Introduction: Type II endometrial cancers (EC), mainly including Carcinosarcoma (C), Serous Papillary (SP) and Clear Cell (CC) carcinomas, are considered at high risk for advanced stage of disease and relapse. In type I-II endometrial carcinoma altogether, 23% of preoperative early clinical stage is expected be upstaged with surgical staging. We evaluated upstaging and downstaging in type II endometrial cancer by subgroups histotypes (C, SP and CC) to highlight possible differences.
Materials and methods: In this retrospective study from January 2010 to June 2018, we retrieved data of all patients (pts) submitted to primary surgical treatment for type II endometrial cancer. Inclusion criteria: C, SP and CC carcinoma of endometrium; laparoscopic radical type A/B hysterectomy, bilateral salpingo-oophorectomy, omentectomy; preoperative contrast enhanced CT-scan within 4 weeks before surgery. Exclusion criteria type I, mucinous, neuroendocrine and undifferentiated carcinoma histotype; previous malignant disease and neoadjuvant chemotherapy or preoperative radiation.
Primary Outcomes to compare distribution of provisional preoperative clinical stages by CT scan versus distribution of surgical staging. Secondary outcome: If surgical staging influenced adjuvant treatment in the study population in comparison with CT scan provisional stage.
Results: Overall 90 eligible pts including 28 (31%) C, 44 (49%) SP and 18 (20%) CC. Only 12% of pts presented pelvic nodal metastases (Pe N+) at CT scan. In overall C, SP and CC surgical upstaging, in comparison with provisional stage by CT scan, was the same of literature (24%) but in the analysis by subgroup it was 36% in C, 25% in SP and only 5% in CC histotype. On the basis of CT scan alone, due to the low accuracy of this technique for myometrial invasion, all patients indiscriminately would have recommended for CT plus EBRT.
Conclusion: Provisional staging by CT scan confirms to be inaccurate in C and SP but could be trustworthy in CC histotype. In type II EC, only in provisional stage I by CT scan, surgical staging helped to tailor adjuvant treatment avoiding useless aggressive chemotherapy.
type II endometrial cancer, carcinosarcoma, Clear Cell carcinoma, Serous Papillary carcinomas, Computed tomography, surgical staging, clinical stage
Abbreviations
CT: contrast enhanced Computerised Tomography
EBRT: external beam radio therapy
BRACHY: brachytherapy alone
LVS: lymph vascular space
BSO: hysterectomy and Bilateral Salpingo-Oophorectomy
Pe LA: Pelvic Lymphadenectomy
Pe N: Pelvic Nodes
FIGO: International Federation of Gynaecology and Obstetrics
Type II endometrial cancers, mainly including Carcinosarcomas, Serous Papillary and Clear Cells carcinomas, occur in 15% of patients with malignancies of endometrium [1-5]. This kind of cancers are considered at high risk for advanced stage of disease at presentation and for relapse. In type I and type II endometrial carcinoma altogether, clinical staging alone is considered inadequate, as 23% of preoperative clinical stage I-II patients is expected be upstaged with extensive surgical staging [6].
Several imaging techniques have been used as diagnostic tools for preoperative staging of endometrial cancer, such as: transvaginal ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and more recently Positron emission tomography (PET), PET/CT, and PET/MRI [7-14]. Currently, MRI imaging is the most widely used modality for preoperative local staging, while CT scan is used in all level centres mainly in the assessment of nodal and distant metastases [7].
Several studies already investigated preoperative clinical staging by CT scan in the initial assessment of endometrial cancer [8-10]. However, all these reports either did not distinguish between various endometrial cancer subtypes or included only few types II altogether with type I histotypes. Actually, the three most common subtypes included in type II endometrial carcinoma are heterogeneous. For example, an emerging theory suggests that Clear Cell endometrial carcinoma should be regarded as a Type I, instead of type II endometrial cancer, because it has similar immunohistochemical features with endometrioid adenocarcinoma [15,16]. On the other side, carcinosarcoma of the uterus, could prefer the haematologic way of dissemination while the serous papillary uterine carcinoma could reproduce the intraperitoneal dissemination like the ovarian serous papillary carcinoma.
Therefore, we decided to evaluate the percentage of upstaging and downstaging in type II endometrial cancer any stage by each most common subgroups histotypes (carcinosarcoma, serous papillary and clear cell carcinoma) in order to highlight the possible differences among the three groups.
