Abstract
Imidazolium salts can be classed as ionic liquids (ILs) and used as antimicrobials. Before widespread use in clinical and industrial release of ILs into the environment, their toxicology and antimicrobial studies must be performed. In this study, new imidazolium salts are synthesized, and their antimicrobial and antifungal activities were tested against Gram-positive (Staphylococcus aureus), Gram-negative (Escherichia coli, Pseudomonas aeruginosa) bacteria and yeast (Candida albicans). Among these compounds studied, IL5 indicated also inhibitor activities against the bacteria (E. coli and S. aureus) and low antifungal activity. The obtained experimental results suggested that compound IL5 may be interesting candidate for development of new antimicrobials.
Keywords
antimicrobial activities, imidazolium salts, ionic liquids
Introduction
Ionic liquids (ILs) are described as thermally stable salts that are consisting of an organic cation and organic anion. In the past several years ionic liquids have been used such as alternative solvents in organic synthesis, polymer chemistry, enzyme catalysis, drug delivery systems and liquid-liquid extractions etc. Therewithal ILs have found numerous applications infuel cells, nanomaterials, fabrication of solar cells and number of biological processes due to their extraordinary physicochemical properties, low vapor pressure, high polarity, high ionic conductivity and tunability etc. [1-5]. Their biological properties, such as toxicity and environmental impacts (accumulation and biodegradability) make them attractive in biological applications. Moreover, many researchers have observed excellent antimicrobial activity of ILs. Thus, it is believed that they could be used as biocidal agents in control of growth of microorganism [6-9].
Imidazolium salts (IMSs) which are well-known as a type of ionic liquids, could potentially be included in antimicrobial, antitumor and other therapeutic agents. It has been found that antimicrobial activity of imidazolium salts is closely related to their structures. The structure-activity relationship showed that cation alkyl side chain length affected in their antimicrobial activity, but the type of anion has little affected in them. Furthermore, in comparison with
N-substituted imidazolium oximes, imidazolium pyridinium, quaternary ammonium and 1-alkyl-3-methylimidazolium halides (CI, Br) were found to possess much broader range of antimicrobial activity. Moreover the antimicrobial potency of imidazolium salts isbased on partly to their lipophilicities which correlate to the alkyl chain length. In this case antimicrobial activity increases with length of the substituent alkyl chain [10-16]. Besides in vitro antimicrobial activity of Mn(II)- and Fe(III) saldach-imidazolium salts against Gram-positive, Gram-negative bacteria and fungus were evaluated and it is observed that its Fe(III) complex depicted high antimicrobial activity. The data showed that the activity depends on the type of metal ion and varies in the following order of the metal ion [17].
In this study, newly synthesized imidazolium salts were investigated for antimicrobial activity against Gram-positive (Staphylococcus aureus), Gram-negative (Escherichia coli, Pseudomonas aeruginosa) and yeast (Candida albicans).
Methods and Materials
All chemicals are purchased from commercial sources, and used as received. All organic solvents used are of high quality from Fluka and J.T.Baker. 1,2-Bis-(2-iodoethoxy) ethane, 1-methylimidazole, 1-(3-aminopropyl)imidazole, 1,8-naphthalic anhydride, 1-bromooctane, 1,4- diiodobutane, 1,8-Diiodooctane and bis(trifluoromethane)sulfonimide lithium salt are purchased from Fluka and used without any treatment.1H-NMRspectrawas measured on a Bruker 400 MHz spectrometer.
Synthesis of ionic liquids
N-(3-propylimidazole)-1,8-naphthalene monoimide (1) was synthesized according to the previously reported method (Figure 1) [1]. IL1, IL2, IL3 and IL4 were prepared according to the literature [2-3]. The synthetic details of IL5 synthesis are given below (Figure 2).
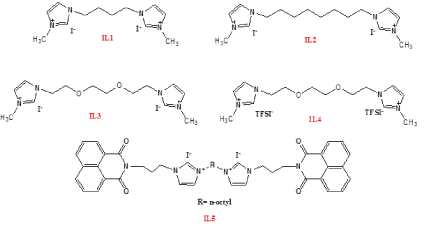
Figure 1. Molecular structures of the compounds.
