Substituting sugar (sucrose and related fermentable saccharides) with low-calorie carbohydrates and sugar alcohols constitutes a well-founded approach to controlling energy intake in the prevention of obesity and certain diseases, and to limiting the occurrence of bacteria-associated diseases such as dental caries. The present concise review discusses selected sugar-substitute applications of alditol-type sugar alcohols and disaccharide polyols. In the former category, erythritol (a tetritol) and xylitol (a pentitol) will receive special attention. Xylitol has been shown to exert interesting biochemical effects―such as formation of ammonia and basic amino acids―in dental biofilm (dental plaque). These nitrogen-associated metabolic features have remained unnoticed because data concerning them were published already in the 1970s in supplements to regular journal volumes. Thus, the present review highlights the oral biologic significance of the xylitol-associated shift observed in the biology of oral biofilm: from carbohydrate dominance to one where nitrogen metabolism plays an important role. This review also comments on the synergy between certain oral health adjuvants (e.g., chlorhexidine and fluoride) and alditols, and on the use of alditols in periodontal treatments (i.e., non-sweet applications of alditols), and provides insights to European Union regulations on sugar substitutes. The article also attempts to rectify defective information regarding these sweeteners, including that contained in a recent Cochrane Xylitol Review. A brief discussion on certain physicochemical aspects of alditols will be provided to facilitate an understanding of the oral biological processes involved.
sugar alcohols, alditols, sugar substitution, dental caries, periodontal disease, oral biology
Sugar substitutes are used to reduce energy intake and to prevent coronary heart disease, diabetes, obesity, dental caries, and other conditions. Scientific literature is replete with articles and reviews [1‒3] on sugar substitutes, reflecting the increasing popularity of low-calorie, sugar-free foods. Various non-sweet applications of sweeteners have also been proposed, for example, in periodontal therapy and as dentine primers. The sugar substitutes discussed in the present review include sugar alcohols, among which simple alditols are currently used in numerous medical, cosmetic, techno-chemical, and related applications. Xylitol-based infusion therapy currently comprises one of the most significant single applications of this alditol. Xylitol-containing chewing gums have also been employed in studies related to cognitive function, mastication, drug delivery, physiologic tests, and others [4].
Researchers and authorities involved in the promotion of health should be aware of the different dietary applications of sugar substitutes and of the advent of new sweeteners. Some alditol-type sugar substitutes, such as xylitol, provide only about 2.4 kcal/g of sweetener, whereas the energy available to the human body from erythritol is virtually zero, as compared to the approximately 4 kcal/g for sucrose (“sugar”). Owing to the lower sweetness of D-arabitol and ribitol, these alditols have received less attention in food applications, although both pentitols play important roles in various biologic processes.
The objective of this article is to discuss selected developments in the study of alditol-type sugar substitutes. This review will also re-evaluate previous data that have escaped the attention of current investigators, and rectify previous reviews. In the former case, certain biochemical plaque effects of xylitol must be revisited, while in the latter, a recent Cochrane Review [5] on the dental effects of xylitol serves as an example.
Understanding alditol-associated oral biologic effects requires referring to physicochemical properties of alditols, which have been reviewed in detail previously [6]. Owing to current interest in erythritol (a tetritol) and xylitol (a pentitol), these alditols will receive more attention. Finally, a brief discussion will examine carbohydrates with deviating configuration.
The validity of some study designs may be called into question because of possible synergy between fluoride and xylitol effects on the cells of mutans streptococci (MS) [7]. An earlier study showed that xylitol augmented the metabolic effects on MS of low levels of fluoride [8]. Petin et al. [9] designed a mathematical model to describe, optimize, and predict a synergistic interaction between fluoride and xylitol on acid production by MS. These considerations receive support from an earlier proposal that the cells of MS possess at least two glucose transport systems, one of which is fluoride-insensitive [10]. Later studies reported that xylitol prolonged the effect of chlorhexidine therapy on MS [11]. Chlorhexidine and xylitol appeared to act synergistically on Streptococcus sanguis and MS [12]. Synergistic inhibition of streptococcal biofilm by D-ribose and xylitol has been reported [13]. In another field of dentistry, Han et al. [14] showed that xylitol inhibits inflammatory cytokine expression induced by Porphyromonas gingivalis. This organism may eventually be involved in surprisingly extensive array of conditions, including various general diseases and infertility problems.
Synergy between xylitol and erythritol may also have been involved in their effects on the growth of oral bacteria [15,16]; the mechanism of the inhibitory effect of erythritol versus xylitol on the growth of MS is different. It is possible that their combinations are effective in caries limitation. Meurman [17], in his study on synergy between chlorhexidine and fluoride, referred to one Norwegian and one Dutch study whose authors concluded that the synergy between fluoride and xylitol was a true phenomenon in the oral cavity. Combinations of chlorhexidine and xylitol were suggested to be of value in maternal post-partum caries therapy [18]. Xylitol and fluoride had an additive effect in the reduction of dental erosion in vitro [19], and xylitol and funoran (a sulphated polysaccharide present in certain seaweeds such as Cloiopeltis furcata) had a similar effect in the promotion of tooth remineralization [20].
“Blind” reliance on the crossover study designs employed in clinical trials with sweeteners may invalidate their conclusions. This stems from the reported long-term effects of xylitol (reviewed in [6]). In studies comparing sugar alcohols, the crossover requirement calls for changing the regimens of the study cohorts following treatments of suitable duration. In case the substances tested exert long-term effects, no washout period will completely nullify the effects of the previous treatment; the effects may overlap. Reservations against such blind reliance thus originate in observations that xylitol, antibiotic agents, and fluoride may exert long-term effects on oral bacteria. Such instances call into question the appropriateness of the washout periods between treatments.
