |
|
LV fractional shortening |
Depressed (< 25%) |
LV radial systolic dysfunction |
|
LV regional function |
Hypokinesia, akinesia and dyskinesia |
Myocardial infarction or ischemia, cardiomyopathy, myocarditis |
|
LV end-diastolic size |
Increased diameter (≥ 60mm/ 32 mm/m2)and volume (> 97 mL/m2) |
Volume overload |
|
LV end-systolic size |
Increased diameter (≥ 45mm/ 25 mm/m2)and volume (> 43 mL/m2) |
Volume overload |
|
LV outflow tract velocity time integral |
Reduced (< 15 cm) |
Reduced LV stroke volume |
If echocardiography images are suboptimal or unusual, and diagnosis is inconclusive, other complementary imaging methods should be considered to confirm diagnosis. Cardiac magnetic resonance imaging (MRI) is the gold standard of assessing ventricular volumes, mass and ejection fraction but its high cost and non-availability limits its use in routine clinical practice [6]. It is the best alternative imaging method for SHF patients when echocardiography is unavailable or inconclusive and the method of choice in patients with complex congenital heart diseases because of high anatomical resolution of all cardiac aspects [77,78]. In addition, cardiac MRI may provide more information about myocardial perfusion, viability and fibrosis useful for identifying SHF etiology and assessing prognosis [5,6]. Chest x-ray may at times be used as part of the initial clinical evaluation of HF but it has limited diagnostic utility in SHF patients. It is useful in the identification of alternative explanation for pulmonary symptoms and signs and for the diagnosis of asthma and COPD. Chest x-ray may also reveal pulmonary venous congestion or edema in SHF patients and more useful in acute settings [79].
Acute setting: Diagnosis of SHF in acute settings differs with that in non-acute settings. Acute settings deal with a rapid onset or worsening symptoms and/or signs of SHF. Acute SHF is a life-threatening medical condition that requires immediate clinical evaluation and treatment often leading to an urgent need for hospitalization. It may occur as a de novo (new) HF or because of acute decompensation of chronic SHF caused by acute MI, valve insufficiency or pericardial tamponade. Although it may occur in the absent of known precipitating factors, typical co-occurring conditions include hypertension, rhythm disturbances or non-adherence to medication [6]. Diagnostic work-up needs to begin in the pre-hospital setting and continues in the emergency department to establish timely diagnosis and institute prompt management. The benefits of early treatment is well established in acute care settings, which should be considered in an acute case of SHF [80,81]. In addition, co-occurring life-threatening conditions or HF precipitants that need urgent medical attention should be immediately identified and managed. The initial step in acute care setting in the diagnosis of SHF is ruling out alternative causes of symptoms and signs such as pulmonary infection, acute renal failure and severe anemia [5,6].
The 2016 ESC HF guidelines recommend the initial diagnosis of SHF in acute settings should be based on a detailed prior clinical history of the patient to assess the presence of symptoms, and to determine prior cardiovascular history and the presence of potential cardiac and extra-cardiac precipitants. Initial assessment should also determine the presence of symptoms and signs of congestion or hypoperfusion by physical examination and additional examinations including ECG abnormalities, chest x-ray, laboratory assessment of serum natriuretic peptides and cardiac imaging to confirm SHF. Typical symptoms of acute onset of SHF include fluid overload (leading to pulmonary congestion and/or peripheral edema) or less often decreased cardiac output with peripheral hypoperfusion (Table 9).
Table 9. Signs and symptoms of acute onset of systolic heart failure
|
Signs/Symptoms |
Description/Interpretation |
|
Left –sided congestion |
Orthopnea, paroxysmal nocturnal dyspnea, bilateral pulmonary rales and bilateral peripheral edema. |
|
Hypoperfusion |
Cold extremities, oliguria, mental confusion, dizziness and narrow pulse pressure. Laboratory measures – metabolic acidosis, elevated serum lactate and elevated serum creatinine. |
|
Hypotension |
Systolic blood pressure (< 90 mmHg) |
|
Bradycardia |
Heart rate < 40 beats per minute |
|
Tachycardia |
Heart rate > 120 beats per minute |
|
Abnormal respiratory effort |
Respiratory rate > 25 breaths/minute or < 8 breaths/minute despite dyspnea |
|
Low oxygen saturation |
Oxygen saturation < 90% in pulse oximetry |
|
Hypoxemia |
Oxygen partial pressure in arterial blood < 80 mmHg |
|
Acidosis |
pH < 7.35 |
|
Oliguria |
Urine output < 0.5 Ml/kg/h |
Chest x-ray and ECG are the initial tests in acute settings for patients suspected with acute onset of SHF. Although normal in about 20% of the patients with acute SHF, chest x-ray is a useful test to diagnose SHF in acute settings by providing information on pulmonary venous congestion, pleural effusion, interstitial or alveolar edema and cardiomegaly are specific findings for acute onset of SHF [82]. Chest x-ray may also be useful to detect alternative underlying non-cardiac diseases precipitating or exacerbating patient symptoms such as pneumonia and non-consolidated pulmonary infections [6]. On the other hand, ECG is rarely normal in acute SHF with a high negative predictive value (important for the exclusion of SHF or for informing the need for cardiac imaging). ECG is also useful in detecting underlying cardiac disease and potential precipitants such as rapid atrial fibrillation and acute myocardial ischemia.
Upon presentation to the emergency department, natriuretic peptides (BNP, NT-proBNP or MR-proANP) should be assessed in all suspected SHF patients with acute dyspnea or with at least one abnormal ECG or chest x-ray finding. Measurement of natriuretic peptides helps to distinguish acute onset of SHF from non-cardia causes of acute dyspnea. Natriuretic peptides have high sensitivity for the exclusion of SHF when there levels are beyond the set thresholds of BNP < 100 pg/mL or NT-proBNP < 300 pg/mL, which are higher than thresholds for non-acute settings [83-89]. However, elevated levels of natriuretic peptides in isolation do not confirm the diagnosis of SHF because they may be associated with a wide variety of cardiac and extra-cardiac causes (Table 10). Some patients with decompensated end-stage HF or flash pulmonary edema may present unexpectedly low levels of natriuretic peptides.
Table 10. Causes of elevated natriuretic peptides in acute onset of systolic heart failure
|
Categories |
Specific Causes |
|
Cardiac |
Heart failure, acute coronary syndrome, pulmonary embolism, myocarditis, LV hypertrophy, hypertrophic/restrictive cardiomyopathy, valvular heart disease, congenital heart disease, atrial and ventricular tachyarrhythmias, heart contusion, cardioversion, ICD shock, surgical cardiac procedures, pulmonary hypertension. |
|
Extra-cardiac |
Advanced age, ischemic stroke, subarachnoid hemorrhage, renal dysfunction, liver dysfunction, paraneoplastic syndrome, chronic obstructive pulmonary disease, severe infections (pneumonia or sepsis), severe burns, anemia or severe metabolic and hormone abnormalities such as thyrotoxicosis or diabetic ketosis. |
In patients with abnormal ECG and/or natriuretic peptides above thresholds, cardiac imaging is recommended to confirm diagnosis. Echocardiography is the widespread non-invasive imaging method. Immediate echocardiography must be considered for patients with hemodynamic instability (those in cardiogenic shock) and for patients suspected with acute life-threatening structural or functional cardiac abnormalities such as mechanical complications, acute valvular regurgitation or aortic dissection. Echocardiography should also be considered with all patients with de novo acute SHF and patients with previously unknown cardiac function. Repeated echocardiography is not usually required unless there is relevant deterioration in clinical status. Other laboratory tests that may be considered in acute onset of SHF include the assessment of cardiac troponin levels, blood urea nitrogen, serum creatinine, electrolytes sodium and potassium, and liver function tests [6].