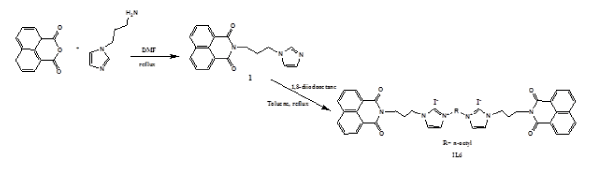
Figure 2. Synthesis of compound IL6.
Synthesis of 1,8-naphthalene monoimidedimer bearing imidazolium iodide salt (IL5)
N-(3-propylimidazole)-1,8-naphthalene monoimide (1) (100 mg, 0.3 mmol) was dissolved in toluene under nitrogen atmosphere, and then 1,8-diiodooctane (33 µL) was added. The mixture was heated overnight at reflux. The crude product was obtained as a yellow solid (32% yield).1H NMR (δH, ppm, 400 MHz, DMSO): 9.18 (s, 2H, imidazole), 8.48 (d, 8H, J= 8 Hz, Ar), 7.9-7.7 (m, 8H, Ar, imidazole) 4.28 (bs, 4H), 4.15-40.8 (bd, 8H), 2.2-2.1 (m, 4H), 1.8-1.7 (m, 4H), 1.4-1.1 (m, 8H).
Determination of minimum inhibitory concentrations
The antimicrobial activities of compounds were tested by the microdilution method according to the
Minimum inhibitory concentrations (MICs) of compounds were determined against American Type Culture Collection (ATCC) reference strains (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and Staphylococcus aureus ATCC 29213and Candidaalbicans ATCC 90028). The antibacterial and antifungal assays were performed in Mueller Hinton broth (Merck, Germany) and Sabouraud dextrose broth (Merck, Germany) respectively. The compounds were solved in dimethylsulfoxide (DMSO) or distilled water and diluted with water to prepare the stock solutions. The two fold serial dilution of the compounds from 0.015 to17 mM was made in a 96-well plate. The final DMSO concentration was occurred as 1:16. According to the CLSI, the microorganism suspensions adjust to 0,5McFarland turbidimetric standard at 530 nm wavelength. 50μL of a bacterial or yeast suspension, obtained from a 24 h culture was added to each well. The final inoculum size was 5x105 CFU/mL for the antibacterial assay and 5x102 CFU/mL for the antifungal assay. The plates were incubated at 35°C for 24 h. The MIC was defined as the lowest compound concentration that inhibits the visible growth of a microorganism after overnight incubation. We tested levofloxacin and fluconazole as standard antimicrobial agents under the same experimental conditions. Each experiment was carried out in duplicate (Table 1).
Table 1. MIC values (µM) of compounds against bacterial and yeast strains. *Acceptable quality control ranges of minimum inhibitory concentrations (MICs) (μM) for reference strains [18,19]. Concentration range for Levofloxacin (22.1-0.04), Concentration range for Fluconazole (52-0.09).
Compound No |
Escherichia coli ATCC 25922 |
Pseudomonas aeruginosa
ATCC 27853 |
Staphylococcus aureus
ATCC 29213 |
Candida albicans ATCC 90028 |
IL1 |
17000 |
> 17000 |
17000 |
17000 |
IL2 |
7500 |
2021 Copyright OAT. All rights reserv
>15000 |
15000 |
1800 |
IL3 |
>14600 |
>14600 |
>14600 |
>14600 |
IL4 |
9500 |
>9500 |
>9500 |
9500 |
IL5 |
1030 |
>8240 |
64 |
>8240 |
Levofloxacin |
0.69(0.16-1.38)* |
0.08(0.02-0.16)* |
1.38(1.38-11.08)* |
- |
Fluconazole |
- |
- |
- |
3.26(0.81-3.26)* |
Results
Synthesis of IL5
The crude product of IL5 was obtained as a yellow solid (32% yield). 1H NMR (δH, ppm, 400 MHz, DMSO): 9.18 (s, 2H, imidazole), 8.48 (d, 8H, J= 8 Hz, Ar), 7.9-7.7 (m, 8H, Ar, imidazole) 4.28 (bs, 4H), 4.15-40.8 (bd, 8H), 2.2-2.1 (m, 4H), 1.8-1.7 (m, 4H), 1.4-1.1 (m, 8H).
Antimicrobial activity
All synthesized imidazolium salts were tested for antimicrobial activity against bacteria and yeast. Minimum inhibitory concentration (MIC) values of the ILs and reference antimicrobial agents were shown in Table 1. It was determined that the DMSO had no activity against test microorganisms.
