The present report describes a case of a neuroendocrine tumor of the rectum with liver metastasis in a 35-year-old woman. The rectum is the second most common location of gastrointestinal neuroendocrine tumors and is typically diagnosed through a screening colonoscopy finding for non-tumor related symptoms. Surgical treatment is indicated when the disease is localized, but due to its characteristics, in most cases, this cancer is diagnosed in advanced stages. When the tumor is advanced and/or metastasis is present, therapeutic options include embolization, somatostatin analogs, interventional radiology, chemotherapy, and radiometabolic peptide therapy. 177-Lutecio-DOTATE therapy has a positive impact on the quality of life of patients with inoperable metastatic tumors. The patient described in the case was initially treated with surgery and chemotherapy and later with liver segmentectomies, embolization, and octreotide due to liver tumor recurrence. With the persistence of the metastasis, she started treatment with 177-Lutecio-DOTATE, and saw, after the second cycle of administration, partial remission of the tumor. This case describes how 177-Lutecio-DOTATE is a viable treatment option and has shown significant results in inoperable and/or metastatic disease.
neuroendocrine tumors, (177lutetium-DOTATE, rectum neuroendocrine tumors, organometallic compounds, octreotide/analogs and derivatives
Neuroendocrine tumors are a heterogeneous set of neoplasms developed from endocrine system cells that produce certain hormones responsible for regulating an organism’s physiological processes. These cells are distributed throughout the body, yet are found principally in the intestine, pancreas, lung, and stomach [1].
Neuroendocrine tumors are classified according to localization of the primary tumor, production of hormones, and the histological grade. The localization of the primary tumor is, in turn, used to classify a disease into one of three groups: gastrointestinal neuroendocrine tumors, pancreatic neuroendocrine tumors, and pulmonary neuroendocrine tumors. Gastrointestinal neuroendocrine tumors are the most common group; the most frequently involved sites are the small intestine, then rectum, and stomach, in order of decreasing frequency. These tumors are divided according to primary site, into: foregut (anterior intestine, including lungs, bronchi, gastric, duodenal, thymus, and bile ducts), midgut (median intestine, including the small intestine, appendix, and proximal colon), and hindgut (posterior intestine, including the distal colon, rectum, and genitourinary tract). In addition to the site, tumors are classified in relation to the existence of symptoms produced by released hormones (functioning and non-functioning tumors). The degree of neuroendocrine tumors should be defined by cell proliferation and classified as: G1 (<2 mitoses per 10 fields and/or Ki67 ≤ 2%), G2 (2-20 mitoses per 10 fields and/or Ki67 3-20%), and G3 (>20 mitoses per 10 fields and/or Ki67>20%) [2,3].
When a gastrointestinal neuroendocrine tumor is localized, the first-line treatment is surgery. When an advanced tumor or metastasis are present, surgery may be indicated to treat symptoms, control complications, such as organ obstruction or bleeding, and increase survival. In cases of an advanced tumor or metastasis, treatment can include embolization (a radiological procedure that restricts or provides blood to the tumor), and somatostatin analogs (octreotide and lanreotide). In metastatic and inoperable tumors, radionuclide 177-Lutecio-DOTATE, a substance similar to octreotide, is used for its similar affinity for the somatostatin receptor [4,5].
The objective of this paper is to report a clinical case of a neuroendocrine tumor of the rectum with hepatic metastasis treated with 177-Lutecio-DOTATE radionuclide therapy and to discuss the particularities of this tumor type with emphasis on 177-Lutecio-DOTATE radionuclide therapy.
A 35-year-old female resident of Taubaté, Sao Paulo, sought healthcare services at the A.C. Camargo Cancer Center Hospital in October of 2009. She reported intermittent episodes of diarrhea associated with hematochezia and abdominal cramps starting in April of the same year, with exacerbations lasting about a week. In August, she had sought medical attention in Taubaté, where she received a colonoscopy. Her biopsy showed a potentially malignant neuroendocrine tumor and was then referred to the oncology department of the A.C. Camargo Cancer Center Hospital. In her personal history, she reported psoriasis controlled with Clobetazole ointment and a history of previous use of Metrotexate and Acitretin. She denied other diseases, family history of comorbidities, past gestational history, and risky habits such as alcoholism, smoking, or drug abuse.