Meta-analysis of diagnosis of acute SHF
Diagnostic guidelines for SHF in non-acute settings is well established. However, in acute settings diagnosis continues to pose considerable challenges. Time to perform diagnostic tests is limited requiring emergency physicians to make rapid diagnosis and treatment plans with limited clinical information [6]. Many patients present with acute decompensated HF (ADHF), which is difficult to diagnose because radiographic and laboratory tests have variable diagnostic values. In addition, many patients in acute settings often present with dual diagnosis of CHF and COPD. Clinically distinguishing the two is often difficult but essential since each requires a different management approach and the use of an incorrect method could cause detrimental cardiovascular outcomes [5,6]. Rapid bedside tests that distinguish between CHF and COPD is extremely useful to emergency physicians, out-of-hospital emergency care providers and intensive care specialists. Multi-organ ultrasound, which evaluates both the lungs and heart, has emerged as an accurate method outperforming clinical gestalt for the diagnosis of ADHF [6]. This meta-analysis therefore seeks to determine the diagnostic value of multi-organ ultrasound for patients suspected with SHF acute settings.
Search strategy and inclusion criteria: The search and analysis of studies examining the diagnosis of SHF in acute settings using (lung and cardiac ultrasound (LUCUS), also referred to as thoracic ultrasound, adhered to the systematic review protocol proposed by the Cochrane Handbook guidelines. The search was conducted on the following online databases: PubMed, EMBASE, Ovid MEDLINE, and non-indexed citations – Google Scholar and the Cochrane Database of Systematic Reviews. In addition, search for grey literature included a manual search for potentially eligible articles from review articles and electronic search for conference abstracts of major emergency medicine, cardiology and critical care conferences. Conferences searched included the Society for Academic Emergency Medicine, American College of Emergency Physicians, Canadian Association of Emergency Physicians, American Heart Association, European Society of Cardiology, Society of Critical Care Medicine, Canadian Cardiovascular Congress, Canadian Critical Care Forum, and the International Symposium on Intensive Care and Emergency Medicine.
Studies were included based on the following inclusion criteria: (a) prospective cohort and prospective case-controlled studies; (b) recruited patients presenting with symptomatic, acute dyspnea or with a clinical suspicion of CHF; (c) used multi-organ ultrasound to assess for SHF; and (d) reported diagnostic outcomes confirming or excluding SHF. No restriction was made on publication period and language. Two reviewed independently reviewed all qualifying citations to assess inclusion, abstracted data and screened included studies for methodological quality using the QUADAS-2 tool. Data abstracted from the included studies included first author and reference number, publication year, patient sample, mean age and female percentage, and outcomes – cardiogenic dyspnea, sensitive and specificity in distinguishing COPD from AHF (Table 11).
Table 11. Summary of included studies on diagnosis of systolic heart failure
|
Study Author [Ref #] |
Publication Year |
Study Sample |
Mean Age (yrs.) |
Female (%) |
Cardiogenic Dyspnea |
Sensitivity |
Specificity |
Lichtenstein et al. [90] |
1998 |
146 |
73.0 |
42 |
NR |
100 |
92 |
Gargani et al. [91] |
2008 |
149 |
72.0 |
26 |
82 |
81 |
85 |
Lichtenstein et al. [92] |
2008 |
260 |
68.0 |
46 |
NA |
89 |
97 |
Liteplo et al. [93] |
2009 |
94 |
74.0 |
41 |
43 |
58 |
85 |
Nazerian et al [94] |
2010 |
145 |
81.0 |
54 |
44 |
63 |
76 |
Prosen et al. [95] |
2011 |
129 |
70.9 |
27 |
NR |
100 |
95 |
Vitturi et al. [96] |
2011 |
152 |
NR |
NR |
97 |
79 |
81 |
Cibinel et al. [97] |
2012 |
56 |
82.1 |
38 |
48 |
94 |
84 |
Spinelli et al [98]. |
2014 |
70 |
NR |
NR |
NR |
92 |
93 |
Russell et al. [99] |
2015 |
99 |
56.0 |
45 |
36 |
83 |
83 |
Golshani et al. [100] |
2016 |
48 |
66.9 |
46 |
NA |
86 |
66 |
Study characteristics and outcomes: The online search and manual screening of bibliographies yielded 5,452 citations. After a review of titles and abstracts, 42 articles were selected for a full text review. Finally, eleven (11) articles [90-100] meeting the inclusion criteria were included in this meta-analysis. The combined patient population from the 11 studies was 1,348. The patients were older (mean age = 72 years) with a slightly lower female proportion (41%). All the studies were prospective and the main presenting symptom in emergency department was acute onset of dyspnea. The primary diagnosis method used was LUCUS. The diagnosis target was to differentiate cardiogenic from non-cardiogenic (usually pulmonary) dyspnea. In six studies [91,93,94,96,97,99] that reported the percentage of diagnosis (who had cardiogenic dyspnea or dyspnea of cardiac origin), out of 350 patients, 58% were had a positive diagnosis. The findings reveal LUCUS has a high mean sensitivity (84%) and mean specificity (85%) in the differential diagnosis of SHF in acute settings.
Discussion of findings: Acute onset of dyspnea is a common symptom in patients suspected with SHF admitted to the Emergency Department (ED) [101]. A consensus paper by the American Thoracic Society defines dyspnea as “a subjective experience of breathing discomfort that consists of qualitatively distinct sensation that vary in intensity…” [102]. The main cause is the inability of the circulatory (cardiac causes) and/or respiratory system (non-cardiac causes) to maintain a balanced gas exchange and acid-base status with overlapping clinical presentations in comorbid diseases such as SHF and COPD [103]. Discriminating between dyspnea due to SHF and COPD is a clinical dilemma in the acute care setting [101,102]. While echocardiographic parameters are well established for the differential diagnosis of SHF, prompt determination of the etiology of dyspnea has remained a challenge in ED. Lung and cardiac ultrasound has been shown to be an important test to confirm or exclude the diagnosis of dyspnea of cardiac origin (caused by SHF) but current guidelines does not include LUCUS but other tests such as medical history and clinical examination, pulse oximetry, NPs, ECG and echocardiography [103]. However, a recent meta-analysis on the diagnosis of acute HF in the ED reports that clinical gestalt based on an aggregate effect of patient medical history, presenting symptoms and physical examination are not reliable for excluding or establishing SHF diagnosis in the acute care setting but determine pretest probability of acute SHF [104]. Thus, the specific objective of this meta-analysis was to assess the accuracy of lung and cardiac ultrasonography in the diagnosis of SHF in patients presenting with non-differentiated acute dyspnea in acute care setting.
This meta-analysis demonstrates that LUCUS is both a sensitive and specific method for the diagnosis and exclusion of SHF. It is a useful test for discriminating between dyspnea of cardiac or pulmonary origin to support the diagnose SHF. Other supporting findings reported in individual studies suggest that the LUCUS protocol in undifferentiated dyspneic ED patient achieves a better diagnostic accuracy than clinical gestalt alone as well as increases physician confidence in the diagnosis of acute SHF [99]. The sensitivity of LUCUS has also been shown to be similar to that of plasma NPs [91,96] or higher [95], suggesting that both LUCUS and NPs are a useful mean of excluding cardiac causes of pulmonary edema. Although operator dependent, LUCUS is rapid and easy to perform as well as its use is justified by a high intra- and inter operator agreement [96]. In addition, the LUCUS protocol can provide additional predictive power to plasma NPs assessment in the immediate evaluation of dyspneic patients presenting to ED to discriminate acute SHF-related dyspnea from that of COPD/asthma-related dyspnea in pre-hospital emergency setting [93,95]. Lung ultrasound alone has also been shown to have a high predictive power to exclude dyspnea of cardiac origin [91] as well as to assist the clinician to make a rapid diagnosis in patients with acute respiratory failure to meet the priority objective of saving time [92].