We retrieved retrospectively the clinical, surgical and pathological data from the prospectively maintained database of the Department of Anatomy and of Multidisciplinary Meeting of Gynaecologic Oncologic Unit of the Oxford University Hospital (tertiary referral hospital). From January 2010 to June 2018, all patients collected underwent preoperative contrast enhanced CT scanning and were therefore submitted to primary surgical treatment for type II endometrial cancer.
Inclusion criteria were as follow: histotype including carcinosarcoma, serous papillary and clear cell carcinoma of the endometrium; surgery including laparoscopic radical type A/B hysterectomy, bilateral salpingo-oophorectomy, total or subtotal omentectomy; preoperative imaging contrast enhanced CT-scan within 4 weeks before surgery discussed and reported in the weekly local multi-disciplinary team (MDT) meeting.
Exclusion criteria were: endometrial endometrioid, mucinous, neuroendocrine and undifferentiated carcinoma histotype; previous diagnosis of another malignant disease and primary treatment other than surgery (neoadjuvant chemotherapy or preoperative radiation). This study protocol was approved by the institutional review board. All patients received informed consent after which verbal and written consent was obtained.
Image analysis and interpretation: CT scans were performed on GE Lightspeed helical scanners (GE Medical Systems). Images were reconstructed at 5-mm intervals. Images from all CT scan were sent to a picture archiving and communication system, PACS (Centricity, GE Healthcare) for interpretation. Imaging findings regarding cervical uterine involvement and corpus uteri serosal extension were assessed as either present or absent. Imaging features of extra-uterine dissemination, such as adnexal involvement, pelvic and/or PA lymphadenopathy, omental, peritoneal implants, and distant metastases, were assessed with a 3-point scale as follows: 1 = no tumour present; 2 = presence of tumour indeterminate/possible; 3 = tumour definitely present. Pelvic lymph nodes were considered enlarged if they measured over 0.8 cm in the short axis, while PA lymph nodes were judged abnormal if they measured more than 1 cm in the short axis. Additionally, a lymph node was considered metastatic regardless of size if it demonstrated central necrosis, heterogeneous contrast enhancement, and/or irregular borders. At the weekly Multidisciplinary Cancer Meeting a dedicated radiologist, expert in gynaecological cancers, reviewed each CT scan preoperatively and the provisional clinical staging, according to the 2009 FIGO criteria, was established and recorded [17].
Primary outcomes: to compare the distribution of provisional preoperative clinical stages by CT scan versus the distribution of surgical staging.
Secondary outcomes: a) The percentage of patients (overall and by each subgroup histotype) in which an additional adjuvant treatment was indicated due to the surgical upstaging in comparison with the treatment planned by the provisional stage by CT scan. b) To compare the rate of patients (overall and by each subgroup histotype) in which the adjuvant chemotherapy and radiotherapy could be avoided thanks to the surgical downstaging.
Outcomes measures: pulmonary, abdominal, pelvic, lymph nodal, parametrial, vagina, cervical and omental metastases at CT scan; at final report disease in uterine serosal, uterine adnexa, uterine cervix, bowel serosa, omentum, pelvic lymph nodes, parametria, vagina, diaphragm and peritoneum. Number of patients in which would be indicated adjuvant chemotherapy and/or radiotherapy and/or brachytherapy. Omental and nodal metastases in the pathology final report were defined microscopic if the size was inferior than 2 mm. After surgery, at the postoperative weekly multidisciplinary meeting the final histology was reviewed by gynaecologic oncologic dedicated pathologists. Adjuvant treatment was considered as well as recommended by the ESMO (European Society for Medical Oncology) / ESGO (European Society of Gynaecological Oncology) / ESTRO (European Society for Radiotherapy & Oncology) consensus conference. For Carcinosarcoma histotype in all stages was recommended adjuvant chemotherapy (CT) and External Beam Radio-Therapy (EBRT) [7]. For Serous Papillary and Clear Cell histotype: in case of myometrial invasion less than 50% and no lymph vascular space (LVS) involvement, adjuvant treatment brachytherapy alone was recommended. In all the stages except IA LVS negative, the consensus conference recommended CT and EBRT [7].
Statistical methods: Clinical parameters were summarized using descriptive statistics. Means, medians, ranges, and standard deviations were used for continuous variables; frequencies with corresponding percentages were used for discrete variables.