On October 6, 2009, she underwent a new colonoscopy with the biopsy results being described as “undifferentiated malignant neoplasia compatible with neuroendocrine neoplasia of undetermined potential for malignancy.” The immunohistochemical panel of the neoplastic cells tested: anti-cytokeratin (CAM 5.2) positive, synaptophysin positive, cytokeratin-7 (CK-7) negative, chromogranin A positive, cytokeratin 20 (CK 20) negative, estrogen receptor (ER), progesterone receptor (PR) negative, and antigen KI-67 <5%. Initial laboratory tests showed normal ranges for complete blood count, white blood cell count, electrolytes, renal function, and carcinoembryonic antigen (CEA) (Table 1).
Table 1. Initial laboratory tests
TesTest |
Result |
Test |
Result |
Hemoglobin |
14,1 g/dL |
Leucocytes |
8500 mil/mm3 |
Platelets |
363000 /mm3 |
Calcium |
8,7 mEq/L |
Sodium |
141 mEq/L |
Potassium |
4,3 mEq/L |
Magnesium |
2,1 mEq/L |
Creatinine |
0,73 mg/dL |
Urea |
18 mg/dL |
CEA |
2,8 ng/ml |
The patient started chemotherapy with a combination treatment of cisplatin and vepeside (CDDP+VP) concomitantly with radiotherapy for five cycles. On April 5, 2010, the patient underwent rectosigmoidectomy with resection of a meso-sigmoid nodule, hepatic nodule, distal margin, cecal appendix, and gallbladder. The pathologist described a well-differentiated neuroendocrine tumor of 1.5 cm in size along its largest axis; seven of fifteen lymph nodes were positive; there was a hepatic nodule compatible with neuroendocrine neoplasia metastasis; 5% ki 67 was detected through immunohistochemical staining. The tumor was, therefore, staged at ypT3yN2M1.
A new colonoscopy was performed the following year (2011) and found no signs of tumor activity. However, new hepatic nodules emerged in May 2013, suggesting tumor recurrence. The positron emission tomography (PET) scan performed with 68-Gallium DOTATATE showed an area of anomalous concentration in the abdominal view corresponding to hypodense nodules in segments IV, V, VI, and VIII cm with standardized uptake value between 10.5 and 13.8. In November 2013, right posterior hepatic segmentectomy (segments VI and VII) and unregulated segmentectomies in segments II, III, IV, V, VII, and VIII were performed. Pathological evaluations of these segmentectomies confirmed the continuing presence of well-differentiated neuroendocrine neoplasia.
In subsequent years, the patient continued with clinical follow-up visits and periodic imaging exams. These exams showed, in 2014, the appearance of new liver nodules and, in December 2015, that these nodules had increased in size. Therefore, chemoembolization was proposed to be performed from February 2016 to September 2017, in a total of seven procedures. The patient concomitantly received cycles of Octreotide LAR 30 mg. However, the absence of necessary intrahepatic arteries meant that the embolizations had to be interrupted. Octreotide continued to be administered even after interrupting the embolizations.
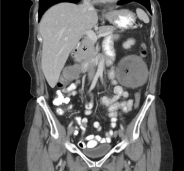
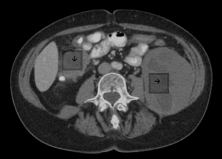
In February 2018, the patient received another 68-Gallium DOTATATE PET-CT scan, which showed an area of concentrated anomalies in the thoracic topography. This concentration corresponded to the enlargement of the right perihilar lymph node with an SUV of 7.8, multiple hepatic lesions in the abdomen with an SUV up to 27.4, and lymph node conglomerates and retroperitoneal lymph node enlargement with an SUV of 23.7. As liver damage was noted during the examination, the treating physicians indicated treatment with Lutetium. The patient began this treatment at the Beneficência Portuguesa Hospital in May 2018 with a planned initial course of four administrations. 200 mCi of the medication was administered per hospital protocol with a prior infusion of renal protection factors (amino acids L-lysine and L-arginine). Two days after the first administration, the patient underwent a therapeutic post-dose scintigraphy study with radiolabeled somatostatin analog (whole-body radioiodine scan). This study demonstrated multiple areas of marked radiopharmaceutical hyper-concentration in the upper abdomen topography related to multiple hepatic lesions, lymph node enlargement, and lymph node conglomerates (Figure 1).
