Poly(1-vinylimidazole) (PVIm) with aminopropyl groups, that is, PVIm-Pr-NH2, has been synthesized as an efficient plasmid DNA (pDNA) carrier. Then, the PVIm-Pr-NH2 has been modified with lactose molecules as a cell-specific pDNA carrier. The resulting PVIm with lactose molecules, that is, PVIm-Pr-Lac, possessed approximately 12 mol% modified lactose units and formed the polyion complex (PIC) with pDNA at the positive/negative charge ratio of 2, 4, 8, or 16. The size of the resulting PVIm-Pr-Lac/pDNA PICs was within the range from approximately 100 nm to 200 nm. The PVIm-Pr-Lac/pDNA PICs specifically mediated the pDNA expression for human hepatoma HepG2 cells with asialoglycoprotein receptors. These results suggest that the PVIm-Pr-Lac worked as cell-specific pDNA carrier to deliver pDNA inside the cells for efficient gene expression.
poly(1-vinylimidazole), lactose, plasmid DNA, cell-specific carrier, drug delivery system
In the field of plasmid DNA (pDNA) delivery, the polyion complex (PIC) formation of between pDNA and polycation has widely been demonstrated as a new design of pDNA carrier [1,2]. The pDNA carriers to deliver pDNA inside cells are internalized into acidic endosome, where the carriers are subjected to a pH change from pH 7 to 5 [3]. Therefore, the escape from the endosome is one of the important factors for the design of efficient pDNA carrier. Poly(ethylenimine) (PEI) is one of pH-sensitive polycations with proton sponge effect to capture protons entering an endosome, resulting in the swelling of the endosomes to lead to membrane disruption for escape from endosomes [4].
On the other hand, the histidine-modified polycations or the polycations containing imidazole (Im) groups have enhanced pDNA expression [5-7]. In this case, the escape of the polycation/pDNA PIC from the endosome has been achieved by the proton sponge effect of Im groups including histidine. The pKa of the Im group is around 6, furthermore, the buffering capacity of Im groups around pH 6 in endosome induces the destabilization of cell membrane after their protonation. A similar effect is also observed with liposomes that include Im polar head [8]. The pH-sensitivity of the resulting Im groups in the pDNA carrier is considered to be critical for the release of the pDNA to cytosol. Based on these backgrounds, we have already reported several pDNA delivery systems based on Im groups [9-14]. Especially, we have already reported a poly(1-vinylimidazole) (PVIm) with several aminoethyl (Et-NH2) groups, that is, PVIm-Et-NH2, for a pH-sensitive polycation to enhance cell specific pDNA delivery [14]. By using PVIm-Et-NH2 as a pH sensitive pDNA carrier, as well as a lactosylated poly(L-lysine) as a cell-targeting pDNA carrier, the resulting ternary complexes specifically mediate pDNA expression. pDNA expression depends on our original concept that pDNA ternary complexes dissociate ligand polycations in response to endosomal pH [14,15]. However, PVIm-Et-NH2/pDNA binary complexes mediate no significant gene expression.
In this study, to develop PVIm-Et-NH2 for the realization of efficient pDNA expression as well as alkylated PVIm (PVIm-R) [12], and to modify PVIm with lactose (Lac) covalently as a cell-targeting ligand, we have synthesized PVIm with several aminopropyl (Pr-NH2) groups, that is, PVIm-Pr-NH2. The resulting PVIm-Pr-NH2 has been modified with lactose by the reductive amination between the amino group of PVIm-Pr-NH2 and the reducing end of lactose. The final product of lactosylated PVIm, that is, PVIm-Pr-Lac, is expected to mediate the cell-specific efficient pDNA expression.
Materials
3-Bromopropylamine hydrobromide and PEI solution were purchased from Sigma–Aldrich Co. LLC, St. Louis, MO, USA. All other chemicals of a special grade were used without further purification.
Synthesis of PVIm-Pr-lac
The synthetic route of PVIm-Pr-Lac is shown in Scheme 1. Poly(1-vinylimidazole) (PVIm) was synthesized according to our previous paper [16]. The PVIm (60 mg) and 3-bromopropylamine (20 mg) were dissolved in 8 mL dimethyl sulfoxide (DMSO). The reaction mixture was incubated at 40°C for 2 days, followed by dialysis against distilled water using a Spectra/Por 7 membrane (molecular weight cutoff=103) to remove unreacted 3-bromopropylamine. The resulting polymer PVIm-Pr-NH2 was obtained by freeze-drying.