Two previous meta-analysis on the diagnosing acute heart failure in the emergency department [104] and in point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea [105] have reported consistent findings. Martindale et al. [104] in a review of 52 studies (totaling 17,893 patients) report that lung ultrasound has the best combination of test characteristics with either the presence or absence of diffuse B-lines to provide reliable information to confirm or exclude ED diagnosis of acute SHF. Ling ultrasound alone provides more specific findings compared to clinical history, symptoms, physical examination, chest radiography and electrocardiography that lack discriminatory in establishing or excluding the diagnosis of acute SHF. In the study, although the assessment of plasms NPs are valuable in excluding acute SHF when values are lower than the recommended cut off – 100 (BNP) and 300 pg/mL (NT-proBNP), values above these thresholds are less helpful in establishing diagnosis. While other diagnostic modalities such as bio-impedance may have future utility in the acute care setting, additional studies are required to confirm their diagnostic values [104]. Al Deeb [105] meta-analysis of seven studies including 1,075 patients in intensive care unit, prehospital settings and in hospital wards reports high sensitivity of lung ultrasound in diagnostic sensitivity (94%) and specificity (84%) for acute cardiogenic pulmonary edema (ACPE). In summary, in the present meta-analysis findings, supported by findings from previous related meta-analyses, strongly suggest the diagnostic value of LUCUS in the timely establishment or exclusion of the diagnosis of SHF in emergency department and pre-hospital acute care setting, and the value of including LUCUS in the current differential diagnosis of SHF in acute care settings.
Clinical management
Therapeutic goals: Clinical management of SHF has had extensive research support on therapeutic efficacy of both pharmacological and device therapies. The evidentiary support has improved prognosis, hospitalization and mortality rates [5,6,52,59]. Pharmacological management remains the frontline therapy in patients diagnosed with SHF [106-108]. The goal of clinical management of SHF include (a) the relief of symptoms and signs such as edema; (b) prevent hospital admission related to HF; and (c) improve survival. While previous focus of clinical trials was on minimizing mortality, preventing HF-associated hospitalization has emerged as an important therapeutic goal for both patients and healthcare systems. Reduction in the rates of mortality and hospitalization reflect the ability of effective treatment to slow or prevent the progression of SHF, which is documented by reverse LV remodeling and a reduction in serum concentration of natriuretic peptides [109,110]. Although relief of symptoms, improvement in quality of life and improved functional capacity are important for SHF patients, they have not been the primary outcomes in most clinical trials [111]. The major reasons have been difficulty in measurement and some treatment previously reported to improve these outcomes showed a decrease in survival [112,113]. However, pharmacological support and device therapies such as cardiac resynchronization therapy (CRT) have shown improvement in both mortality rates and hospitalization [52].
Treatment algorithm: The optimal frontline treatment of SHF includes a combination of medication targeting favorable myocardial remodeling by affecting neurohormonal (SNS and RAAS) activation, which is the leading contributory factor to maladaptive pathophysiological pathways [107]. The main medication recommended for SHF include three neurohumoral antagonist modulators: angiotensin-converting enzyme inhibitors (ACE-I), angiotensin receptor blockers (ARBs), mineralocorticoid receptor antagonists (MRAs) and beta-adrenergic receptor blockers (beta-blockers). These medications modify the course of SHF and should be considered in every patients with SHF. In addition to these conventional HF medication, a distinct and complementary mechanisms of bradycardic action – the selective pacemaker current (If) inhibitor ivabradine, is recommended to provide further reduction of heart rate. The conventional HF medication are often used with diuretics to relieve symptoms and signs of congestion [6,52]. Figure 2 illustrates algorithm for pharmacological support and device therapies for patients diagnosed with SHF.
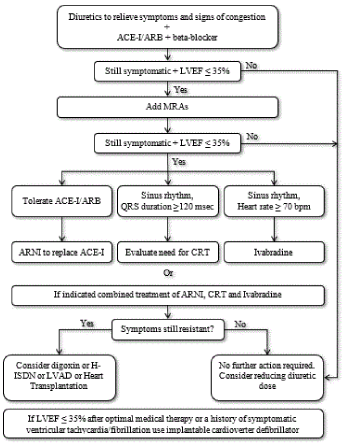
Figure 2.Treatment algorithm for chronic systolic heart failure
Frontline medical therapy for HFrEF are ACE-I and beta-blocker, and diuretis for symptom relief. If still symptomatic and LVEF ≤35%, MR antagonist should be added. If still symptomatic and LVEF ≤35%, ARNI should replace ACE-I, Ivabradine or cardiac resynchronization therapy should be considered. If still asymptomatic, digoxin, LV assist device or heart transplantation should be considered. Adapted from McMurray et al. 2012, p. 1805 [52]
Recommended pharmacological management of SHF begins with a dual therapy of diuretics (to relive symptoms of congestion), and ACE-I/ARBs and/or beta-blockers. If symptoms continue to worsen or are refractory and LVEF remains significantly depressed (≤ 35%), an addition of MRAs is recommended. If patients continue to remain symptomatic with LVEF ≤ 35%, additional medication should be considered. In these patients, angiotensin receptor neprilysin inhibitor (ARNI) should be considered in patients tolerant to ACE-I/ARBs. For patients with persistent symptoms and sinus rhythm with QRS duration ≥ 120 msec the need for CRT should be evaluated but those with heart rate ≥ 70 beats per minute should be considered for ivabradine therapy. If patients remain symptomatic despite an optimal combination therapy of ARNI, CRT and Ivabradine, digoxin, LV assist devices (LVAD) or heart transplantation should be considered. However, if LVEF remains ≤ 35% following optimal medical therapy or patients with a history of symptomatic ventricular tachycardia or atrial fibrillation, the use of implantable cardioverter defibrillator (ICD) therapy is highly recommended [6].
Meta-analysis of the effect of medication on mortality
Systolic heart failure is a life-threatening medical condition. Early diagnosis and prompt institution of appropriate therapy greatly improves treatment efficacy [52]. The goals of treatment is to improve clinical status, functional capacity and quality of life, and to prevent hospital admission and mortality [6]. However, several medications for HF have shown long-term detrimental effects despite beneficial outcomes on short-term surrogate markers, regulatory bodies and clinical guidelines are using mortality and/or hospitalization data for approving and recommending HF medication [114-116]. Thus, the aim of the present meta-analysis is to pool data from previous studies on the effect of HF medication on mortality and/or hospitalization of SHF patients compared to placebo.
Study search and selection: Prospective randomized placebo-controlled trials were identified through electronic search on online databases (EMBASE and MEDLINE), and scrutiny of bibliographies and review articles. The search was performed using various combinations of the following key terms: heart failure, systolic failure, medication, beta-blocker, mineralocorticoid receptor antagonists, angiotensin receptor blockers and angiotensin-converting enzyme – inhibitors. Studies were included if they (a) were prospective, randomized controlled trials (RCTs), case-controlled studies or cohort studies; (b) investigated HF medication; (c) compared treatment group and placebo group; (d) reported all-cause mortality; and (e) followed patients for at least six months. Studies were excluded if they (a) reported composite clinical endpoints of mortality and hospitalization with no specific data on all-cause mortality; (b) were available only in abstract form; or (c) recruited all HF patients including diastolic or bi-ventricular HF patients. Studies were eligible irrespective of publication year and language.