As shown by the results, MIC values of IL2were between 1.8 mM for fungus and >15 mM for bacteriae. Nevertheless, it was observed low antifungal and antibacterial activities in studies with IL1 (MIC values >2 mM), and it was nontoxic to all bacteriae and yeast (MIC value: 17 mM). The MIC values of IL5 were 64 µM for S.aureus, 1.03 mM for E.coli and >8.24 mM for P. aeruginosa and C.albicans.
Discussion
The imidazolium salts substituted with alkyl chains of a shorter length had poor surfactant properties, and resultant poor MIC values as expected [6,14,20,21]. Only IL2 showed good antifungal activity as other ionic liquids containing long alkyl chains. It is observed that the MIC values of the imidazolium salt bearing ethoxy ether group (IL3), was no change against bacteriae and fungus (>14.6 mM). On the other hand, MIC values of IL2 were 1.8-15mM. The antimicrobial activities of IL3 were not good when compared IL2. This situation can be explained by the length of the alkyl chain; both compounds have same chain length. This has been shown in previous study [Cn-Im-3OEG][CI] compounds showed better activities with longer alkyl chain against bacteriae [5]. Besides it is indicated that antimicrobial activities against bacteriae increased depending on the length of alkyl chain in imidazolium salts [22].
On the other hand, both compounds displayed increase on toxicity towards the Gram positive type bacteria (S. aureus) compared to Gram negative. These results may be interpreted as the different structural properties present in the both types of bacteriae. The outer membrane in bacterial cell walls is present in Gram positive, whereas the outer membrane is not existent in Gram negative. Some components (lipopolysaccharide later etch) of Gram negative walls can guard the bacteriae from chemicals [4,21].
Our results revealed that antimicrobial and antifungal activities of IL3 and IL4 changed by the presence of different anionic groups. The MIC values for IL3 and IL4 were >14.6 mM and >9.5 mM for all the bacteriae and yeast, respectively. This improvement in the antimicrobial activity may be due to the inclusion of TFSI group in IL4 structure. Nonetheless, both ILs have low antimicrobial and antifungal activities (MIC values >2mM). The antimicrobial activities of a series of novel chiral ionic liquids, containing amino acid ester and dipeptidyl functionalities, were evaluated and by Coleman, et. al. [21]. In this study the CILs containing a single peptide moiety in the cation side chain, low bacterial potency was reported ( 2mM) and the ionic liquid with a D-Val-D-Val sequence, showed no antibacterial toxicity (up to the maximum concentration, 2 mM) [21]. Our results are similar to theirs. The antimicrobial and antifungal activities of the synthesized ILs were evaluated against a microorganism. Some of the compounds showed quite strong antimicrobial and antifungal activities. It was found that the activities were related to the alkyl chain length and type of the anionic groups.
2mM) and the ionic liquid with a D-Val-D-Val sequence, showed no antibacterial toxicity (up to the maximum concentration, 2 mM) [21]. Our results are similar to theirs. The antimicrobial and antifungal activities of the synthesized ILs were evaluated against a microorganism. Some of the compounds showed quite strong antimicrobial and antifungal activities. It was found that the activities were related to the alkyl chain length and type of the anionic groups.
In this study, different ILs were synthesized and their antimicrobial toxicity was evaluated against Gram negative and Gram positive bacterial and yeast strains. IL6 also showed inhibitor activities on the bacteriae (E.coli and S. aureus) and low antifungal activity (MIC value >2mM). On the other hand, other compounds (IL1, IL2, IL3, IL4) have low antimicrobial and antifungal toxicities (MIC values >2mM). Our study indicates that some ILs are toxic to microorganisms. These results suggest that antimicrobial activity should be considered in design of ILs and evaluation in clinical and industrial applications.
Author Contributions
All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Acknowledgments
This research has not received any financial support and is not an article that is carried out within the scope of the project or the student’s thesis.
Conflict of Interest Statement
The authors declared no conflict of interest.
References
- Ozdemir S, Varlikli C, Oner I, Ocakoglu K, ICLI S (2010) The synthesis of 1,8-naphthalimide groups containing imidazolium salts/ionic liquids using I-, PF6-, TFSI- anions and their photophysical, electrochemical and thermal properties. Dyes Pigm 86: 206-216.