We identified overall 90 eligible patients including 28 (31%) Carcinosarcoma, 44 (49%) Serous Papillary carcinoma and 18 (20%) Clear Cell carcinoma. Patient’s median age and surgical characteristics were summarized in Table 1. All patients were submitted to laparoscopic hysterectomy, bilateral salpingo-oophorectomy, subtotal/total omentectomy and pelvic lymphadenectomy. Patients with preoperative suspicious of cervical involvement by disease were submitted to radical hysterectomy type B, instead of type A [16]. Four patients did not undergo lymphadenectomy due to comorbid conditions contraindicating prolonged operative time and/or advanced disease and in four patients were not retrieved lymph nodes at final histology and were excluded from the analysis. Total omentectomy (or subtotal omentectomy in case of obviously abdominal or cervical involvement at the time of surgery) was performed accordingly to the intraoperative examination and decision of surgeon. Overall, among 82 patients submitted to pelvic lymphadenectomy, 10 (12%) presented pelvic nodal metastases at CT scan (one of these patients was stage IV) and 72 did not.
Table 1. Surgical and Histologic characteristics of 90 patients type II endometrial cancer by histologic subgroups
|
carcinosarcomas
(28 pts)
|
serous
cell
(44 pts) |
clear
cell
(18 pts) |
Overall
(90 pts) |
Median age ys (range) |
74 (51-87) |
71 (52-88) |
67 (54-66) |
71(51-88). |
Type A hysterectomy [16]
+ BSO |
27 (97%) |
39 (89%) |
17 (95%) |
83 (92%)
|
Type B hysterectomy [16]
+ BSO |
1 ( 3%) |
5 (11%) |
1 ( 5%) |
7 ( 8%) |
subtotal omentectomy |
13 ( 46%) |
14 (32%) |
2 (11%) |
29 ( 32%) |
total omentectomy |
15 ( 54%) |
30 (68%) |
16 (89%) |
61 ( 68%) |
No. of Pe LA |
26 ( 93%) |
40 (91%) |
16 (89%) |
82 ( 91%) |
Median no. of Pe N
(range)
|
14 (2-33) |
14 (1-33) |
10 (3-24) |
14(2-33) |
No. of pts with Pe N+ |
5/26 (19%) |
9/40 (22%) |
2/16 (12%) |
16/82 (19%) |
Nodal metastases
micro metastases
intracapsular > 2 mm
bulky/extracapsular |
2/5 (40%)
-
3/5 (60%) |
2/9 (22%)
5/9 (56%)
2/9(22%) |
1/2( 50%)
1/2 (50%)
- |
5/16 (31%)
6/16 (38%)
5/16 (31%) |
Lymph vascular Space
positive
negative
unknown |
17/28 (61%)
9/28 (32%)
2/28 ( 7%) |
14/44 (32%)
24/44 (54%)
6/44 (14%) |
8/18 (44%)
7/18 (39%)
3/18 (17%) |
39/90(43%)
40/90 (45%)
11/90 (12%) |
Patients with involved omentum |
3/28 (11%) |
4/44( 9%) |
0 |
7 ( 8%) |
Number of microscopic omental involvement |
2/3(66%) |
4/4(100%) |
0 |
6/7(86%) |
Distribution of provisional preoperative clinical stages by CT scan versus by surgical staging. In Table 2 are detailed the preoperative provisional clinical stage assessed by CT scan and the final surgical stage of study population, overall and by each histologic subgroup, respectively. Around 70% of patients with clear cell and serous papillary histotype showed a confirmed surgical staging, with a lower rate in carcinosarcoma subgroup (61% confirmed surgical stage, see Table 2).We can observe that among all subgroups, Clear Cell histotype showed the lower median age and the lower rate of advanced stage of disease and of nodal metastases at final histology. As expected, Clear cell histotype subgroup showed even the lower rate of surgical upstaging and the higher rate of surgical downstaging in comparison with the other subgroups histotype (Table 2). Of note, no patients with Clear Cell sub-types had omental metastases. In Serous Papillary subgroup, at presentation only 1 patient (2%) was suspected for nodal metastases by CT scan, but at final histology 20% of pelvic nodes showed metastases (Table 2). At final histology, among 7 omental metastases, 6/7 (86%) occurred in radiologically advanced stage of disease. Among 68 patients presenting by CT scan at stage I, only 1 (1.5%) had microscopic omental metastases: other histological findings were myometrial invasion > 50%, cervical surface glandular epithelium involved (but not the cervical stroma), the background endometrium inactive and atrophic, ovarian capsular involvement and extrauterine diseases on the sigmoid appendices (that contraindicated the pelvic lymphadenectomy) and the final histology was Serous Papillary endometrial carcinoma FIGO stage IVB with LVS invasion. Regarding pelvic nodal metastases, we made the calculation on the 82 patients submitted to pelvic lymphadenectomy and, as expected from the data in literature supporting surgical staging in endometrial cancer, overall accuracy of CT scan was very low: 82% (Table 3). However, in the analysis by each subgroup, accuracy of CT scan for pelvic nodal metastases was low only in serous papillary and clear cell while is good in Carcinosarcoma (92%). Out of the 10 patients with nodal metastases at CT scan (one is included in stage IV), only 5/10 (50%) were confirmed at final histology, while out the 72 patients with CT scan negative for pelvic nodal spread of disease, 11 (15%) of them at final histology were found with positive pelvic nodes.