Methodological quality of studies was assessed using the 12-item Methodological Index for Non-Randomized Studies (MINORS) [101]. A score system with a maximum of 24 points (each scored 0 to 2) assessed the following aspects: study aim, patient inclusion, prospective data collection, clinical endpoint, unbiased evaluation of endpoints, appropriate follow up period, loss to follow-up of <5%, comparable control group, baseline equivalence, sample size, and adequate statistical analysis. Two reviewers independently scored all the potential studies and the average MINORS score used for inclusion. MINORS scores of <16 were considered low quality while ≥16 considered high quality [117]. Data abstracted from the included studies included author, publication year, patient population (control and placebo), patient characteristics (mean age and male %), mean follow up period and clinical endpoint (all-cause mortality or hospitalization) (Table 12).
Table 12. Summary of included studies on clinical management of systolic heart failure
|
First Author [Ref#] |
Year |
Study Name |
Control Group |
Placebo Group |
Mean Age (yrs.) |
Male (%) |
Medication Investigated |
Mean Follow-up Period (Months) |
Mortality or Composite Mortality-Hospitalization |
|
Control Group (N) |
Placebo Group (N) |
Risk Reduction (%) |
Swedberg et al. [118] |
1988 |
CONSENSUS |
127 |
126 |
-- |
-- |
ACE-I (Enalapril) |
6.2 |
50 |
68 |
40 |
SOLVD Investigators [119] |
1991 |
SOLVD-TREATMENT |
1285 |
1284 |
60.7 |
81 |
ACE-I (Enalapril) |
41.4 |
452 |
510 |
16 |
Granger et al. [120] |
2003 |
CHARM-Alternative |
1013 |
1015 |
66.3 |
68 |
ARB (Candesartan) |
33.7 |
334 |
406 |
23 |
Yusuf, et al. [121] |
2003 |
CHARM-Preserved |
1514 |
1509 |
67.2 |
61 |
ARB (Candesartan) |
36.6 |
333 |
366 |
11 |
McMurray et al. [122] |
2003 |
CHARM-Added |
1276 |
1272 |
64.0 |
79 |
ARB (Candesartan) |
41.0 |
483 |
538 |
15 |
Packer et al. [123] |
1996 |
-- |
696 |
398 |
57.9 |
77 |
Beta-blocker (Carvedilol) |
6.5 |
22 |
31 |
65 |
Merit-HF Study [124] |
1999 |
MERIT-HF |
1990 |
2001 |
-- |
-- |
Beta-blocker (Metoprolol) |
12.0 |
145 |
217 |
34 |
CIBIS-II Investigators [125] |
1999 |
CIBIS-II |
1327 |
1320 |
61.0 |
81 |
Beta-blocker (Bisoprolol) |
16.9 |
156 |
228 |
34 |
Packer et al. [126] |
2002 |
COPERNICUS |
1156 |
1133 |
63.2 |
79 |
Beta-blocker (Carvedilol) |
10.4 |
271 |
357 |
27 |
Flather et al. [127] |
2005 |
SENIORS |
1067 |
1061 |
76.1 |
62 |
Beta-blocker (Nebivolol) |
21.0 |
332 |
375 |
14 |
Willenheimer et al. [128] |
2005 |
CIBIS III |
505 |
505 |
72.4 |
66 |
Beta-blocker (Bisoprolol) |
24.0 |
178 |
186 |
12 |
Pitt et al. [129] |
1999 |
RALES |
822 |
841 |
65.0 |
73 |
MRAs (Spironolactone) |
24.0 |
284 |
386 |
30 |
Zannad et al. [130] |
2011 |
EMPHASIS-HF |
1364 |
1373 |
68.7 |
77 |
MRAs (Eplerenone) |
21.0 |
249 |
356 |
24 |
McMurray et al. [131] |
2014 |
PARADIGM-HF |
4187 |
4212 |
63.8 |
79 |
ARNI (LCZ696) vs Enalapril |
27.0 |
914 |
1117 |
16 |
Swedberg et al. [132] |
2010 |
SHIFT |
3241 |
3264 |
60.7 |
76 |
If Channel Blocker (Ivabradine) |
22.9 |
793 |
937 |
18 |
ACE-I: Angiotensin-Converting Enzyme – Inhibitors; ARB: Angiotensin Receptor Blockers; ARNI: Angiotensin Receptor Neprilysin Inhibitor; MRAs: Mineralocorticoid Receptor Antagonists
Study characteristics and outcomes: Following the screening of titles, abstracts, and full text of articles retrieved from the online search and a review of bibliographies against the inclusion criteria, fifteen (15) prospective randomized controlled trials were eventually included in the present meta-analysis [118-132]. The publication period of the studies ranged between 1988 [118] and 2014 [131]. The 11 studies recruited patients diagnosed with symptomatic SHF and LVEF < 40% who were on pharmacological treatment. The combined patient sample in the 15 studies was 42,844 consisting of 21,570 patients in the treatment group and 21,314 patients in the placebo group. The patients were generally older (mean = 65 years) with a greater proportion of males (74%). The specific HF medication investigated were ACE-inhibitors (Enalapril) [118,119], ARB (Candesartan) [120-122], beta-blockers (Carvedilol, Metoprolol, Bisoprolol or Nebivolol) [123,128], MRAs (Spironolactone or Eplerenone) [129,130], ARNI [131] and If Channel Blocker (Ivabradine) [132]. The mean follow-up period was 23.97 months (range = 6.20 to 41.40).
The number of deaths and/or hospitalization for SHF patients on medication remained high relative to placebo group. There were 4,996 deaths or hospitalization (23%) in the treatment group compared to 6,078 (29%) in the placebo group. Risk reduction in mortality and/or hospitalization on SHF patients on a combination of HF medication reported in the individual studies ranged between 11% [121] and 65% [123]. Pooling together mortality/hospitalization data from individual studies, the combined risk reduction was 18% (RR: 0.82; 95% CI, 0.796 to 0.848; p=0.0009) (Table 13; Figure 3). The results reveal HF medication (ACE-I, ARBs, beta-blockers, MRAs, ARNI and If channel blockers) if used as prescribed significantly reduces the risk of mortality and/or hospitalization in patients diagnosed with SHF.
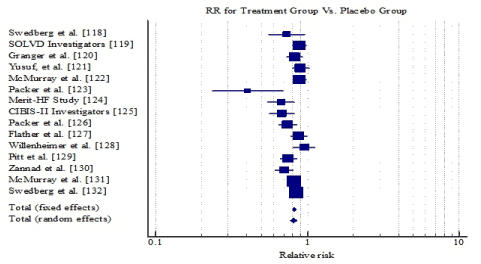
Figure 3. Forest plot of relative risk of HF medication on SHF mortality/hospitalization.