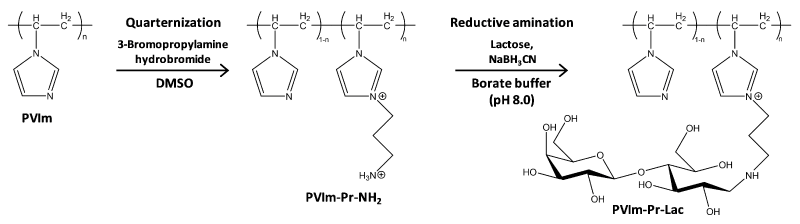
Scheme 1. Synthesis scheme of PVIm-Pr-Lac
Then, the PVIm-Pr-NH2 (20 mg) and lactose monohydrate (288 mg) were preincubated at 37°C for 3 days in 2.5 mL of sodium borate buffer (0.1 M, pH 8.5) [17]. Subsequently, a reducing agent, NaBH3CN (193 mg), was added to the mixture of PVIm-Pr-NH2 and lactose in 5 mL of the sodium borate buffer. The reaction mixture was further incubated at 37 °C for 3 days. The degree of amino group modification was examined by the primary amino group determination with fluorescamine [18]. The reaction mixture was dialyzed against distilled water using a Spectra/Por 7 membrane (molecular weight cutoff = 103) to remove unreacted lactose, followed by freeze-drying.
Agarose gel retardation assay
The resulting PVIm-Pr-Lac as well as PVIm-Pr-NH2 and pDNA (pGL3-control vector; from Promega Co.) were mixed in PBS(-) at a positive/negative charge ratio of 2, 4, 8 or 16. After the incubation at 37 °C for 1 h, each sample (corresponding to 300 ng pDNA) was mixed with a loading buffer (BlueJuice™, Invitrogen/Life Technologies) and loaded onto a 1% agarose gel containing 1 μg/mL ethidium bromide (EtBr). Gel electrophoresis was run at room temperature in TAE buffer (Tris-acetate, EDTA) at 50 V for 30 min. The pDNA bands were visualized under UV irradiation.
Size and z-potential measurement
The PVIm-Pr-Lac as well as PVIm-Pr-NH2 and the pDNA were mixed in PBS(-) at a positive/negative charge ratio of 2, 4, 8 or 16. After the incubation at 37 °C for 2 h, the size of each sample was measured by a dynamic light scattering (DLS) method using an electrophoresis light scattering spectrophotometer (ELS-Z2, Otsuka Electronics Co., Ltd., Tokyo, Japan) and the zeta potential was measured by ELS with electrodes.
Cell viability assay
HepG2 cells (from Riken Bioresource Center Cell Bank), human hepatoblastoma cell line, were cultured in tissue culture flasks containing Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated of fetal bovine serum (FBS). The cells were seeded at 1×104 cells/well (100 µL/well) in a 96-well plate and incubated at 37°C in a 5% CO2 incubator, overnight. After the addition of 15 µL PBS (-) containing the complex between 200 ng pDNA and PVIm-Pr-Lac as well as PVIm-Pr-NH2 at a positive/negative charge ratio of 2, 4, 8 or 16, the cells were incubated at 37°C for 24 h. After changing the medium, the cells were further incubated for 48 h, followed by the Alamar Blue assay [19] in triplicate.
Transfection procedure
According to the cell viability assay, 1×104 cells/well HepG2 cells were transfected in DMEM supplemented with 10% heat-inactivated FBS by the addition of 15 µL PBS (-) containing 200 ng of each pDNA encoding the modified firefly luciferase and PVIm-Pr-Lac as well as PVIm-Pr-NH2 at a positive/negative charge ratio of 2, 4, 8 or 16. PEI (positive/negative = 8) was used as a positive control. After 1 day of incubation, the medium was removed and the cells were further incubated for 2 days in the DMEM supplemented with 10% FBS. After washing the cells with PBS (-) twice, the cells were assayed by the luciferase protocol (Promega kit), according to the manufacturer’s instructions. Luciferase activities were normalized to protein concentrations and the results were presented as relative light units (RLU). Protein concentrations were determined by the bicinchonic acid (BCA) protein assay kit (Pierce), according to the manufacturer’s instructions.