Table 2. Characteristics of 90 patients with type II endometrial cancer by histologic subgroups.
|
Carcinosarcoma
(28 patients) |
Serous Cell
(44 patients) |
Clear Cell
(18 patients) |
Overall
(90 patients) |
CT scan Provisional
FIGO stage
I
II
IIIA-B
IIIC
IVB |
21 (76%)
1 ( 3%)
-
5 (18%)
1 ( 3%) |
36 (82%)
2 ( 4.5%)
3 ( 7%)
1 ( 2%)
2 (4.5%) |
11(63%)
-
1 ( 5%)
3 (16%)
3 (16%) |
68 (76%)
3 ( 3%)
4 ( 4%)
9 (10%)
6 ( 7%) |
Surgical
FIGO stage
I
IA
IB
II
IIIA-B
IIIC1
IVB |
13/28(46%)
11/13(86%)
2/13(14%)
5/28(18%)
3/28(11%)
4/28(14%)
3/28 11%) |
27/44(62%)
23/27(85%)
4/27(15%)
2/44 (4,5%)
2/44 (4,5%)
9/44 (20%)
4/44( 9%) |
12/18 (67%)
9/12 (75%)
3/12 (25%)
1/18 ( 5%)
3/18 (17%)
2/18(11%)
- |
52/90 (58%)
43/52 (83%)
9/52 (17%)
8/90 (9%)
8/90 ( 9%)
15/90 (16%)
7/90 ( 8%) |
Upstaged* |
10/28 (36%) |
11/44(25%) |
1/18( 5%) |
22/90(24%) |
Downstage* |
1/28 ( 3%) |
1/44( 2%) |
5/18(28%) |
7/90 ( 8%) |
Confirmed stage* |
17/28 (61%) |
32/44 (73%) |
12/18(67%) |
61/90 (68%) |
*: At final histology report
Percentage of patients (overall and by each subgroup histotype) in which an additional adjuvant treatment was indicated due to the surgical upstaging in comparison with the treatment planned by the provisional stage by CT scan. Considering the suggestion of ESMO/ESGO/ESTRO consensus conference, CT plus EBRT could be avoided only in serous papillary and clear cell histotype stage IA with LVS negative. On the basis of CT scan alone, due to the low accuracy of this technique for myometrial invasion, all patients would have recommended for CT plus EBRT.
Rate of patients (overall and by each subgroup histotype) in which the adjuvant chemotherapy and radiotherapy could be avoided thanks to the surgical downstaging. In Figure 1 is presented, in overall type II endometrial cancer, the distribution of surgical stages with the corresponding adjuvant treatment suggested by consensus conference ESMO/ESGO/ESTRO in the patients with provisional CT scan stage I. Detailing by each subgroup histotype, for the carcinosarcoma subgroup was recommended CT plus EBRT in all stages, and therefore no differences were reported due to the CT scan provisional stage or the surgical stage. Regarding Serous Papillary and Clear Cell histotypes, in the Figure 2a and 2b is detailed the distribution of surgical stages and the relative adjuvant treatment suggested by ESMO/ESGO/ESTRO conference, in all the patients with provisional CT scan stage I. In Figure 3 is presented among patients with provisional CT scan advanced stage II-IV (any subgroup histotype) the distribution of surgical stages and the corresponding adjuvant treatment: thanks to the surgical downstaging, only one case with clear cell histotype avoided CT plus EBRT.