Table 13. Relative risk of HF medication on mortality/hospitalization of SHF patients
Study |
Intervention |
Controls |
Relative risk |
95% CI |
Swedberg et al. [118] |
50/127 |
68/126 |
0.730 |
0.557 to 0.955 |
SOLVD Investigators [119] |
452/1285 |
510/1284 |
0.886 |
0.801 to 0.979 |
Granger et al. [120] |
334/1013 |
406/1015 |
0.824 |
0.734 to 0.925 |
Yusuf, et al. [121] |
333/1514 |
366/1509 |
0.907 |
0.796 to 1.033 |
McMurray et al. [122] |
483/1276 |
538/1272 |
0.895 |
0.814 to 0.984 |
Packer et al. [123] |
22/696 |
31/398 |
0.406 |
0.238 to 0.691 |
Merit-HF Study [124] |
145/1990 |
217/2001 |
0.672 |
0.550 to 0.821 |
CIBIS-II Investigators [125] |
156/1327 |
228/1320 |
0.681 |
0.563 to 0.822 |
Packer et al. [126] |
271/1156 |
357/1133 |
0.744 |
0.650 to 0.852 |
Flather et al. [127] |
332/1067 |
375/1061 |
0.880 |
0.780 to 0.993 |
Willenheimer et al. [128] |
178/505 |
186/505 |
0.957 |
0.812 to 1.128 |
Pitt et al. [129] |
284/822 |
386/841 |
0.753 |
0.668 to 0.848 |
Zannad et al. [130] |
249/1364 |
356/1373 |
0.704 |
0.610 to 0.813 |
McMurray et al. [131] |
914/4187 |
1117/4212 |
0.823 |
0.763 to 0.888 |
Swedberg et al. [132] |
793/3241 |
937/3264 |
0.852 |
0.786 to 0.924 |
Total (fixed effects) |
4996/21570 |
6078/21314 |
0.821 |
0.796 to 0.848 |
Total (random effects) |
4996/21570 |
6078/21314 |
0.810 |
0.767 to 0.856 |
Further analysis of individual HF medication shows beta-blocker have the greatest risk reduction of composite morality and hospitalization by 22% (RR: 0.778; 95% CI, 0.726 to 0.833); followed by MRAs by 21% (RR: 0.794; 95% CI, 0.743 to 0.850) and finally ACEI/ARBs by 13% (RR: 0.874; 95% CI, 0.828 to 0.921). However, HF medications are usually prescribed in combination and thus the efficacy of individual medication is not additive.
Pharmacotherapy remains the frontline and widely prescribed therapy for the clinical management of patients diagnosed with SHF. The present meta-analysis aimed to determine therapeutic efficacy of HF medication in the prevention of hospitalization and/or mortality in SHF patients. The findings show that HF medication is clinically effective by significantly reducing the risk of composite mortality and hospitalization. Therapeutic efficacy of HF medication is underscored by the ESC [6] and the AHA/ACC [5] HF clinical guidelines recommending the use of HF medication as the initial therapy for all SHF patients unless contra-indicated.
Beta-blocker, ACE-I/ARB, and MRAs are neurohumoral antagonist modulators reduce the risk of mortality rate by modifying the natural course of SHF while the If channel inhibitors such as Ivabradine provide additional reduction on the heart rate but only recommended for SHF patients in sinus rhythm [52]. In particular, current ESC guidelines recommend ACE-I to all symptomatic patients unless contra-indicated to reduce mortality and morbidity through the inhibition of the renin-angiotensin-aldosterone system (RAAS). While the guidelines recommend titration of ACE-I to the maximum tolerated doses to achieve adequate inhibition, Packer et al. [133] observed no significant difference in efficacy between intermediate and high doses. However higher doses had a significant reduction on the risk of death or hospitalization for any reason but non-significant 8% reduction on the risk of death.
Beta-blockers has a complementary effect on ACE-I and the two could be started together. Beta-blocker is usually recommended for patients with a history of MI and asymptomatic LV systolic dysfunction to minimize the risk of death [5]. MRAs such as spironolactone block receptors that bind aldosterone is recommended in asymptomatic patients or as an additional medication to ACE-I/ARBs and beta-blockers in non-responsive patients to reduce the risk of death. In summary, the traditional HF medication, ACE-I, ARBs, beta-blocker and MRAs are effective in reducing the risk of death or hospitalization and their use is recommended unless contraindicated or intolerant.
Systolic heart failure (SHF) is a debilitating clinical syndrome characterized by typical symptoms of dyspnea, lower extremities edema and fatigue usually accompanied by signs of elevated jugular pressure and pulmonary rales caused by structural and/or functional cardiac abnormality. It is distinguishable from other HF subsets by a depressed LVEF (< 40%). It has an ominous prognosis predicted by older age, the male sex, worsening symptoms, severity of LV functional dysfunction, increased levels of plasma BNP and NT-proBNP and the presence of cardiovascular comorbidities. Its etiology is heterogeneous but often includes conditions causing myocardial injury such as coronary artery disease, cardiomyopathy, viral myocarditis and environmental toxins; abnormal loading conditions such as arterial hypertension, aortic stenosis and mitral regurgitation; and arrhythmias. Frequent clinical manifestations of SHF include typical symptoms of heart failure (HF) – dyspnea, orthopnea, paroxysmal nocturnal dyspnea, fatigue, and edema, and typical symptoms of tachycardia, pedal edema, increased hepatojugular pressure, pulmonary crackles, gallop rhythm, hepatojugular reflux and ascites. Diagnosis is based on careful assessment of the patient’s medical history, physical examination, assessment of serum natriuretic peptides and cardiac imaging. Echocardiography is the most common cardiac imaging modality recommended following inconclusive findings from electrocardiograph and/or plasma NPs. If echocardiography is inconclusive, cardiac magnetic resonance imaging is the best alternative to confirm diagnosis. Frontline treatment for SHF is pharmacological management mainly based on neurohumoral antagonist modulators (ACE-I/ARBs, beta-blocker and MRAs). If symptoms persists and LV ejection fraction remains < 40% despite optimal pharmacological support, device therapy such as implantable cardioverter defibrillator, cardiac resynchronization therapy or heart transplantation may be considered.
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. (2015) American heart association statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circ 131: e29-322. [Crossref]
- Lam CS, Lyass A, Kraigher-Krainer E, Massaro JM, Lee DS, et al. (2011) Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circ 124: 24-30. [Crossref]
- Correction (2015) Circ 131: e535. [Crossref]
- Yeboah J, Rodriguez CJ, Stacey B, Lima JA, Liu S, et al. (2012) Prognosis of individuals with asymptomatic left ventricular systolic dysfunction in the multi-ethnic study of atherosclerosis (MESA). Circ 126: 2713-2719. [Crossref]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, et al. (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62: e147-e239. [Crossref]
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, et al. (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37: 2129-2200. [Crossref]
- Sidebotham D, Le Grice IJ (2007) Physiology and pathophysiology. In Cardiothoracic Critical Care (p. 9). Butterworth-Heinemann Elsevier, Philadelphia, PA.
- Calvert JW, Lefer DJ (2012) Overview of Cardiac Muscle Physiology. In Muscle (pp. 57-66).
- Rushmer RF (1955) Initial phase of ventricular systole: asynchronous contraction. American Journal of Physiology-Legacy Content 184: 188-94. [Crossref]
- Federmann M, Hess OM (1994) Differentiation between systolic and diastolic dysfunction. Eur Heart J 15: 2-6. [Crossref]
- Hess OM (1993). Hemodynamics in heart failure: systolic and diastolic dysfunction. Therapeutische Umschau. Revue Therapeutique 50: 414-418. [Crossref]
- Yamamoto K, Sakata Y, Ohtani T, Takeda Y, Mano T (2009) Heart failure with preserved ejection fraction. Circ 73: 404-410. [Crossref]
- Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, et al. (2003) Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circ 108: 977-982. [Crossref]
- McDonagh TA, Morrison CE, Lawrence A, Ford I, Tunstall-Pedoe H, et al. (1997) Symptomatic and asymptomatic left-ventricular systolic dysfunction in an urban population. The Lancet 350: 829-333. [Crossref]
- Lewis T (1933) Diseases of the heart. London, UK: MacMillan.
- Braunwald E (1980) Clinical manifestations of heart failure. Heart disease. In: Braunwald E, editor. Heart disease: a textbook of cardiovascular medicine (1st Ed.). Philadelphia: WB Saunders.