Synthesis of PVIm-Pr-Lac as well as PVIm-Pr-NH2
As shown in Scheme 1, PVIm was reacted with 3-bromopropylamine for alkylation to obtain primary amino groups as well as quaternary imidazole groups. The amino groups of the resulting PVIm-Pr-NH2 were subsequently reacted with the reducing end of lactose by reductive amination to obtain alkylated PVIm with lactose, PVIm-Pr-Lac. As shown in Figure 1, the 1H NMR spectrum showed the characteristic signals of PVIm backbone (Figure 1) and propylene. From the signal ratio, the modification ratio of aminopropylated imidazole groups of PVIm-Pr-NH2 was calculated to be 12 mol%. Furthermore, as shown in Figure 1, the 1H NMR spectrum showed the characteristic signals of PVIm-Pr-NH2 backbone and lactose. By the primary amino group determination with fluorescamine (results not shown), as well as 1H NMR signals, only 5 mol% amino groups were remained after the reductive amination reaction, suggesting the almost complete modification (95 mol%) of the PVIm-Pr-NH2 with lactose. Thus, we have synthesized the lactosylated PVIm, that is, PVIm-Pr-Lac, as well as the PVIm with several aminopropyl groups, that is, PVIm-Pr-NH2.
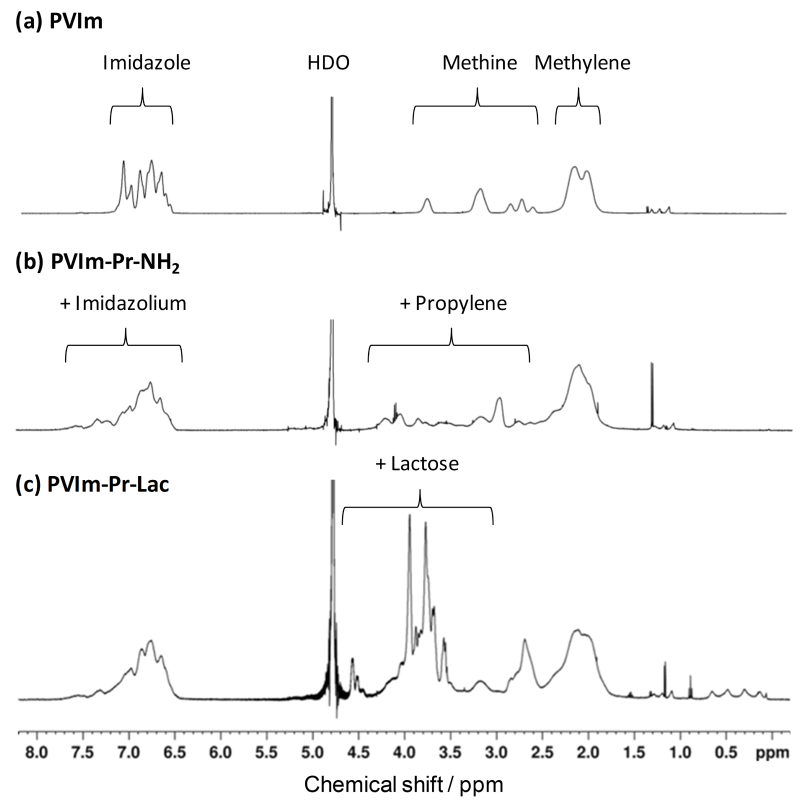
Figure 1. 1H NMR spectra of PVIm derivatives
PIC formation between pDNA and PVIm-Pr-Lac as well as PVIm-Pr-NH2
As shown in Figure 2, we examined whether the PVIm-Pr-Lac as well as PVIm-Pr-NH2 formed the PIC with pDNA by agarose gel electrophoresis. The complete retardation of pDNA in the agarose gel proved that the PVIm-Pr-Lac as well as PVIm-Pr-NH2 formed the PIC with pDNA above positive/negative charge ratio of 2. Because of the coil-globule transition of pDNA and the resulting inhibition of the EtBr intercalation [20,21], no fluorescence owing to EtBr staining was observed in case of the PEI/pDNA PIC at the positive/negative charge ratio of 8. On the other hands, slight fluorescence was observed in case of the PVIm-Pr-NH2/pDNA PIC at the positive/negative charge ratio of 8. The nonionic PVIm backbone is considered to solubilize the pDNA PICs and to reduce the coil-globule transition of pDNA, that is, pDNA compaction, at the charge ratio [14,22]. Furthermore, a significant fluorescence was observed in case of the PVIm-Pr-Lac/pDNA PICs. A similar observation was previously reported in the case of the soluble complexes between pDNA and the polycation grafted with water-soluble carbohydrate chains such as dextran [23,24]. In this study, the water-soluble carbohydrate lactose seems to the pDNA PICs and to reduce pDNA compaction.