Not all dental xylitol studies have reached positive clinical and oral biologic findings. Long-term field experience has shown that in most cases, failure to demonstrate such effects can be explained in terms of the following features of the studies in question:
- Use of caries-resistant study cohorts or cohorts with extremely low caries experience. In these cases, the study subjects are not “sufficiently sick”; they are not caries-prone.
- Use of too-small study cohorts. This means that although the intervention causes differences between study groups, the small number of subjects does not make the differences statistically significant. The direction is “correct”, but the results have no statistical power. The significance of using large enough study cohorts was exemplified by the Costa Rica toothpaste trials: the 10% and 12.3% differences (in favor of xylitol-containing products) were statistically significant owing to the use of more than 2,000 subjects [21,22].
- Use of too-low concentrations of xylitol in experimental products, or use of too-small daily consumption levels of xylitol. The earlier 5 to 7 g per day recommendations of the present author may have been too conservative, while 10 to 15 g per day xylitol levels are currently regarded as preferable. A recent study by Campus et al. [23] used 11.6 g of xylitol with good results.
- Use of too-short intervention; low caries experience presumes longer intervention.
- Use of too-short or too-infrequent daily exposure to xylitol. Instead of three chewing episodes per day―which has been the case in several clinical chewing gum trials―the present author recommends 5 (minimum) to 8 daily episodes.
- Simultaneous use of other caries-limiting agents and strategies (e.g. fluorides).
- Use of too-insensitive analytical or diagnostic procedures.
- Inadequate compliance of subjects and/or families.
- Use of a single analytical procedure (e.g. total protein or nitrogen assay) to assess the growth of dental plaque. Ideally, gravimetry, clinical, microbiological, biochemical, and other methods are used simultaneously (vide infra).
Owing to the above requirements, it is too early to conduct meta-analyses on sugar alcohols and dental caries; the number of trials that meet all requirements of desk theorists is currently too small. Thus, the recent conclusions by Marghalani et al. [24], judging the caries-preventive action of xylitol uncertain, were too hasty. A candid and scientifically balanced analysis should also compare xylitol with sucrose and include the successful mother-child trials on xylitol [25‒27]. It is a pity that these types of negative analyses are being carried out in haste, when all efforts should be directed at making noncariogenic sucrose substitutes available to infants and juveniles. Meta-analyses receive further comment in a paragraph that deals with a recent Cochrane Xylitol Review [6] (vide infra).
Gum chewing and cognition have been the subject of some recent studies [4,28]. While the robustness of reported effects of gum chewing on cognition have been questioned, gum chewing has been unequivocally shown to reduce gastric acidity; the first results of this nature were published already in 1946 [29].
Studies on the effect of sweeteners on dental biofilm (dental plaque) are necessary surrogate investigations of long-term, expensive clinical trials; the quantity and quality of dental biofilm normally reflect its cariogenic potential. Plaque studies can lead to misinformation unless the entire, complex biochemistry of the biofilm is considered. In some studies, plaque quantification has been based on its nitrogen or protein content―a measure that should never be exploited in plaque mass assessments. During xylitol consumption, the levels of protein and nitrogen present in plaque increase owing to biochemical expedience. In the presence of xylitol, and when the microorganisms are deprived of their normal six-carbon-based energy sources (e.g. glucose), plaque-forming oral bacteria increase their overall nitrogen metabolism and the formation of ammonia, urea, and free amino acids, and induce the liberation of increased amounts of proteolytic enzymes [30‒33].
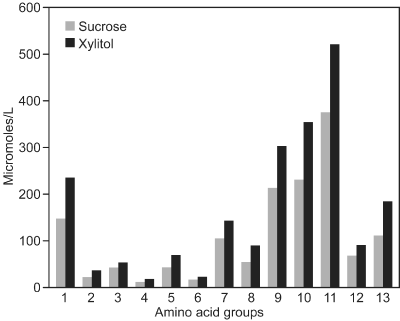
The xylitol-associated increase in the levels of free amino acids and ammonia was for the first time demonstrated in the whole-mouth saliva of subjects who were fed large quantities of xylitol (approximately 67f g daily) over a period of two years [32‒35]. The ammonia levels in the xylitol group were 46% higher compared with the group receiving no xylitol. Amino acid analyses of whole-mouth saliva of the same subjects showed that the consumption of xylitol was associated with increased amounts of amino acids in saliva, regardless of the chemical type of amino acid involved (Figure 1). The levels of basic amino acids (e.g. arginine, histidine, and lysine) increased remarkably. The increase in serine and threonine levels was also remarkable. The xylitol-associated increase in whole-mouth saliva ammonia levels during xylitol consumption is also depicted in Table 1: 60-day consumption of relatively small amounts of xylitol increased the ammonia levels significantly regardless of the way the ammonia levels were calculated. It has been suggested that the elevated ammonia and amino acid levels may partly counteract the reduction of pH values in the dental plaque interface. It is possible that such biochemical reactions in dental biofilm contribute to the reduced cariogenicity of the xylitol diet.
Table 1: Evidence for the xylitol-associated increase in whole-mouth saliva ammonia levels. Human subjects consumed, on average, 20g xylitol daily in the form of chewable troches (99.9% xylitol and 0.1% sodium stearate) after main meals. Whole-mouth saliva was collected by paraffin-stimulation at baseline, and after 30 and 60 days.
Ammonia, mean±SD n = 14 |
Beginning |
30 days |
60 days |
µmol/ml saliva |
3.7 ±1.1 |
5.0 ± 2.5* |
5.3 ± 1.8** |
µmol/mg protein |
3.2 ± 1.2 |
3.9 ±1.7 |
4.1 ± 1.3º |
Ammonia, pooled samples of 14 subjects |
|
|
|
µmol/ml saliva |
2.6 |
2.8 |
5.4 |
µmol/mg protein |
1.6 |
1.8 |
3.7 |
ºApproaches significance; * p<0.05; ** p<0.01. The comparisons were made with baseline
values. Adapted from Pakkala et al. [35].