- Chatterjee K, Massie B (2007) Systolic and diastolic heart failure: differences and similarities. J Card Fail 13: 569-576. [Crossref]
- Van Riet EE, Hoes AW, Limburg A, Landman MA, van der Hoeven H, et al. (2014) Prevalence of unrecognized heart failure in older persons with shortness of breath on exertion. Eur J Heart Fail 16:v772-777. [Crossref]
- Rutten FH, Cramer MJ, Grobbee DE, Sachs AP, Kirkels JH, et al. (2005) Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J 26: 1887-1894. [Crossref]
- Boonman-de Winter LJ, Rutten FH, Cramer MJ, Landman MJ, Liem AH, et al. (2012) High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia 55: 2154-2162. [Crossref]
- Van Riet EE, Hoes AW, Wagenaar KP, Limburg A, Landman MA, et al. (2016) Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail 18: 242-252. [Crossref]
- Abhayaratna WP, Smith WT, Becker NG, Marwick TH, Jeffery IM, (2006) Prevalence of heart failure and systolic ventricular dysfunction in older Australians: the Canberra Heart Study. Medical Journal of Australia 184: 151-154. [Crossref]
- Tiller D, Russ M, Greiser KH, Nuding S, Ebelt H, et al. (2013) Prevalence of symptomatic heart failure with reduced and with normal ejection fraction in an elderly general population–the CARLA study. PLoS One 8 :e59225. [Crossref]
- Mureddu GF, Agabiti N, Rizzello V, Forastiere F, Latini R, et al. (2012) Prevalence of preclinical and clinical heart failure in the elderly. A population‐based study in Central Italy. Eur J Heart Fail 14: 718-729. [Crossref]
- Philbin EF, Rocco Jr TA, Lindenmuth NW, Ulrich K, Jenkins PL (2000) Systolic versus diastolic heart failure in community practice: clinical features, outcomes, and the use of angiotensin-converting enzyme inhibitors. Am J Med 109: 605-613. [Crossref]
- Mosterd A, Hoes AW (2007) Clinical epidemiology of heart failure. Heart 93: 1137-1146. [Crossref]
- Redfield, M. M., Jacobsen, S. J., Burnett Jr, J. C., Mahoney, D. W., Bailey, K. R., & Rodeheffer, R. J. (2003). Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama, 289(2), 194-202.
- Redfield MM, Jacobsen SJ, Burnett Jr JC, Mahoney DW, Bailey KR, et al. (2003) Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama 289: 194-202. [Crossref]
- Ceia F, Fonseca C, Mota T, Morais H, Matias F, et al. (2002) Prevalence of chronic heart failure in Southwestern Europe: the EPICA study. Eur J Heart Fail 4: 531-539. [Crossref]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman et al. (2013) Heart Disease and Stroke Statistics - 2016 Update. A Report From the American Heart Association. Circ 2015 [Crossref]
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, et al. (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355: 251-259. [Crossref]
- Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, et al. (2015) A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA internal medicine 175: 996-1004. [Crossref]
- Savarese G, Lund LH (2017) Global public health burden of heart failure. Cardiac failure review 3: 7-11. [Crossref]
- Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, et al. (2002) Long-term trends in the incidence of and survival with heart failure. N Engl J Med 347: 1397-1402. [Crossref]
- Najafi F, Jamrozik K, Dobson AJ (2009) Understanding the ‘epidemic of heart failure’: a systematic review of trends in determinants of heart failure. Eur J Heart Fail 11: 472-479. [Crossref]
- Kapoor JR, Kapoor R, Ju C, Heidenreich PA, Eapen ZJ, et al. (2016) Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. JACC: Heart Failure 4: 464-472. [Crossref]
- Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo‐Leiro MG, et al, (2017) Epidemiology and one‐year outcomes in patients with chronic heart failure and preserved, mid‐range and reduced ejection fraction: an analysis of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 19: 1574-1585. [Crossref]
- Löfman I, Szummer K, Dahlström U, Jernberg T, Lund LH (2017) Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid‐range, and reduced ejection fraction. Eur J Heart Fail 19: 1606-1614. [Crossref]
- The Criteria Committee of the New York Heart Association. (1994). Nomenclature and criteria for diagnosis of diseases of the heart and great vessels (9th ED.) Boston, Ma: Little & Brown.
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, et al. (200) 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 53: e1-90. [Crossref]
- Goldman L, Hashimoto B, Cook EF, Loscalzo A (1981) Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circ 64 :1227-1234. [Crossref]
- Madsen BK, Hansen JF, Stokholm KH, Brøns J, Husum D, et al. (1994) Chrome congestive heart failure: Description and survival of 190 consecutive patients with a diagnosis of chronic congestive heart failure based on clinical signs and symptoms. Eur Heart J 15: 303-310. [Crossref]
- Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, et al. (2007) Prevalence and prognostic significance of heart failure stages: application of the ACC/AHA heart failure staging criteria in the community. Circ 115: 1563-1570. [Crossref]
- Aronow WS (2006) Epidemiology, pathophysiology, prognosis, and treatment of systolic and diastolic heart failure. Cardiology in review 14: 108-124. [Crossref]
- Javaheri S, Shukla R, Zeigler H, Wexler L (2007) Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol 49: 2028-2034. [Crossref]
- Aronow WS, Ahn C, Kronzon I (1990) Prognosis of congestive heart failure in elderly patients with normal versus abnormal left ventricular systolic function associated with coronary artery disease. Am J Cardiol 66: 1257-1259. [Crossref]
- Pernenkil R, Vinson JM, Shah AS, Beckham V, Wittenberg C, et al. (1997) Course and prognosis in patients> or= 70 years of age with congestive heart failure and normal versus abnormal left ventricular ejection fraction. Am J Cardiol 79: 216-219. [Crossref]
- Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, (1999) Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol 33: 1948-1955. [Crossref]
- Aronow WS, Ahn C, Kronzon I (2000) Prognosis of congestive heart failure after prior myocardial infarction in older men and women with abnormal versus normal left ventricular ejection fraction. Am J Cardiol 85:1382-1384. [Crossref]
- Gustafsson F, Torp-Pedersen C, Brendorp B, Seibæk M, Burchardt H, et al. (2003) Long-term survival in patients hospitalized with congestive heart failure: relation to preserved and reduced left ventricular systolic function. Eur Heart J 24: 863-870. [Crossref]
- Aronow WS, Ahn C, Kronzon I (2001) Prognosis of congestive heart failure after prior myocardial infarction in older persons with atrial fibrillation versus sinus rhythm. Am J Cardiol 87: 224-225. [Crossref]
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, et al. (2012) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 14: 803-869. [Crossref]
- Sutton MG, Sharpe N (2000) Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circ 101: 2981-2988. [Crossref]
- Shah RU, Lloyd-Jones DM (2013) Heart failure in women. In Women and Health (2nd Ed.).