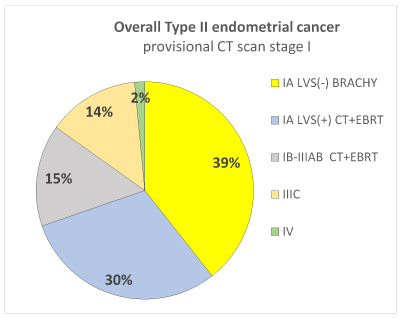
Figure 1. Distribution of surgical stage and corresponded adjuvant treatment among 68 patients with provisional CT scan stage I type II endometrial cancer (carcinosarcoma, serous papillary and clear cell).
Thanks to the surgical staging, overall 39% of patients avoided adjuvant CT + EBRT. Only on the basis of CT scan, which has a low accuracy on nodal and myometrial involvement, all these patients could be counselled for CT plus EBRT.
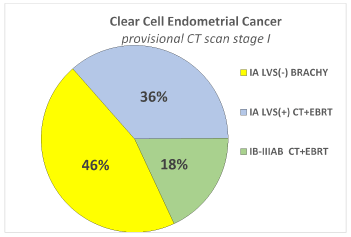
Figure 2a. Distribution of surgical stage and corresponded adjuvant treatment among 11 patients with Clear Cell histotype and provisional preoperative stage I by CT scan.
Thanks to the surgical staging 46% of patients avoided adjuvant CT + EBRT. Only on the basis of CT scan, which has a low accuracy on nodal and myometrial involvement, all these patients could be counselled for CT and EBRT.
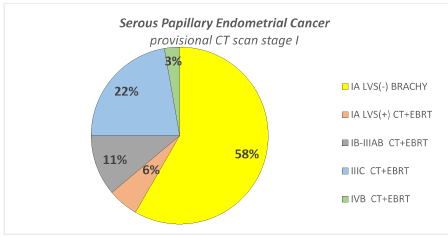
Figure 2b. Distribution of surgical stage and corresponded adjuvant treatment among 36 patients with Serous Papillary histotype and provisional preoperative stage I by CT scan.
Thanks to surgical staging 58% of patients avoided adjuvant CT + EBRT. Only on the basis of CT scan, which has a low accuracy on nodal and myometrial involvement, all these patients could be counselled for CT and EBRT.
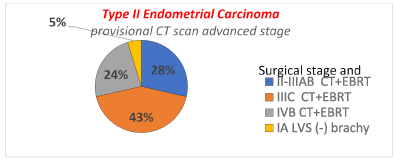
Figure 3. Distribution of surgical stage and corresponded adjuvant treatment among 21 patients with provisional CT scan advanced stage in type II endometrial cancer (carcinosarcoma, serous papillary and clear cell).
Thanks to surgical staging overall only 5% of patients avoided adjuvant CT + EBRT.
In our study on type II endometrial cancer, among 90 patients preoperatively provisional staged by CT scan, overall 22 (24%) of them were surgically upstaged. Considering more in detail by each subgroup histotype, one can note that the rate of upstaging was particularly high in Carcinosarcoma (36%) and in the Serous Papillary (25%) while in Clear Cell the rate of upstaging was very low (5%). In Clear Cell subgroup histotype the rate of down staging was very high (28%). Roughly, we can conclude that approximatively for 70% of patients, the surgical staging will confirm the provisional stage by CT scan and that the Clear Cell subgroup histotype has the higher chances to have the early stage confirmed after surgical treatment. This data can be useful during informed consent before surgery in order to plan the adjuvant treatment. Usually, almost 80% of type I endometrial cancer at presentation are diagnosed at early stage. From many authors, this rate falls to 46% for overall type II endometrial cancers, and to 55-63% in particular for Carcinosarcoma, to 50% for Serous Papillary and to 42% for Clear Cell [18-21]. In our study, unexpectedly, the overall rate of patients with type II endometrial cancer with provisional stage I by CT scan at presentation was 68 (76%), similar to the correspondent figure in type I endometrial cancers. In addition, at final histology, stage I was confirmed in our overall study population in 52 (58%), a rate quite higher than reported in literature for these high risk histotypes. This unexpected result, could be explained by an efficient initial investigation and referral pathway at our Institution taking advantage of the office hysteroscopy plus biopsy and transvaginal ultrasound. Actually, more in detail, in the analysis by subgroups, final histology stage I incidence of Carcinosarcoma corresponded to the literature (49%), while it is for the Serous Papillary Cells and Clear Cells subgroups that final histology stage I incidence was higher than reported by literature, particularly for Clear Cell carcinoma (67% surgical stage I, Table 1). This could be explained by the emerging theory that Clear Cell endometrial carcinoma maybe should be regarded as a Type I, instead of type II endometrial cancer, because its similar immunohistochemical features with endometrioid adenocarcinoma [15]. Reinforcing this theory, in our study, at final histology, no patients with Clear Cell carcinoma had omental metastases