At the same time, an increase occurred in overall carbohydrate metabolism, including the activity levels of invertase-sucrase enzymes [6,32‒34]. These enzymes may be regarded as caries markers, since the reaction products of these enzymes are acid-forming sugars. However, from the cariologic point of view, the most important properties of plaque―its quantity, volume, and adhesiveness―decrease simultaneously as ammonia levels increase. Therefore, although plaque protein and nitrogen assays are excellent methods in the characterization of plaque chemistry, such procedures cannot be used to evaluate the mass, volume, or cariogenicity of oral biofilm. Instead, a combination of gravimetry (of fresh plaque), clinical plaque indices, and use of disclosing stains with photography, microbiologic MS tests, and related procedures should be applied simultaneously [6].
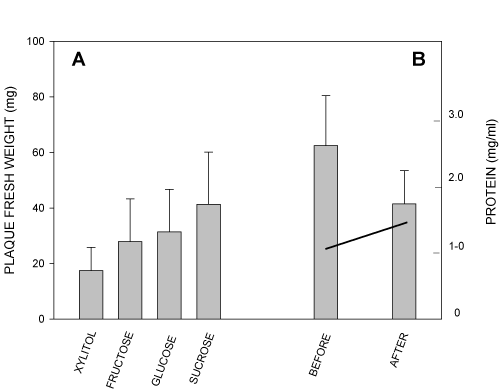
The above situation is graphically depicted in Figure 2 (left panel), which offers a historic view of the effects of dietary sweeteners on the quantity of dental plaque [30]. Consequently, this example warns against using a single chemical method, though impeccable per se, to quantify dental plaque. Sugar substitutes can have remarkable effects on oral biology, such as the balance between carbohydrate and nitrogen metabolism of dental biofilm. For example, storing cells of MS in the presence of 0.25% xylitol resulted in an up to ten-fold increase in overall extracellular proteolytic activity of the cells compared with storage in 0.25% glucose [31]. These observations on ammonia, amino acids, proteolytic activity, and sucrose-splitting enzymes have passed unnoticed, since most data were published in supplements to regular journal volumes in the 1970s. Revisiting these early observations is therefore justifiable. Although other polyols were not tested, it is possible that other non-glucose polyols exert similar effects on dental plaque.
Studies carried out with dental biofilm have resulted in at least two internationally significant resolutions. On April 27, 2009, a European Union Scientific Panel on the substantiation of health claims related to sugar-free chewing gum approved the following claim: “Chewing gum sweetened with 100% xylitol has been shown to reduce dental plaque. High content/level of dental plaque is a risk factor in the development of caries in children”. The International Association of Paediatric Dentistry (IAPD) published the following resolution: “Policy on the Use of Xylitol in Caries Prevention is intended to assist oral health-care professionals make informed decisions about the use of xylitol-based products in caries prevention”.
Some caloric sweetening agents have gained use in applications where the sweetness of the molecule plays no or only a minor role. Traditional subgingival root scaling with hand tools has been regarded as technically demanding. Air-polishing with erythritol powder can reduce tissue loss on root surfaces while causing less pain for patients [36‒39]. Erythritol can also be used with chlorhexidine [39]. Erythritol powder provided comparable or better results than the frequently used glycine powder. Erythritol-treated tooth sites were less frequently positive for Aggregatibacter actinomycetemcomitans [38]. The non-cariogenicity and sweetness of erythritol may offer additional benefits in these treatments.
Erythritol, xylitol, and D-glucitol were used as dentine primers [40]. Contraction gap formation was completely prevented in aqueous solutions of 37.5% erythritol; alditols are always used at very high concentrations in this type of research. Ethylene glycol was most effective. Earlier studies showed that esterification of methacrylate with erythritol prevented the formation of a contraction gap by a commercial light-activated resin composite [41]. Xylitol‒farnesol combinations can be of value in root-canal rinsing. In a study investigating the biocompatibility of dental restorative materials, ascorbic acid increased, in a dose-dependent manner, the toxic effects of most of the restorative materials tested [42]. However, D-mannitol was found to neutralize the toxicity of ascorbic acid.
Xylitol was successfully tested in a nasal spray for the alleviation of cystic fibrosis conditions [43], middle-ear infections, asthma, sinus infections, and infections of the upper respiratory tract [44]. In the case of cystic fibrosis, xylitol reduced the salt concentration of airway surface liquid, and was suggested to enhance bacterial killing. These and other medical uses of xylitol have been summarized elsewhere [45,46]. The first use of xylitol in the prevention of otitis media in children was implemented by the team of Uhari [47], spurred by the Turku Sugar Studies showing xylitol to reduce the growth of MS [31‒34].
In another medical field, early findings such as those of Smith et al. [29] more than 70 years ago, showing gum chewing to reduce gastric acidity, may speak to the advantages of the use of sugar-free chewing gum as a versatile approach which has found support in later gastric fluid studies, e.g. by Schoenfelder et al. [48].
The carbohydrate portion of the human diet is normally based on D-sugars, such as D-glucose and D-fructose. The use of D and L is based on the configurational differences between monosaccharides. Such sugars are not necessarily dextrorotatory and levorotatory. Therefore, D and L do not designate optical rotational properties. Aldoses and ketoses of the L series are mirror images of their D counterparts. If the sign of rotation of a specific monosaccharide is to be included in naming the compound, it is designated by the italic letters d and l, or by (+) and (‒). This particular nomenclature detail must be emphasized, since the literature on sweeteners has included papers on “levorotary” sugars, leaving readers uncertain as to whether configurational differences or true rotationary properties (i.e., the ability to rotate the plane of polarized light) were meant. L sugars are found in nature, but they are not as abundant as D-sugars. The L-forms may interfere with metabolic reactions involving D-sugars. This possibility must have constituted the impetus for a recent study on “levorotatory" carbohydrates and xylitol [49] which were advocated as potential agents for controlling dietary oral biofilms. The authors regarded their test compounds as enantiomers, a term which indicates configurational differences, not rotatory ones. In any case, adhesion of the cells of S. mutans and Candida albicans was subdued in the presence of xylitol and certain L-sugars, notably L-glucose and L-mannose. This line of research is well-grounded and suggests that certain L-sugars may have potential uses in dental applications.