- Cleutjens JP, Kandala JC, Guarda E, Guntaka RV, Weber KT (1995) Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol 27: 1281-1292. [Crossref]
- Warren SE, Royal HD, Markis JE, Grossman W, McKay RG (1988) Time course of left ventricular dilation after myocardial infarction: influence of infarct-related artery and success of coronary thrombolysis. J Am Coll Cardiol 11: 12-19. [Crossref]
- Sadoshima JI, Jahn L, Takahashi T, Kulik TJ, Izumo S (1992) Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. Journal of Biological Chemistry 267: 10551-10560. [Crossref]
- Lew WY, Chen ZY, Guth B, Covell JW (1985) Mechanisms of augmented segment shortening in nonischemic areas during acute ischemia of the canine left ventricle. Circ Res 56: 351-358. [Crossref]
- McKelvie RS, Moe GW, Ezekowitz JA, Heckman GA, Costigan J, et al. (2013) The 2012 Canadian Cardiovascular Society heart failure management guidelines update: focus on acute and chronic heart failure. Canadian Journal of Cardiology 29: 168-181. [Crossref]
- Henes J, Rosenberger P (2016) Systolic heart failure: diagnosis and therapy. Current Opinion in Anesthesiology 29: 55-60. [Crossref]
- Watson RD, Gibbs CR, Lip GY (2000) ABC of heart failure: clinical features and complications. BMJ: BMJ 320: 236-239. [Crossref]
- Gibbs CR, Davies MK, Lip GY (2000) ABC of heart failure-Clinical features and complications. Student BMJ 8. [Crossref]
- Inamdar AA, Inamdar AC (2016) Heart failure: diagnosis, management and utilization. Journal of clinical medicine 5: 62. [Crossref]
- Heart Failure Society of America (2010) Executive summary: HFSA 2010 comprehensive heart failure practice guideline. Journal of cardiac failure 16: 475-539. [Crossref]
- Marti CN, Georgiopoulou VV, Kalogeropoulos AP (2013) Acute heart failure: patient characteristics and pathophysiology. Current heart failure reports 10: 427-433. [Crossref]
- Wu AH, Apple FS, Gibler WB, Jesse RL, Warshaw MM, et al. (1999) National Academy of Clinical Biochemistry Standards of Laboratory Practice: recommendations for the use of cardiac markers in coronary artery diseases. Clinical chemistry 45: 1104-1121. [Crossref]
- Maisel A, Hollander JE, Guss D, McCullough P, Nowak R, et al. (2004) Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT): a multicenter study of B-type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath. J Am Coll Cardiol 44: 1328-1333. [Crossref]
- Murtagh G, Canniffe C, Mahgoub M, Blake L, McCarroll N, et al. (2009) Crowley V, Bennett K, Silke B. Introduction of an NT-proBNP assay to an acute admission unit—A 2-year audit. European journal of internal medicine 20: 58-62. [Crossref]
- Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, et al. (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28: 2539-2550. [Crossref]
- Marwick TH, Raman SV, Carrió I, Bax JJ (2010) Recent developments in heart failure imaging. JACC: Cardiovascular Imaging 3: 429-439. [Crossref]
- Dokainish H, Nguyen JS, Bobek J, Goswami R, Lakkis NM (2011) Assessment of the American Society of Echocardiography-European Association of Echocardiography guidelines for diastolic function in patients with depressed ejection fraction: an echocardiographic and invasive haemodynamic study. Eur J Echocardiogr 12: 857-864. [Crossref]
- Kirkpatrick JN, Vannan MA, Narula J, Lang RM (2007) Echocardiography in heart failure: applications, utility, and new horizons. J Am Coll Cardiol 50: 381-396. [Crossref]
- Nagueh SF, Bhatt R, Vivo RP, Krim SR, Sarvari SI, et al. (2011) Echocardiographic evaluation of hemodynamics in patients with decompensated systolic heart failure. Circulation: Cardiovascular Imaging 4: 220-227. [Crossref]
- Caballero L, Kou S, Dulgheru R, Gonjilashvili N, Athanassopoulos GD, et al. (2015) Echocardiographic reference ranges for normal cardiac Doppler data: results from the NORRE Study. European Heart Journal-Cardiovascular Imaging 16: 1031-1041. [Crossref]
- Garbi M, McDonagh T, Cosyns B, Bucciarelli-Ducci C, Edvardsen T, et al. (2014) Appropriateness criteria for cardiovascular imaging use in heart failure: report of literature review. European Heart Journal-Cardiovascular Imaging 16: 147-153. [Crossref]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, et al. (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart Journal-Cardiovascular Imaging 16: 233-271. [Crossref]
- Kilner PJ, Geva T, Kaemmerer H, Trindade PT, Schwitter J, et al. (2010) Recommendations for cardiovascular magnetic resonance in adults with congenital heart disease from the respective working groups of the European Society of Cardiology. Eur Heart J 31: 794-805. [Crossref]
- Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, et al. (2010) ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol 55: 2614-2662. [Crossref]
- Thomas JT, Kelly RF, Thomas SJ, Stamos TD, Albasha K, et al. (2002) Utility of history, physical examination, electrocardiogram, and chest radiograph for differentiating normal from decreased systolic function in patients with heart failure. American journal of medicine 112: 437-445. [Crossref]
- Maisel AS, Peacock WF, McMullin N, Jessie R, Fonarow GC, et al. (2008) Wynne J, Mills RM. Timing of immunoreactive B-type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE (Acute Decompensated Heart Failure National Registry) analysis. J Am Coll Cardiol 52: 534-540. [Crossref]
- Peacock WF, Emerman C, Costanzo MR, Diercks DB, Lopatin M, et al.. (2009) Fonarow GC. Early vasoactive drugs improve heart failure outcomes. Congestive Heart Failure 15: 256-264. [Crossref]
- Chakko S, Woska D, Martinez H, De Marchena E, Futterman L, et al. (1991) Clinical, radiographic, and hemodynamic correlations in chronic congestive heart failure: conflicting results may lead to inappropriate care. Am J Med 90: 353-359. [Crossref]
- Yamamoto K, Burnett Jr JC, Bermudez EA, Jougasaki M, Bailey KR, et al. (2000) Clinical criteria and biochemical markers for the detection of systolic dysfunction. Journal of cardiac failure 6: 194-200. [Crossref]
- Fuat A, Murphy JJ, Hungin AP, Curry J, Mehrzad AA, et al. (2006) The diagnostic accuracy and utility of a B-type natriuretic peptide test in a community population of patients with suspected heart failure. Br J Gen Pract 56: 327-333. [Crossref]
- Cowie MR, Struthers AD, Wood DA, Coats AJ, Thompson SG, et al. (1997) Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. The Lancet 350: 1349-1353. [Crossref]
- Kelder JC, Cramer MJ, Verweij WM, Grobbee DE, Hoes AW (2011) Clinical utility of three B-type natriuretic peptide assays for the initial diagnostic assessment of new slow-onset heart failure. Journal of cardiac failure 17: 729-734. [Crossref]
- Krishnaswamy P, Lubien E, Clopton P, Koon J, Kazanegra R, et al. (2001) Utility of B-natriuretic peptide levels in identifying patients with left ventricular systolic or diastolic dysfunction. Am J Med 111: 274-279. [Crossref]
- Gustafsson F, Steensgaard-Hansen F, Badskjær J, Poulsen AH, Corell P, et al. (2005) Diagnostic and prognostic performance of N-terminal ProBNP in primary care patients with suspected heart failure. Journal of cardiac failure 11: S15-20. [Crossref]
- Nielsen OW, Rasmussen V, Christensen NJ, Hansen JF (2004) Neuroendocrine testing in community patients with heart disease: plasma N-terminal proatrial natriuretic peptide predicts morbidity and mortality stronger than catecholamines and heart rate variability. Scandinavian journal of clinical and laboratory investigation 64: 619-628. [Crosssref]
- Lichtenstein D, Meziere G (1998) A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: the comet-tail artifact. Intensive care medicine 24: 1331-134. [Crossref]
- Gargani L, Frassi F, Soldati G, Tesorio P, Gheorghiade M, et al. (2008) Ultrasound lung comets for the differential diagnosis of acute cardiogenic dyspnoea: A comparison with natriuretic peptides☆. Eur J Heart Fail 10: 70-77. [Crossref]
- Lichtenstein DA, Meziere GA (2008) Relevance of lung ultrasound in the diagnosis of acute respiratory failure*: The BLUE Protocol. Chest 134: 117-125. [Crossref]
- Liteplo AS, Marill KA, Villen T, Miller RM, Murray AF, et al. (2009) Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): Sonographic B‐lines and N‐terminal Pro‐brain‐type Natriuretic Peptide in diagnosing congestive heart failure. Academic Emergency Medicine 16: 201-210. [Crossref]
- Nazerian P, Vanni S, Zanobetti M, Polidori G, Pepe G, et al. (2010) Diagnostic accuracy of emergency Doppler echocardiography for identification of acute left ventricular heart failure in patients with acute dyspnea: comparison with Boston criteria and N‐terminal prohormone brain natriuretic peptide. Academic Emergency Medicine 17: 18-26. [Crossref]
- Prosen G, Klemen P, Strnad M, Grmec Š (2011) Combination of lung ultrasound (a comet-tail sign) and N-terminal pro-brain natriuretic peptide in differentiating acute heart failure from chronic obstructive pulmonary disease and asthma as cause of acute dyspnea in prehospital emergency setting. Critical Care 15:vR114. [Crossref]
- Vitturi, N., Soattin, M., Allemand, E., Simoni, F., & Realdi, G. (2011). Thoracic ultrasonography: a new method for the work-up of patients with dyspnea. Journal of ultrasound, 14(3), 147-151.