The patient received a second dose in July 2018, with a stable response, and then a third dose in September 2018 (Figure 2).
After the second cycle of Lutetium in July 2018, the whole-body radioiodine scan showed regression of avidity foci in the thorax and right iliac fossa and a reduction in avidity in liver lesions [6-10]. These findings on the analog scan were in line with comparative imaging exams (MRI January and August 2018 – Table 2).
Table 2. Magnetic resonance imaging (MRI) in January and August of 2018
Abdomen MRI (magnetic resonance imaging) January / 2018 |
Abdomen MRI (magnetic resonance imaging) August / 2018 |
This scan revealed a liver with multiple areas of subcapsular resection, multiple heterogeneous hypervascular nodules of the predominately T2 type with the following characteristics:
- In segment IVb with an area of central necrosis measuring 3.1x2.4 cm in.
-In segment VI measuring 2.3x2.2 cm.
-In the subcapsular segment VII, solid, adjacent to the inferior vena cava, measuring 4.0x3.9 cm.
-In segment VIII, measuring 2.5x2.1 cm.
-Intra-aortocaval lymph node enlargement, measuring 2.7x1.3 cm. |
This scan revealed a liver of normal dimensions, showing a relative reduction in the posterior segment of the right lobe and lobulated contours.
Multiple sparse nodular formations with well-demarcated borders were identified throughout the liver parenchyma. The largest nodule was located in segment V, measuring 2.2 cm.
Enlarged lymph nodes were found in the hepatic hilum and portocaval space, with the largest measuring 1.5x1.3 cm. |
The incidence of rectal neuroendocrine tumors has increased in recent decades, in part due to improved research and diagnostic techniques. The majority of cases of rectal neuroendocrine tumors are diagnosed incidentally by screening colonoscopies performed for non-tumor complaints [11-13]. Symptoms of rectal neuroendocrine tumors include hematochezia, tenesmus, abdominal discomfort or pain, changes in bowel habits (diarrhea), and weight loss [14,15]. When metastasis is present, symptomatology may include right upper quadrant pain, lethargy, cachexia, or generalized symptoms of carcinomatosis. Carcinoid syndrome manifests in 10% of patients with hepatic metastatic disease, retroperitoneal disease, or when the primary site is extra-intestinal (bronchi, testis, and ovaries). This syndrome is characterized by flush, diarrhea, bronchospasm, heart valve injury, and pellagra. Five-year survival in localized disease is 90%, while metastatic disease has a limited prognosis, with survival of approximately 24% [16,17].
Only about 20% of patients are diagnosed at a stage that allows therapeutic planning at the time of diagnosis with curative intent. This late diagnosis is because neuroendocrine tumors are initially asymptomatic or present symptoms common in other more prevalent pathologies. Therefore, neuroendocrine tumors are mostly diagnosed in advanced stages, implying metastases and/or unresectable tumors [11,14]. In the case of unresectable primary and/or metastatic disease, the therapeutic approach is most often palliative, with therapeutic options including embolization, somatostatin analogs, interventional radiology, chemotherapy, and peptide receptor radionuclide therapy. Ideal candidates for peptide receptor radionuclide therapy are people with well- or moderately-differentiated gastrointestinal and pulmonary neuroendocrine tumors with progressing unresectable and/or metastatic disease [6,10].
Well-differentiated neuroendocrine tumors have overexpressed somatostatin receptors, mainly of subtype 2 (sstr2). Nuclear medicine uses somatostatin analogs with an affinity for these chelated-bound receptors (DOTA) and radionuclide radiators with diagnostic (y or B +) or therapeutic (B-) application. 68-Ga-DOTA is the radiopharmaceutical commonly used for PET for diagnostic purposes; 68-Ga-DOTA is also used to evaluate the expression of somatostatin receptor by tumor lesions. 68-Ga-DOTA is made up of an emitting radionuclide B + (Ga) with an affinity for sstr2, sstr3, and sstr5. 177-Lutecio-DOTATE is a radiopharmaceutical consisting of a radionuclide B- (Lu 177), bound to a somatostatin analog (TATE) with an sstr2 affinity. The drug is administered intravenously to provide a cytotoxic dose to the tumor cells that express these receptors. Thus, somatostatin receptor expression in tumor cells is a predictive factor of favorable therapeutic response. However, sstr2 expression must be confirmed through an octreotide scan or PET-CT before administering peptide receptor radionuclide therapy [7-9].