In spite of the possible advent of L-sugars in dentally relevant consumer products, new information on “old” D-sugars, such as D-tagatose and D-psicose, continues to emerge. Both occur in small amounts in natural products and have been promoted as tooth-friendly dietary sweeteners. The United States Food and Drug Administration (FDA) regards each as a safe dietary ingredient.
Erythritol is currently approved and marketed in more than sixty countries world-wide [15]. This level of authorization implies the oral safety of erythritol, as demonstrated in toxicological tests in experimental animals and humans. The recent dental erythritol trial in Estonia [50] stemmed from earlier animal studies [51] and prior theoretical consideration of dietary alditols as potential sugar surrogates [6,46]. At the same time, erythritol was shown to decrease the adherence of polysaccharide-forming oral streptococci [52]: adherence of cells onto glass surfaces declined in the presence of 0.13 mol/L (2%) and 0.26 mol/L (4%) erythritol and xylitol. A Chinese study suggested that, compared with xylitol, erythritol in low concentrations had a weaker effect on bacterial growth and acid production of S. mutans, while having a stronger effect at high concentrations [53]; the low concentrations ranged from 0.5% to 2%, while the high concentrations ranged from 8% to 16%.
Erythritol at 10% had an inhibitory effect on the metabolomic profiles and microstructure of biofilm composed of Porphyromonas gingivalis and S. gordonii [54]. The most effective reagent to reduce the substrata of these organisms was erythritol, compared with xylitol and D-glucitol (sorbitol). It was suggested that erythritol functions via several pathways. These include suppression of bacterial growth resulting from DNA and RNA depletion, attenuated extracellular matrix production, and alterations of dipeptide acquisition and amino acid metabolism.
The above-mentioned [50] school program revealed that a lower number of dentin caries teeth and surfaces was found in the erythritol group than in the xylitol or sorbitol groups. The time it took for caries lesions to develop was longest in the erythritol group. The study featured certain drawbacks: use of polyol candies within a relatively short 5-h period daily, restriction of the number of sucking episodes to three per day, and use of the test items on only about 200 days/year. Tests on dental plaque and whole-mouth saliva suggested, however, that erythritol reduced their cariogenic potential.
Duane [55] reviewed a study of another group of researchers [56] and stated that there was no evidence of caries reduction in a school program with xylitol and erythritol lozenges. However, the subjects used the test items only three times a day, the overall consumption level of xylitol being 4.7 g (with 4.6 g maltitol), and the daily erythritol level being 4.5 g (with 4.2 g maltitol). Both the frequency of use and the amount of xylitol and erythritol seemed to be too low in this child cohort. The study subjects lived in a fluoridated area and exhibited low caries activity. The intervention lasted only 9 to 21 months (the final caries diagnoses were made 48 months after the start of the program). These study features call into question the conclusions [55]. The shortcomings resulted largely from the local conditions at the study site.
The nonprofit organization Cochrane has been regarded as a leader in evidence-based medicine― systematic Cochrane Reviews are conducted in accordance with the organization's widely recognized handbook. A Cochrane Review entitled Xylitol-containing products for preventing dental caries in children and adults [5] was published in 2015 and attracted attention in the media. These Reviews are generally top quality and often provide thoroughly analyzed information on research papers in a particular scientific discipline. Unfortunately, in this 2015 instance, the opposite was true, as shown below.
● The Review included a total of ten xylitol-related dental studies. In five of them, the daily xylitol doses were significantly smaller than the recommended lowest amounts found in several studies to be caries-limiting. Five- to ten-gram daily consumption has been frequently recommended for adults. (The present author currently recommends 10- to 15-gram daily consumption levels for adults and older children―i.e., seven years and older―and 3- to 10-gram levels for younger children, depending on age. In Finland, supervised xylitol use has become routine practice in families and at numerous public and private day-care centers; infants have started receiving xylitol products before the age of two years.)
● Three of the Cochrane Review trials used xylitol toothpaste. Such products normally provide only negligible amounts of xylitol for caries prevention. Toothpaste studies should never be compared with oral consumption of xylitol, such as use of chewable gums and troches. In such trials comparisons can be made between different toothpaste brands only.
● The Review listed two dentifrice trials carried out in Costa Rica [21,22]. Neither specified the xylitol dosage used. Two questionable customs were involved: 1) An objective appraisal should not have included studies that failed to reveal the amounts of xylitol used; and 2) The editors and reviewers involved should have demanded publication of those amounts. (The present author's written attempts to query these points have remained unanswered.)
● One of the remaining five studies used adults while four studies employed children. One study was carried out in children with excellent dental health. In such cases, it is impossible to observe any xylitol effect, or the effect of any other intervention procedure. In two studies, a xylitol syrup and “xylitol tooth wipes” were tested in infants. The results were encouraging, supporting the use of xylitol. However, tooth wipes and dentifrices can be regarded as cosmetic devices. Their use should not be compared with enteral administration of food items. In two further studies, xylitol troches were employed. One of them was groundlessly regarded by the Cochrane Review as suffering from “high overall risk of bias”. An explanation for this opinion was not offered.
● The review included the Swedish Lycksele study in infants, where the daily xylitol levels were understandably quite low.