at any stage and the incidence of nodal metastases was the lowest among the three histologic subgroups. Unfortunately, notwithstanding the preoperative imaging assessment has been conducted by dedicated gynaecologic oncologic radiologists, it is remarkable that in our series 22 (24%) were surgically up-staged, most of them due to nodal involvement, particularly in Serous Papillary subgroup. This data confirms the low accuracy of CT scan for staging in this high-risk endometrial cancer patients, as already well been established by literature for endometrial cancer, in this case especially for Serous Papillary subtypes. Out of 82 procedures of lymphadenectomies, 11(12%) of patients showed nodal metastases at final histology not detected by CT scan and, out of these, 8/11 (73%) were microscopic (< 2mm) while 3/11 (27%) were bulky, therefore theoretically detectable by CT scan. Nevertheless, analysing by each histologic subgroup, the accuracy of CT scan for nodal metastases, in our study was low in Serous Papillary and Clear Cell subgroups while it was better in Carcinosarcoma subgroup (92%) and unexpectedly favorably comparable with the median values of 91% reported by PET/CT scan in literature [22,23]. This data maybe could be explained by the high rate of bulky nodes in carcinosarcoma subgroup and maybe by the propensity of carcinosarcoma to form bulky nodal metastases (Table 1). Unfortunately, one limitation of this study is that, in our Institution, paraaortic lymphadenectomy was not included routinely in the surgical staging: this was due to the fact that a more extensive lymphadenectomy could have increased the treatment-related morbidity while, in literature, the incidence of solitary aortic nodes metastases is negligible in case of negative pelvic nodes at final histology and the therapeutic value of aortic lymphadenectomy has not yet been proven.
Percentage of patients in which an additional adjuvant treatment was indicated due to the surgical upstaging in comparison with the treatment planned by the provisional stage by CT scan. It is interesting to note that, notwithstanding the higher rate of surgical upstaging compared with CT scan preoperative provisional stage, after all following the consensus conference recommendations, no patients needed additional adjuvant treatment that had not already been planned on the merely basis of CT scan alone.
Rate of patients in which the adjuvant chemotherapy and radiotherapy could be avoided thanks to the surgical downstaging. In type II endometrial cancer provisional stage, I by CT scan, overall 39% of patients avoided CT and EBRT thanks to the surgical staging. More in detail, in serous cell subgroup surprisingly 58% could avoid chemotherapy after surgical treatment. Due to the strict indication to chemotherapy even in early stages of disease, in carcinosarcoma subgroup no patients avoided chemotherapy and EBRT. This result reinforces indication to perform the pelvic lymphadenectomy in type II endometrial cancer because in case of negative nodes, in addition to the absence of the other two prognostic factors (myometrial invasion more than 50% and lymph vascular invasion) allows avoiding chemotherapy and EBRT in a large part of patient at early stage.
In type II endometrial cancer with provisional stage I by CT scan, the surgical staging gives precious additional information that can help to tailor adjuvant treatment in order to avoid useless aggressive chemotherapy regimens. In the patients with provisional advanced stage by CT scan, surgical staging fails to add any additional information. In particular in the carcinosarcoma subgroup histotype the surgical staging did not change adjuvant treatment in any case, due to the homogeneous adjuvant treatment at any stage.
The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article
RG contributed to the study design, the data analysis and wrote the manuscript
HSM performed surgery and revised manuscript
GV, YH, SM, SD contributed to data collection, analysis and wrote the manuscript.
RCG performed the surgery
RT performed the surgery and contributed to study design and the data analysis.