- Zafer C, Ocakoglu K, Ozsoy C, ICLI S (2009) Dicationic bis-imidazolium molten salts for efficient dye sensitized solar cells: Synthesis and photovoltaic properties. Electrochim Acta 54: 5709-5714.
- Erten-Ela S, Ocakoglu K (2014) Iridium dimer complex for dye sensitized solar cells using electrolyte combinations with different ionic liquids. Mat Sci Semicon Proces 27: 532-540.
- Huang RTW, Peng KC, Shih HN, Lin GH, Chang TF, et al. (2011) Antimicrobial properties of ethoxyether-functionalized imidazolium salts. Soft Matter 7: 8392-8400.
- Des. Hodyna D, Kovalishyn V, Rogalsky S, Blagodatnyi V, Petko K, et al. (2016) Antibacterial Activity of Imidazolium-Based Ionic Liquids Investigated by QSAR Modeling and Experimental Studies. Chem Biol Drug 88: 422-433.
- Messali M, Moussa Z, Alzahrani AY, EL-Naggar MY, Eldouhaibi AS, et al. (2013) Synthesis, characterization and the antimicrobial activity of new eco-friendly ionic liquids. Chemosphere 91: 1627-34.
- Garcia MT, Gathergood N, Scammells PJ (2005) Biodegradable ionic liquids Part II. Effect of the anion and toxicology. Green Chem 7: 9-14.
- Pernak J, Goc I, Mirska I (2004) Anti-microbial activities of protic ionic liquids with lactate anion. Green Chem 6: 323-329.
- Docherty KM, Kulpa JCF (2005) Toxicity and antimicrobial activity of imidazolium and pyridinium ionic liquids. Green Chem 7: 185-189.
- Odzak R, Skocibusic M, Maravic A (2013) Synthesis and antimicrobial profile of N-substituted imidazolium oximes and their monoquaternary salts against multidrug resistant bacteria. Bioorg Med Chem 21: 7499-506.
- Dalla Lana DF, Donato RK, Bundchen C, Guez CM, Bergamo VZ, et al. (2015) Imidazolium salts with antifungal potential against multidrug-resistant dermatophytes. J Appl Microbiol 119: 377-88.
- Bergamo VZ, Donato RK, Dalla Lana DF, Donato KJ, Ortega GG, et al. (2015) Imidazolium salts as antifungal agents: strong antibiofilm activity against multidrug-resistant Candida tropicalis isolates. Lett Appl Microbiol 60: 66-71.
- Bergamo VZ, Balbueno EA, Hatwig C, Pippi B, Dalla Lana DF, et al. (2015) 1-n-Hexadecyl-3-methylimidazolium methanesulfonate and chloride salts with effective activities against Candida tropicalis biofilms. Lett Appl Microbiol 61: 504-510.
- Pernak J, Sobaszkiewicz K, Mirska I (2003) Anti-microbial activities of ionic liquids. Green Chem 5: 52-56.
- Carson L, Chau PKW, Earle MJ, Gilea MA, Gilmore BF, et al. (2009) Antibiofilm activities of 1-alkyl-3-methylimidazolium chloride ionic liquids. Green Chem 11: 492-497.
- De Luca L (2006) Naturally occurring and synthetic imidazoles: their chemistry and their biological activities. Curr Med Chem 13: 1-23.
- Elshaarawy RF, Janiak C (2014) Toward new classes of potent antibiotics: synthesis and antimicrobial activity of novel metallosaldach-imidazolium salts. Eur J Med Chem 75: 31-42.
- CLSI (2008) Performance Standards for antimicrobial susceptibility testing; 18th Informational supplement M100–S18. Clinical and Laboratory Standards Institute, Wayne Pa.
- NCCLS (2002) Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast; Approved Standard (2nd Edn) M27-A2. The National Committee for Clinical Laboratory Standards, Wayne Pa.
- Riduan SN, Zhang Y (2013) Imidazolium salts and their polymeric materials for biological applications. Chem Soc Rev 42: 9055-9070.
- Coleman D, Spulak M, Garcia MT, Gathergood N (2012) Antimicrobial toxicity studies of ionic liquids leading to a 'hit' MRSA selective antibacterial imidazolium salt. Green Chem 14: 1350-1356.
- Demberelnyamba D, Kim KS, Choi S, Park SY, Lee H, et al. (2004) Synthesis and antimicrobial properties of imidazolium and pyrrolidinonium salts. Bioorg Med Chem 12: 853-857. [Crossref]