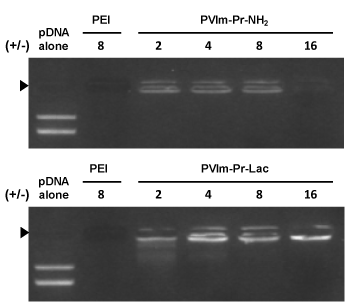
Figure 2. Analysis of the PIC formation between pDNA and the PVIm-Pr-Lac as well as PVIm-Pr-NH2 by agarose gel electrophoresis. Positive/negative (+/-) mixing charge ratios are indicated. PEI was used as a control. Solid arrowhead indicates the well where each sample was loaded
Accordingly, we measure the particle size and z-potential of the PVIm-Pr-Lac/pDNA PICs (Table 1) as well as the PVIm-Pr-NH2/pDNA PICs (Table 2). The particle size of the PVIm-Pr-Lac/pDNA PICs is almost similar to that of the PVIm-Pr-NH2/pDNA PICs in the rage around 200 nm. On the other hand, the z-potential of PVIm-Pr-Lac/pDNA PICs seems to be a little lower than that of the PVIm-Pr-NH2/pDNA PICs, suggesting the slight shielding of surface positive charges by nonionic carbohydrate lactose.
Table 1. Particle size and z-potential of PVIm-Pr-NH2/pDNA PICs
PVIm-Pr-NH2/pDNA |
Particle size /nm |
z- potential /mV |
+/- = 2 |
121.5 |
+43.17 |
+/- = 4 |
102.1 |
+44.05 |
+/- = 8 |
149.5 |
+38.97 |
+/- = 16 |
250.4 |
+43.34 |
Table 2. Particle size and z-potential of PVIm-Pr-Lac/pDNA PICs
PVIm-Pr-Lac/pDNA |
Particle size /nm |
z- potential /mV |
+/- = 2 |
123.9 |
+25.32 |
+/- = 4 |
219.5 |
+25.63 |
+/- = 8 |
119.0 |
+36.06 |
+/- = 16 |
154.5 |
+36.27 |
Cell-Specific pDNA delivery by PVIm-Pr-Lac/pDNA PICs
To confirm the required properties as pDNA carrier, as shown in Figure 3, we first carried out a cytotoxicity assay. The cell viability of HepG2 hepatoma cells kept over 90% in the presence of the PVIm-Pr-Lac/pDNA PIC at the positive/negative charge ratio of 16. On the other hand, the cell viability decreased up to 80% in the presence of the PVIm-Pr-NH2/pDNA PIC at the positive/negative charge ratio of 16, when the cell viability decreased up to 60% in the presence of the PEI/pDNA PIC as a control even at the positive/negative charge ratio of 8. These results suggest that the highest positive z-potential of the the PVIm-Pr-NH2/pDNA PIC at the positive/negative charge ratio of 16 (Tables 1 and 2) damaged a negative-charged cell membrane. Therefore, the PVIm-Pr-Lac with no apparent cytotoxicity promises to be an effective pDNA carrier.