A qualified scientific review should establish inclusion and exclusion criteria. This decision normally results in a selection of studies that have been executed “correctly”, i.e., their review will generally include only controlled, randomized and blinded studies. Some indexing services list dentally-related xylitol studies that currently amount to more than 750, with “xylitol and caries” studies amounting to at least 500. Xylitol data have been accepted by the European Food Safety Authority, EFSA [57,58] (vide infra), and the International Association of Paediatric Dentistry (IAPD). For caries-limiting xylitol effects to occur, the daily xylitol levels and the daily frequency of use must be sufficiently high, i.e., at least 5 g, and preferably more (10‒15 g in adults with poor oral hygiene and craving sweets). The xylitol regimen has been unnecessarily impoverished in several studies (vide supra regarding shortcomings in study planning). Researchers planning clinical trials with sugar substitutes should consult reviews discussing failures to demonstrate caries reduction [4,6,46]. Xylitol selectively affects the metabolism of caries-inducive MS [6].
Consequently, the ten xylitol studies chosen for the Cochrane Review were not comparable. It is likely, of course, that Cochrane editors were fully aware of the above “shortcomings”. One of those is the categorical omission of several school programs that the authors of the review obviously regarded as "weak studies”, providing “weak evidence” or “suffering from experimental bias”. This categorical omission is unfortunate, since this type of omission practice may echo another kind of bias: the unconditional negation of a large number of studies that have indicated success in xylitol-based caries prevention. Absolutely unbiased school programs cannot be conducted anywhere, except perhaps in North Korea. A further concern in the above xylitol Review is the problem of comparing sucrose versus xylitol; no serious cariology expert would rank these carbohydrates as equal.
That meta-analyses can also underestimate medical procedures became evident when the effect of aerobic exercise on mental health was reviewed [59]. When the meta-analysis in question was adjusted to account for “weak studies” ―those prone to some kind of experimental bias―a strong positive effect was found. Another cause for concern was the observation that it would have taken at least 1,000 contradictory studies to negate the affirming evidence that had piled up. Such findings eclipse blind trust on some meta-analyses. According to the most recent meta-analysis “xylitol was found to be an effective strategy as self-applied caries preventive agent” [60]. The very latest controlled clinical trial showed xylitol gum to reduce caries incidence and enhance remineralization in primary dentition and oral hygiene in a cohort of sight- and hearing-impaired subjects [61]. That xylitol application may be associated with remineralization of caries lesions received support from a recent in vitro study [62]. The mother-child xylitol trials in turn seemed to exert long-term caries-reducing effects on children's dental health [25-27,63].
Consumers should be able to make food choices based on reliable and accurate information. The European Food Safety Authority (EFSA) has been influential in establishing the currently accepted health claims on sweeteners used in sugar-free chewing gums (SFCG) [57,58]. The sugar replacers currently permitted in EU-approved health claims for SFCG include intense sweeteners (such as aspartame and sucralose), erythritol, xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, polydextrose, D-tagatose, and isomaltulose. For SFCG, Article 13.1 contains three claims related to tooth mineralization, neutralization of plaque acids, and reduction of oral dryness, and a fourth claim for SFCG with carbamide concerning neutralization of plaque acids. Article 14.1 enounces a “plaque reduction” claim for gum sweetened with 100% xylitol. Two other claims for xylitol-containing SFCG relate to neutralization of plaque acids and reduction of tooth demineralization. The current EU-based legislation allows manufacturers to make claims for 100% xylitol gum to “reduce the risk of tooth decay”. The use of SFCG in medical and oral physiologic tests has become popular. Table-top sweeteners and foods formulated with sugar replacers are useful in achieving healthier diets in relation to overall carbohydrate and energy intake [58]. This is important considering the increasing prevalence of diseases like dental caries, obesity, and diabetes.
For an understanding of oral biologic effects of alditols, it is important to be familiar with their physicochemical properties. Previous articles have discussed physical and bioinorganic processes such as protein stabilization, hydration of alditols, complexation, and hydroxyl radical scavenging, that may help explain the clinical observations made with alditols [6]. The reactions are summarized here as follows:
(i) Alditol-induced stabilization of proteins. It has been known for several decades that sugars and polyols can protect protein structures and biological cells from damage caused by heating, freezing, and loss of solubility during drying [64-66]. Polyols thus stabilize the α-helix and β-structures of proteins.
(ii) Hydration properties of alditols. Alditols have been widely used as sweeteners and preservatives in foods. The use of alditols as “tissue-friendly” humectants in cosmetic products and dentifrices is also popular. Some of these uses depend on the way the alditol molecules interact with water which is the preferred physiological solvent. Studies on hydration have shown that the hydration water can display lower or higher mobility when compared with pure water. In literature, this has been casually referred to as the “structure-making” or “structure-breaking” effect. It has been suggested that “positive” and “negative” hydration are probably more suitable descriptions of the true phenomena involved [67]. Hydration of alditols most likely plays a role in their effects as dentin primers (vide supra); the bonding efficacy of dentin adhesives was investigated in the presence of alditols [68]. Although the required alditol levels are by far higher than those normally used in biomedical studies, this study nevertheless speaks to the existence of important differences between alditols, especially since glycerol, xylitol, or D-glucitol did not display the above preventive effect. Apatite cement containing poragen has been used in the fabrication of biporous apatite which has gained attention as a bone substitute material. Addition of D-mannitol improved the setting reaction and mechanical strength of apatite [68], owing to its satisfactory dissolution behaviour and biocompatibility, and because it did not inhibit the compositional transformation to apatitic material. It should also be mentioned that OH radical reactions with erythritol, D-arabitol, and D-mannitol are much faster than the NO3 and SO4– radical reactions [69].