177-Lutecio-DOTATE therapy has an impact on patients' quality of life, improves symptoms, and improves survival. It does this by causing a reduction in tumor size, total (8%) or partial (21 to 50%) disease remission, and disease stabilization (35-52%). This response is satisfactory, as most patients receiving 177-Lutecio-DOTATE treatment are in advanced stages of the disease or have previously failed other types of attempted therapy [13,18].
The principal side effect of 177-Lutecio-DOTATE is nephrotoxicity. The kidneys are what limit the medication’s dosage because they retain peptides in the renal cortex; this thus leads to a highly absorbed dose which results in toxicity. Patients treated with 177-Lutecio-DOTATE are scheduled to receive three to four cycles of medication based on a calculated maximum tolerated kidney dose of 27 Gy. Therefore, monitoring of renal function is required before, during, and after treatment to prevent and manage adverse effects. Monitoring also helps to protect the kidneys through amino acid infusions (lysine and arginine) that inhibit radiopharmaceutical resorption in the proximal contorted tubules. Under these conditions, the risk of severe nephrotoxicity is low. Other known side effects of 177-Lutecio-DOTATE include nausea (in 30% of all users), vomiting (in 10% of all users), and abdominal pain (in 6% of all users); these effects are all transient during the treatment cycle. Most cases present low myelotoxicity; however, when present, laboratory follow-up before and after each cycle is required [19,20].
This article describes a clinical case of a patient with a neuroendocrine rectal tumor initially treated with surgery and chemotherapy. After two years in remission, the patient presented with hepatic recurrence and underwent hepatic segmentectomies and a posterior embolization with cycles of Octreotide. However, the embolizations had to be interrupted because of the absence of appropriate intrahepatic arteries. The patient was then referred for treatment with 177-Lutecio-DOTATE after obtaining the results of an abnormal PET-CT. The 68-Gallium PET-CT showed the presence of multiple areas of hyperconcentration in the areas above the thorax and abdomen; these areas were more evident in the upper abdomen and related to neuroendocrine neoplastic tissue. The patient commenced the proposed treatment with a previous infusion of renal protection factors (amino acids L-lysine and L-arginine) and, after two cycles of medication, entered into regression of this metastatic disease.
Neuroendocrine tumors are a rare group of neoplasms that can culminate in a variety of prognoses. Neuroendocrine tumors of the rectum are no exception, as they can have a broad spectrum of clinical evolution. Despite increasing evidence about the usefulness of 177-Lutecio-DOTATATE in the treatment of neuroendocrine tumors, it is still an infrequently administered therapy due to both the rarity of the disease and the therapy’s limited availability. However, this medication has emerged as a promising tool for the management of patients with inoperable or metastatic neuroendocrine tumors, as it leads to remission and stabilization of the disease. Thus, it is an effective therapy that increases in survival and quality of life of patients.
- Oberg K, Knigg U, Kwekkeboom D, Perren A (2012) Neuroendocrine gastroenteropancreatic tumors: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23: 124-130.
- Rodrigues A, Castro-Pocas F, Pedroto I (2015) Neuroendocrine rectal tumors: main features and management. Portuguese Journal of Gastroenterology 22: 5.
- Guimaraes N (2014) Neuroendocrine tumors of the rectum: from diagnosis to therapy. Abel Salazar Institute of Biomedical Sciences - University of Porto p: 1-26, 2014.
- Kong G, Grozinsky-Glasberg S, Hofman MS, Akhurst T, Meirovitz A, et al. (2019) Highly favourable outcomes with peptide receptor radionuclide therapy (PRRT) for metastatic rectal neuroendocrine neoplasia (NEN). Eur J Nucl Med Mol Imaging 46: 718-727. [Crossref]
- KIM SJ, Pak K, Koo PJ, Kwak JJ, Chang S (2015) The efficacy of 177Lu-labelled peptide receptor radionuclide therapy in patients with neuroendocrine tumors: a meta-analysis. Eur J Nucl Med Mol Imaging 42: 1964-70.