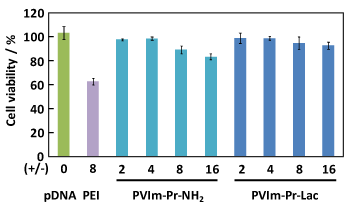
Figure 3. Effect of the PVIm-Pr-Lac/pDNA PICs as well as PVIm-Pr-NH2/pDNA PICs on the viability of HepG2 cells
As a no apparent cytotoxicity, we finally examined the pDNA expression mediated by the PVIm-Pr-Lac/pDNA PICs as well as PVIm-Pr-NH2/pDNA PICs. HepG2 cells are a human hepatoma cell line, so that asialoglycoprotein receptor (ASGP-R) expressed on the cell surface [16]. As shown in Figure 4, the PVIm-Pr-Lac/pDNA PICs mediated remarkable pDNA expression, which was almost the same level as that mediated by the control PEI, on HepG2 cells. On the other hand, the pDNA expression mediated by the PVIm-Pr-NH2/pDNA PICs was lower than that mediated by the PVIm-Pr-Lac/pDNA PICs. On human cervical carcinoma (HeLa) cell line with no ASGP-R on the cell surface, as shown in Figure 4, almost no difference between PVIm-Pr-Lac and PVIm-Pr-NH2 was observed. Namely, the pDNA expression mediated by the PVIm-Pr-Lac/pDNA PICs was almost the same level as that mediated by the PVIm-Pr-NH2/pDNA PICs on HeLa cells. These results suggest that the PVIm-Pr-Lac/pDNA PICs with β-galactose residues recognized the HepG2 cells via ASGP-Rs on the cell surface.
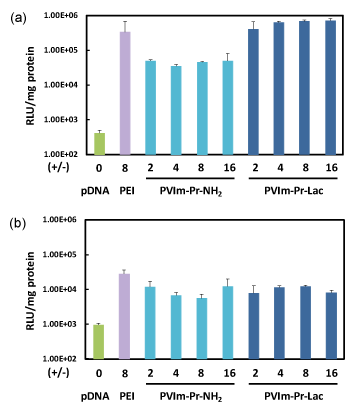
Figure 4. Transfection of luciferase gene to HepG2 (a) or HeLa (b) cells by the PVIm-Pr-Lac/pDNA PICs as well as PVIm-Pr-NH2/pDNA PICs
We have synthesized the PVIm-Pr-NH2 and PVIm-Pr-Lac as derivatives from our pH-sentivive pDNA carrier PVIm-R. Especially, the PVIm-Pr-Lac possessed approximately 12 mol% modified lactose units and formed the PICs with pDNA. The resulting PVIm-Pr-Lac/pDNA PICs specifically mediated the pDNA expression for human hepatoma with asialoglycoprotein receptors. These results suggest that the PVIm-Pr-Lac worked as cell-specific pDNA carrier to deliver pDNA inside the cells for efficient gene expression.
This research was partially supported by a Grant-in-Aid for Scientific Research (B) from Japan Society for the Promotion of Science (JSPS KAKENHI grant no. 16H03183).
- Asayama S (2020) Molecular design of polymer-based carriers for plasmid DNA delivery in vitro and in vivo. Chem Lett 49: 1-9.
- Cabral H, Myata K, Osada K, Kataoka K (2018) Block copolymer micelles in nanomedicine application. Chem Rev 118: 6844-6892.
- Steinman RM, Mellman IS, Muller WA, Cohn ZA (1983) Endocytosis and the recycling of plasma membrane. J Cell Biol 96: 1-27. [Crossref]
- Boussif O, Lezoualcah F, Zanta MA, Margny MD, Scherman D, et al. (1995) A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc Natl Acad Sci USA 92: 7297-7301.
- Midoux P, Pichon C, Yaouanc JJ, Jaffrès PA (2009) Chemical vectors for gene delivery: a current review on polymers, peptides and lipids containing histidine or imidazole as nucleic acids carriers. Br J Pharmacol 157: 166-178. [Crossref]
- Ihm JE, Han KO, Han IK, Ahn KD, Han DK, et al. (2003) High transfection efficiency of poly(4-vinylimidazole) as a new gene carrier. Bioconjugate Chem 14: 707-708.