(iii) Complexation. During the past forty years, a large number of works concerning the complexation of metal cations with sugars and alditols have been published. Complexes of dietary alditols with Fe(II), Fe(III), Ca(II), Cu(II), and other metal cations are interesting owing to their contribution to various biological processes. Alditol molecules can be regarded as carriers of metal ions in the transport of Ca(II) and Fe(II,III) through the gut wall, in remineralization of demineralised enamel caries lesions (by facilitating the flux of Ca(II) from saliva and plaque fluid into calcium-deficient tooth sites), as well as in other reactions. However, there are substantial differences between alditols in forming such metal complexes; alditols are not identical in a sense. The stability constants of alditol-metal complexes depend, among other things, on the size (chain length) and detailed conformation of the alditol molecule. The metal-centered structures may carry a negative or positive charge. In general, alditols form stronger complexes than do monosaccharides [70]. The process of complex formation in aqueous solution is essentially a displacement of one set of ligands, i.e. water molecules of the aqua complex, by another set, e.g., a diol [70].
(iv) Hydroxyl radical scavenging. Reactive oxygen species are constantly formed in biological systems. Tissues and cells both in the animal and plant kingdom are normally protected against this oxidative stress by means of various innate molecular mechanisms. Alditols can play a role in both endogenous and exogenous protection against oxidative stress [6,15]. Exogenous protection in this case means addition of alditol molecules to the reaction environment.
The consumption of fermentable, mostly hexose-based sugars, such as glucose, fructose, and sucrose, can be associated with certain dental problems, such as dental caries. Research and chemical technology have provided synthetic intense sweeteners and special carbohydrates whose consumption has helped curb pathological developments. A recent study suggested that partial substitution of glucose with xylitol may selectively inhibit the proliferation of oral cancer cells [71]. A related anti-tumor effect was noted already 35 years ago in rats bearing hepatocellular carcinomas (referred to in [46]). Erythritol and xylitol have been found to be effective in caries prevention. The sugar alcohol family of sweeteners provides versatile applications within the entire odonto-stomatologic discipline. Evaluation of research publications presumes thorough knowledge of the physicochemical properties of the polyols involved. Evaluation of study papers and review articles on sweeteners also requires vigilant and impartial perusal of the interpretations and data presented; surveys may have been based on a small number of publications that attempt to compare incompatible treatment procedures. Because the manifestation of dental caries and periodontal disease is often “sluggish” and “deceptive”, new and truly long-term clinical trials are warranted using disease-prone patient cohorts in non-fluoridate environments, with incomplete access to dental care, and with poor oral hygiene habits. Obviously, these types of studies can no longer be implemented owing to ethical considerations. The number of clinical caries trials with alditols is at the moment too small to conduct reliable meta-analyses.
- Roshan NM, Sakeenabi B (2011) Practical problems in use of sugar substitutes in preventive dentistry. J Int Soc Prev Community Dent 1: 1-8. [Crossref]
- Roberts MW, Wright JT (2012) Nonnutritive, low-caloric substitutes for food sugars: Clinical implications for addressing the incidence of dental caries and overweight/obesity. Int J Dent 625-701.
- Chattopadhyay S, Raychaudhuri U, Chakraborty R (2014) Artificial sweeteners-a review. J Food Sci Technol 51: 611-621. [Crossref]
- Mäkinen KK (2009) Oral care gum products. In: Food Constituents and Oral Health (Wilson M, ed.). Woodhead Publishing, Cambridge, UK, 433-454.
- Riley P, Moore D, Ahmed F, Sharif MO, Worthington HV (2015) Xylitol-containing products for preventing dental caries in children and adults. Cochrane Database Syst Rev: CD010743. [Crossref]
- Mäkinen KK (2010) Sugar alcohols, caries incidence, and remineralization of caries lesions: a literature review. Int J Dent 2010: 981072. [Crossref]
- Maehara H, Iwami Y, Mayanagi H, Takahashi N (2005) Synergistic inhibition of fluoride and xylitol on glycolysis by mutans streptococci and its biochemical mechanism. Caries Res 39: 521-526. [Crossref]
- Rogers AH, Bert AG (1992) Effects of xylitol and fluoride on the response to glucose pulses of Streptococcus mutans T8 growing in continuous culture. Oral Microbiol Immunol 7: 124-126. [Crossref]
- Petin VG, Kim JK, Kritsky RO, Komarova LN (2008) Mathematical description, optimization and prediction of synergistic interaction of fluoride and xylitol. Chemosphere 72: 844-849. [Crossref]
- Hamilton IR, Ellwood DC (1978) Effects of fluoride on carbohydrate metabolism by washed cells of Streptococcus mutans grown at various pH values in a chemostat. Infect Immun 19: 434-442. [Crossref]
- Hildebrandt GH, Sparks BS (2000) Maintaining mutans streptococci suppression with xylitol chewing gum. J Am Dent Assoc 131: 909-916. [Crossref]
- Decker EM, Maier G, Axmann D, Brecx M, von Ohle C (2008) Effect of xylitol/chlorhexidine versus xylitol or chlorhexidine as single rinses on initial biofilm formation of cariogenic streptococci. Quintessence Int 39: 17-22. [Crossref]
- Lee HJ, Kim SC, Kim J, Do A, Han SY, et al. (2015) Synergistic inhibition of Streptococcal biofilm by ribose and xylitol. Arch Oral Biol 60: 304-312. [Crossref]
- Han SJ, Jeong SY, Nam YJ, Yang KH, Lim HS, et al. (2005) Xylitol inhibits inflammatory cytokine expression induced by lipopolysaccharide from Porphyromonas gingivalis. Clin Diagn Lab Immunol 12: 1285-1291. [Crossref]
- de Cock P, Mäkinen K, Honkala E, Saag M, Kennepohl E, et al. (2016) Erythritol Is More Effective Than Xylitol and Sorbitol in Managing Oral Health Endpoints. Int J Dent 2016: 9868421. [Crossref]
- Mäkinen KK, Saag M, Isotupa KP, Olak J, Nõmmela R, et al. (2005) Similarity of the effects of erythritol and xylitol on some risk factors of dental caries. Caries Res 39: 207-215. [Crossref]
- Meurman JH (1998) Ultrastructure, growth and adherence of Streptococcus mutans after treatment with chlorhexidine and fluoride. Caries Res 22: 283-287. [Crossref]
- Silk H, Douglass AB, Douglass JM, Silk L (2008) Oral health during pregnancy. Am Fam Physician 77: 1139-1144. [Crossref]
- Amaechi BT, Higham SM, Edgar WM (1998) The influence of xylitol and fluoride on dental erosion in vitro. Arch Oral Biol 43: 157-161. [Crossref]
- Saeki Y, Takahashi S, Kamikawa S, Tokumoto T, Miake Y, et al. (2000) Remineralization effects of xylitol chewing gum containing Gloipeltis furcata extract and calcium hydrogenphosphate on initial caries-like enamel lesions. Jap J Oral Biol 42: 590-600.