- Cibinel GA, Casoli G, Elia F, Padoan M, Pivetta E, et al. (2012) Diagnostic accuracy and reproducibility of pleural and lung ultrasound in discriminating cardiogenic causes of acute dyspnea in the emergency department. Internal and emergency medicine 7: 65-70. [Crossref]
- Spinelli A, De Curtis E, Savino A, Michelagnoli G, Magazzini S (2014) Acute cardiogenic dyspnea in the emergency department: accuracy of lung ultrasound. In Critical ultrasound journal 6: A7. [Crossref]
- Russell FM, Ehrman RR, Cosby K, Ansari A, Tseeng S, et al. (2015) Diagnosing Acute Heart Failure in Patients With Undifferentiated Dyspnea: A Lung and Cardiac Ultrasound (Lu CUS) Protocol. Academic Emergency Medicine 22: 182-191. [Crossref]
- Golshani K, Esmailian M, Valikhany A, Zamani M (2016) Bedside Ultrasonography versus Brain Natriuretic Peptide in Detecting Cardiogenic Causes of Acute Dyspnea. Emergency 4: 140. [Crossref]
- Berliner D, Schneider N, Welte T, Bauersachs J (2016) The differential diagnosis of dyspnea. Deutsches Ärzteblatt International 113: 834-845. [Crossref]
- Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, et al. (2012) An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 185: 435-452. [Crossref]
- Kapos I, Tanner FC (2017) Value of echocardiography in differentiation of acute dyspnea. Cardiovascular Medicine 20: 146-153. [Crossref]
- Martindale JL, Wakai A, Collins SP, Levy PD, Diercks D, et al. (2016) Diagnosing acute heart failure in the emergency department: a systematic review and meta‐analysis. Academic emergency medicine 23: 223-242. [Crossref]
- Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D (2014) Point‐of‐care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta‐analysis. Academic Emergency Medicine 21: 843-852. [Crossref]
- Okwuosa IS, Princewill O, Nwabueze C, Mathews L, Hsu S, et al. (2016) The ABCs of managing systolic heart failure: past, present, and future. Cleve Clin J Med 83: 753-765. [Crossref]
- Mancini GJ, Howlett JG, Borer J, Liu PP, Mehra MR, et al. (2015) Pharmacologic options for the management of systolic heart failure: examining underlying mechanisms. Canadian Journal of Cardiology 31: 1282-1292. [Crossref]
- Yurevich O, Borer JS (2017) Role of New Therapies in Reducing Mortality and Major Morbidity in Patients with Systolic Heart Failure. In The Role of the Clinical Cardiac Electrophysiologist in the Management of Congestive Heart Failure 2017. InTech. [Crossref]
- Masson S, Latini R, Anand IS, Barlera S, Angelici L, et al. (2008) Prognostic value of changes in N-terminal pro-brain natriuretic peptide in Val-HeFT (Valsartan Heart Failure Trial). J Am Coll Cardiol 52: 997-1003. [Crossref]
- Wong M, Staszewsky L, Latini R, Barlera S, Volpi A, et al. (2002) Valsartan benefits left ventricular structure and function in heart failure: Val-HeFT echocardiographic study. J Am Coll Cardiol 40: 970-975. [Crossref]
- Ekman I, Cleland JG, Andersson B, Swedberg K (2005) Exploring symptoms in chronic heart failure. Eur J Heart Fail 7: 699-703. [Crossref]
- Packer M, Narahara KA, Elkayam U, Sullivan JM, Pearle DL, et al. (1993) Double-blind, placebo-controlled study of the efficacy of flosequinan in patients with chronic heart failure. J Am Coll Cardiol 22: 65-72. [Crossref]
- Cowley AJ, Stainer K, Wynne RD, Rowley JM, Hampton JR (1989) Comparison of the effects of captopril and enoximone in patients with severe heart failure: a placebo controlled double-blind study. International journal of cardiology 24: 311-316. [Crossref]
- Stewart S, Jenkins A, Buchan S, McGuire A, Capewell S, et al. (2002) The current cost of heart failure to the National Health Service in the UK. Eur J Heart Fail 4: 361-371. [Crossref]
- Gheorghiade M, Shah AN, Vaduganathan M, Butler J, Bonow RO, et al. (2013) Recognizing hospitalized heart failure as an entity and developing new therapies to improve outcomes: academics', clinicians', industry's, regulators', and payers' perspectives. Heart failure clinics 9: 285-290. [Crossref]
- Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, et al. (2014) The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 63: 1123-1133. [Crossref]
- Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, et al. (2003) Methodological index for non‐randomized studies (MINORS): development and validation of a new instrument. ANZ journal of surgery 73: 712-716. [Crossref]
- Swedberg K, Kjekshus J (1988) Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol 62: 60A-66A. [Crossref]
- SOLVD Investigators (1991) Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 325: 293-302. [Crosssref]
- Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, et al. (2003) Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. The Lancet 362: 772-776. [Crossref]
- Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, et al. (2003) Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. The Lancet 362: 777-781. [Crossref]
- McMurray JJ, Östergren J, Swedberg K, Granger CB, Held P, et al. (2003) Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. The Lancet 362: 767-771. [Crossref]
- Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, et al. (1996) The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 334: 1349-1355. [Crossref]
- Merit-HF Study Group (1999) Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in-congestive heart failure (MERIT-HF). The Lancet 353: 2001-2007. [Crossref]
- CIBIS-II Investigators and Committees (1999). The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A randomized trial. Lancet 353: 9-13. [Crossref]
- Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, et a;. (2002) Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circ 106: 2194-2199. [Crossref]
- Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, et al. (2005) Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 26: 215-225. [Crossref]
- Willenheimer R, van Veldhuisen DJ, Silke B, Erdmann E, Follath F, et al. (2005) Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence: results of the randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) III. Circ 112: 2426-2435. [Crossref]
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, et al. (1999) The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 341: 709-717. [Crossref]
- Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, et al. (2011Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 364: 11-21. [Crossref]
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, etal. (2014) Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371: 993-1004. [Crossref]
- Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, et al. (2010) Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. The Lancet 376: 875-885. [Crossref]
- Packer M, Poole-Wilson PA, Armstrong PW, Cleland JG, Horowitz JD, et al. (1999) Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. Circ 100: 2312-2318. [Crossref]