- Roufa MB, Midoux P (2001) Histidylated polylysine as DNA vector: Elevation of the imidazole protonation and reduced cellular uptake without change in the polyfection efficiency of serum stabilized negative polyplexes. Bioconjugate Chem 12: 92-99.
- Mavel M, Neveu C, Gonalves C, Yaouanc JJ, Pichon C, et al. (2008) Novel neutral imidazole-lipophosphoramides for transfection assays. Chem Commun 27: 3124-3126.
- Asayama S, Sakata M, Kawakami H (2017) Structure-activity relationship between Zn2+-chelated alkylated poly(1-vinylimidazole) and gene transfection. J Inorg Biochem 173: 120-125. [Crossref]
- Asayama S, Matsuda K, Negishi Y, Kawakami H (2014) Intracellular co-delivery of zinc ions and plasmid DNA for enhancing gene transfection activity. Metallomics 6: 82-87.
- Asayama S, Nishinohara S, Kawakami H (2011) Zinc-chelated poly(1-vinylimidazole) and a carbohydrate ligand polycation form DNA ternary complexes for gene delivery. Bioconjugate Chem 22: 1864-1868.
- Asayama S, Hakamatani T, Kawakami H (2010) Synthesis and characterization of alkylated poly(1-vinylimidazole) to control the stability of its DNA polyion complexes for gene delivery. Bioconjug Chem 21: 646-652. [Crossref]
- Asayama S, Hakamatani T, Kawakami H (2010) Synthesis and characterization of alkylated poly(1-vinylimidazole) to control the stability of its DNA polyion complexes for gene delivery. Bioconjug Chem 21: 646-652. [Crossref]
- Asayama S, Sudo M, Nagaoka S, Kawakami H (2008) Carboxymethyl poly(L-histidine) as a new pH-sensitive polypeptide to enhance polyplex gene delivery. Mol Pharm 5: 898-901. [Crossref]
- Asayama S, Sekine T, Kawakami H, Nagaoka S (2007) Design of aminated poly(1-vinylimidazole) for a new pH-sensitive polycation to enhance cell-specific gene delivery. Bioconjugate Chem 18: 1662-1667.
- Asayama S, Sekine T, Kawakami H, Nagaoka S (2006) pH-Dependent dissociation of carbohydrate ligand polycations from DNA ternary complexes. Chem Lett 35: 100-101.
- Asayama S, Akaike T, Maruyama A (2001) Bi-phasic polycation for the DNA carrier responding to endosomal pH. Colloids Surf. B: Biointerfaces 22: 183-191.
- Martinez-Fong D, Mullersman JE, Purchio AF, ArmendarizBorunda J, Martinez-Hernandez A (1994) Nonenzymatic glycosylation of poly-L-lysine: a new tool for targeted gene delivery. Hepatology 20: 1602-1608.
- Udenfriend S, Stein S, Bohlen P, Dairman W, Leimgruber W, et al. (1972) Fluorescamine: A reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science 24: 871-872.
- Unsworth JM, Rose FR, Wright E, Scotchford CA, Shakesheff KM (2003) Seeding cells into needled felt scaffolds for tissue engineering applications. J Biomed Mater Res A 66: 425-431.
- Shuai X, Merdan T, Unger F, Kissel T (2005) Supramolecular gene delivery vectors showing enhanced transgene expression and good biocompatibility. Bioconjugate Chem 16, 322-329.
- Pouton CW, Lucas P, Thomas BJ, Uduehi AN, Milroy DA, et al. (1998) Moss, S.H. Polycation-DNA complexes for gene delivery: a comparison of the biopharmaceutical properties of cationic polypeptides and cationic lipids. J Controlled Release 53: 289-299.
- Yamagata M, Kawano T, Shiba K, Mori T, Katayama Y, et al. (2007) Structural advantage of dendritic poly(L-lysine) for gene delivery into cells. Bioorg Med Chem 15: 526-532. [Crossref]
- Maruyama A, Watanabe H, Ferdous A, Katoh M, Ishihara T, et al. (1998) Characterization of interpolyelectrolyte complexes between double-stranded DNA and polylysine comb-type copolymers having hydrophilic side chains. Bioconjugate Chem 9: 292-299.