- Sintes JL, Escalante C, Stewart B, McCool JJ, García L, et al. (1995) Enhanced anticaries efficacy of a 0.243% sodium fluoride/xylitol/silica dentifrice: 3-year clinical results. Am J Dent 8:231-235. [Crossref]
- Sintes JL, Elias-Boneta AS, Stewart B, Volpe AR, Lovett J (2001) Anticaries efficacy of a sodium monofluorophosphate dentifrice containing xylitol in a dicalcium phosphate dihydrate base. A 30-monhth caries clinical study in Costa Rica. Am J Dent 15:215-219. [Crossref]
- Campus G, Cagetti MG, Sale S, Petruzzi M, Solinas G, et al. (2013) Six months of high-dose xylitol in highj-risk caries subjects?a 2-year randomized, clinical trial. Clin Oral Invest 17: 785-791. [Crossref]
- Marghalani AA, Guinto E, Phan M, Dhar V, Tinanoff N (2017) Effectiveness of Xylitol in Reducing Dental Caries in Children. Pediatr Dent 39: 103-110. [Crossref]
- Söderling EM (2009) Xylitol, mutans streptococci, and dental plaque. Adv Dent Res 21: 74-78. [Crossref]
- Milgrom P, Söderling EM, Nelson S, Chi DL, Nakai Y (2012) Clinical evidence for polyol efficacy. Adv Dent Res 24: 112-116. [Crossref]
- Lin H-K, Fang C-E, Huang M-S, Cheng H-C, Huang T-W, et al. (2016) Effect of maternal use of chewing gums containing xylitol on transmission of mutans streptococci in children: a meta-analysis of randomized controlled trials. Int J Paed Dent 26: 35-44. [Crossref]
- Tucha L, Koerts J (2012) Gum chewing and cognition: An overview. Neurosci Med 3: 243-250.
- SMITH CS, WIKOFF HL, SOUTHARD ME (1946) Some effects of gum chewing on gastric acidity in healthy individuals. Am J Dig Dis 13: 245-247. [Crossref]
- Scheinin A, Mäkinen KK (1971) The effect of various sugars on the formation and chemical composition of dental plaque. Int Dent J 21: 302-321. [Crossref]
- Knuuttila ML, Mäkinen KK (1981) Extracellular hydrolase activity of the cells of the oral bacterium Streptococcus mutans isolated from man and grown on glucose or xylitol. Arch Oral Biol 26: 899-904. [Crossref]
- Mäkinen KK, Scheinin A (1982) Xylitol and dental caries. Annu Rev Nutr 2: 133-150. [Crossref]
- Mäkinen KK (1985) New biochemical aspects of sweeteners. Int Dent J 35: 23-35. [Crossref]
- Scheinin A, Mäkinen KK (1975) Turku Sugar Studies I-XXI. Acta Odontol Scand 33 Suppl 70.
- Pakkala U, Liesmaa H, Mäkinen KK (1981) The use of xylitol in the control of oral hygiene in mentally retarded children. A clinical and biochemical study. Proc Finn Dent Soc 77: 271-277. [Crossref]
- Hägi TT, Hofmänner P, Salvi GE, Ramseier CA, Sculean A (2013) Clinical outcomes following subgingival application of a novel erythritol powder by means of air polishing in supportive therapy: a randomized, controlled clinical study. Quintessence Int 44: 753-761. [Crossref]
- Hägi TT, Hofmänner P, Eick S, Donnet M, Salvi GE, et al. (2015) The effects of erythritol air-polishing powder on microbiologic and clinical outcomes during supportive periodontal therapy: Six-month results of a randomized, controlled clinical trial. Quintessence Int 46: 31-41. [Crossref]
- Müller N, Moëne R, Cancela JA, Mombelli A (2014) Subgingival air-polishing with erythritol during periodontal maintenance: randomized clinical trial of twelve months. J Clin Periodontol 41: 883-889. [Crossref]
- Drago L, Del Fabbro M, Bortolin M, Vassena C, De Vecchi E, et al. (2014) Biofilm removal and antimicrobial activity of two different air-polishing powders: an in vitro study. J Periodontol 85: e363-369. [Crossref]
- Ohhashi M, Chigira H, Itoh K, Hisamitsu H, Wakumoto S (1997) Effects of polyvalent alcohol solutions as dentine primers. J Dent 25: 161-166. [Crossref]
- Manabe A, Katsuno K, Itoh K, Wakumoto S, Miyasaka T (1991) Bonding efficacy of erythritol methacrylate solutions as dentin primers. J Dent Res 70: 1294-1298. [Crossref]
- Soheili Majd E, Goldberg M, Stanislawski L (2003) In vitro effects of ascorbate and Trolox on the biocompatibility of dental restorative materials. Biomaterials 24: 3-9. [Crossref]
- Zabner J, Seiler MP, Launspach JL, Karp PH, Kearney WR, et al. (2000) The osmolyte xylitol reduces the salt concentration of airway surface liquid and may enhance bacterial killing. Proc Natl Acad Sci USA 97: 11614-11619. [Crossref]
- Jones L (2010) No More Allergies, Asthma, or Sinus Infections. Freedom Press, LLC. ISBN 978-1-893910-88-1.