No source of funding
- Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68: 7-30. [Crossref]
- ACOG practice bulletin (2005) Clinical management guidelines for obstetrician-gynecologists, management of endometrial cancer. Obstet Gynecol 106: 413– 425. [Crossref]
- Mendivil A, Schuler KM, Gehrig PA (2009) Non-endometrioid adenocarcinoma of the uterine corpus: a review of selected histological subtypes. Cancer Control 16: 46-52. [Crossref]
- Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, et al. (2006) Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer 94: 642-646. [Crossref]
- Varughese J, Hui P, Lu L, Yu H, Schwartz PE (2011) Clear cell cancer of the uterine corpus: the association of clinicopathologic parameters and treatment on disease progression. J Oncol 2011:628084.
- Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, et al. (1987) Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group study. Cancer 60: 2035-2041. [Crossref]
- Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, et al. (2016) ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-up. Ann Oncol 27: 16-41. [Crossref]
- Boruta DM 2nd, Gehrig PA, Fader AN, Olawaiye AB (2009) Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol 115: 142-153. [Crossref]
- Sorosky JI (2015) Endometrial cancer. Obstet Gynecol 125: 1006-1026. [Crossref]
- SGO Clinical Practice Endometrial Cancer Working Group, Burke WM, Orr J, Leitao M, Salom E, et al. (2014) Endometrial cancer: a review and current management strategies: part I. Gynecol Oncol 134: 385-392. [Crossref]
- Lakhman Y, Katz SS, Goldman DA, Yakar D, Vargas HA, et al. (2016) Diagnostic Performance of Computed Tomography for Preoperative Staging of Patients with Non-endometrioid Carcinomas of the Uterine Corpus. Ann Surg Oncol 23: 1271-1278. [Crossref]
- Connor JP, Andrews JI, Anderson B, Buller RE (2000) Computed tomography in endometrial carcinoma. Obstet Gynecol 95: 692-696. [Crossref]
- Bogani G, Gostout BS, Dowdy SC, Multinu F, Casarin J, et al. (2017) Clinical Utility of Preoperative Computed Tomography in Patients With Endometrial Cancer. Int J Gynecol Cancer 27: 1685-1693. [Crossref]
- Zerbe MJ, Bristow R, Grumbine FC, Montz FJ (2000) Inability of preoperative computed tomography scans to accurately predict the extent of myometrial invasion and extracorporal spread in endometrial cancer. Gynecol Oncol 8: 67-70. [Crossref]
- Bae HS, Kim H, Young Kwon S, Kim KR, Song JY, et al. (2015) Should endometrial clear cell carcinoma be classified as Type II endometrial carcinoma? Int J Gynecol Pathol 34: 74-84. [Crossref]
- Querleu D, Morrow CP (2008) Classification of radical hysterectomy. Lancet Oncol 9: 297–303. [Crossref]
- Pecorelli S (2009) Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 105: 103-104. [Crossref]
- Bakkum-Gamez JN, Gonzalez-Bosquet J, Laack NN, Mariani A, Dowdy SC (2008) Current issues in the management of endometrial cancer. Mayo Clin Proc 83: 97-112. [Crossref]
- Mariani A, Dowdy SC, Keeney GL, Long HJ, Lesnick TG, et al. (2004) High-risk endometrial cancer subgroups: candidates for target-based adjuvant therapy. Gynecol oncol 95: 120-126. [Crossref]
- Abdulfatah E, Sakr S, Thomas S, Al-Wahab Z, Mutch DG, et al. (2017) Clear cell carcinoma of endometrium : evaluation of prognostic parameters I a multi-institutional cohort of 165 cases. Int J Gynecol Cancer 27: 1714-1721. [Crossref]
- Nogami Y, Banno K, Irie H, Iida M, Kisu I, et al. (2015) The efficacy of preoperative positron emission tomography-computed tomography (PET-CT) for detection of lymph node metastasis in cervical and endometrial cancer: clinical and pathological factors influencing it. Jpn J Clin Oncol 45: 26-34. [Crossref]
- Stecco A, Buemi F, Cassara A, Matheoud R, Sacchetti GM, et al. (2016) Comparison of retrospective PET and MRI-DWI (PET/MRI-DWI) image fusion with PET/CT and MRI-DWI in detection of cervical and endometrial cancer lymph node metastases. Radiol Med 121: 537-545. [Crossref]
- Atri M, Zhang Z, Dehdashti F, Lee SI, Marques H, et al. [2017] Utility of PET/CT to Evaluate Retroperitoneal Lymph Node Metastasis in High-Risk Endometrial Cancer: Results of ACRIN 6671/GOG 0233 Trial. Radiology 283: 450-459. [Crossref]