- Bodei L, Pepe G, Paganelli G (2010) Peptide receptor radionuclide therapy (PRRT) of neuroendocrine tumors with somatostatin analogues. Eur Rev Med Pharmaco 14: 347-51.
- COSTA AFE (2014) Nuclear medicine treatment of neoruendocrine tumors: Systematic review of efficacy and safety of DOTATE-177Lu radiopharmaceutical. Interdisciplinary Journal of Experimental Studies 6: 15-21.
- Pencharz D, Walker M, Yalchin M, Quigley AM, Caplin M, et al. (2017) Early efficacy of and toxicity from lutetium-177-DOTATATE treatment in patients with progressive metastatic NET. Nucl Med Commun 38: 593-600.
- Forrer F, Uusijärvi H, Storch D, Maecke HR, Mueller-Brand J (2019) Treatment with 177Lu-DOTATOC of Patients with Relapse of Neuroendocrine Tumors After Treatment with 90Y-DOTATOC. The Journal of Nuclear Medicine 46: 1310-1316.
- Wang L, Tang K, Zhang Q, Li H, Wen Z, et al. (2013) Somatostatin Receptor-Based Molecular Imaging and therapy for Neuroendocrine Tumors. Biomed Res Int p. 2013.
- Silva TN, van Velthuysen MLF, H J van Eijck C, Teunissen JJ, Hofland J, et al. (2018) Successful neoadjuvant peptide receptor radionuclide therapy for an inoperable pancreatic neuroendocrine tumour. Endocrinology, Diabetes & Metabolism Case Reports p. 2018.
- Schuffner ROA (2012) Carcinoma neuroendócrino de pequenas células de laringe: relato de caso e revisão de literatura. Arquivos Catarinenses de Medicina 41: 85-88.
- Garkavij M, Nickel M, Sjögreen-Gleisner K, Ljungberg M, Ohlsson T, et al. (2010) 177Lu-[DOTA0,Tyr3] octreotate therapy in patients with disseminated neuroendocrine tumors: Analysis of dosimetry with impact on future therapeutic strategy. Cancer 116: 1084-1092.
- Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, et al. (2012) ENETS Consensus Guidelines for the Management of Patients with Liver and Other Distant Metastases from Neuroendocrine Neoplasms of Foregut, Midgut, Hindgut, and Unknown Primary. Neuroendocrinology 95:157-176.
- Caplin M, Sundin A, Nillson O, Baum RP, Klose KJ, et al. (2012) ENETS Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Neoplasms: Colorectal Neuroendocrine Neoplasms. Neuroendocrinology 95: 88-97.
- Jernman J, Välimäki MJ, Louhimo J, Haglund C, Arola J (2012) The Novel WHO 2010 Classification for Gastrointestinal Neuroendocrine Tumours Correlates Well with the Metastatic Potential of Rectal Neuroendocrine Tumors. Neuroendocrinology 95: 317-324.
- Weinstock B, Harpaz N, Warner RRP, Itzkowitz S, Kim M (2013) Clinical and Prognostic Features of Rectal Neuroendocrine Tumors. Neuroendocrinology 98: 180-187.
- Ezziddin S, Attassi M, Yong-Hing CJ, Ahmadzadehfar H, Willinek W, et al. (2014) Predictors of Long-Term Outcome in Patients with WellDifferentiated Gastroenteropancreatic Neuroendocrine Tumors After Peptide Receptor Radionuclide Therapy with 177Lu-Octreotate. J Nucl Med 55: 183-190. [Crossref]
- Skoura E, Michopoulou S, Mohmaduvesh M, Panagiotidis E, Al Harbi M, et al. (2016) The Impact of 68Ga-DOTATATE PET/CT Imaging on Management of Patients with Neuroendocrine Tumors: Experience from a National Referral Center in the United Kingdom. The Journal Nuclear of Medicine 57: 34-40.
- Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, et al. (2011) Peptide receptor radionuclide therapy with 177Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol I 38: 2125-2135.