- Peldyak J, Mäkinen KK (2002) Xylitol for caries prevention. J Dent Hyg 76: 276-285. [Crossref]
- Mäkinen KK (2011) Sugar alcohol sweeteners as alternatives to sugar with special consideration of xylitol. Med Princ Pract 20: 303-320. [Crossref]
- Uhari M, Kontiokari T, Koskela M, Niemelä M (1996) Xylitol chewing gum in prevention of acute otitis media: double blind randomised trial. Br Med J 313: 1180-1184.
- Schoenfelder RC, Ponnamma CM, Freyle D, Wang SM, Kain ZN (2006) Residual gastric fluid volume and chewing gum before surgery. Anesth Analg 102: 415-417. [Crossref]
- Brambilla E, Ionescu AC, Cazzaniga G, Ottobelli M, Samaranayake LP (2015) Levorotatory carbohydrates and xylitol subdue Streptococcus mutans and Candida albicans adhesion and biofilm formation. J Basic Microbiol 55: 1-13.
- Honkala S, Runnel R, Saag M, Olak J, Nõmmela R, et al. (2014) Effect of erythritol and xylitol on dental caries prevention in children. Caries Res 48: 482-490. [Crossref]
- Kawanabe J, Hirasawa M, Takeuchi T, Oda T, Ikeda T (1992) Noncariogenicity of erythritol as a substrate. Caries Res 26: 358-362. [Crossref]
- Söderling E, Hietala-Lenkkeri AM (2010) Xylitol and erythritol decrease adherence of polysaccharide-producing oral streptococci. Curr Microbiol 60: 22-29. [Crossref]
- Yao J, Zhang JL, Wu YQ, Lu ZJ (2009) [Contrasting study of erythritol and xylitol on Streptococcus mutans]. Hua Xi Kou Qiang Yi Xue Za Zhi 27: 603-605. [Crossref]
- Hashino E, Kuboniwa M, Alghamdi SA, Yamaguchi M, Yamamoto R (2013) Erythritol alters microstructure profiles of biofilm composed of Streptococcus gordonii and Porphyromonas gingivalis. Mol Oral Microbiol 28: 435-451. [Crossref]
- Duane BG (2011) No evidence of caries reduction found in a school xylitol and erythritol lozenge programme. Evid Based Dent 12: 102-103. [Crossref]
- Lenkkeri AM, Pienihäkkinen K, Hurme S, Alanen P (2012) The caries-preventive effect of xylitol/maltitol and erythritol/maltitol lozenges: results of a double-blind, cluster-randomized clinical trial in an area of natural fluoridation. Int J Paediatric Dent 22: 180-190. [Crossref]
- Mäkinen KK (2014) Authorised EU health claims for xylitol and sugar-free chewing gum (SFCG). In: Foods, Nutrients and Food Ingredients with Authorised EU Health Claims, (Sadler MJ, ed). Woodhead Publishing, Cambridge, U.K. 1: 46-72.
- Shortt C (2014) Authorised EU health claims for intense sweeteners and sugar replacers. In: Foods, Nutrients and Food Ingredients with Authorised EU Health Claims, (Sadler MJ, ed). Woodhead Publishing, Cambridge, U.K.1: 151-176.
- Jabr F (2017) Head strong. Scientific American Mind, 27-31.
- Janakiram C, Deepan Kumar CV, Joseph J (2017) Xylitol in preventing dental caries: A systematic review and meta-analyses. J Nat Sci Biol Med 8: 16-21. [Crossref]
- Watthanasaen S, Merchant AT, Luengpailin S, Chansamak N, Pisek A, et al. (2017) Xylitol-containing chewing gum for caries prevention in students with disabilities: A randomized trial. Oral Health Prev Dent.
- Cardoso CA, de Castilho AR, Salomão PM, Costa EN, Magalhães AC, et al. (2014) Effect of xylitol varnishes on remineralization of artificial enamel caries lesions in vitro. J Dent 42: 1495-1501. [Crossref]
- Laitala ML, Alanen P, Isokangas P, Söderling E, Pienihäkkinen K (2013) Long-term effects of maternal prevention on children's dental decay and need for restorative treatment. Community Dent Oral Epidemiol 41: 534-540. [Crossref]
- Back JF, Oakenfull D, Smith MB (1979) Increased thermal stability of proteins in the presence of sugars and polyols. Biochemistry 18: 5191-5196. [Crossref]
- Gekko K (1981) Mechanism of polyol-induced protein stabilization; solubility of amino acids and diglycine in aqueous polyol solutions. J Biochem (Tokyo) 90: 1633-1641. [Crossref]
- Gekko K, Morikawa T (1981) Preferential hydration of bovine serum albumin in polyhydric alcohol-water mixtures. J Biochem (Tokyo) 90: 39-50. [Cossref]
- Carlevaro M, Caffarena ER, Grigera JR (1998) Hydration properties of xylitol: computer simulation. Int J Biol Macromol 23: 149-155. [Crossref]
- Shimogoryo R, Eguro T, Kimura E, Maruta M, Matsuya S, et al. (2009) Effects of added mannitol on the setting reaction and mechanical strength of apatite cement. Dent Mater J 28: 627-633. [Crossref]
- Hoffmann D, Weigert B, Barzaghi P, Herrmann H (2009) Reactivity of poly-alcohols towards OH, NO3 and SO4- in aqueous solution. Phys Chem Chem Phys 11: 9351-9363. [Crossref]
- Briggs J, Finch P, Matulewicz MC, Weigel H (1981) Complexes of copper(II), calcium, and other metal ions with carbohydrates: thin-layer ligand-exchange chromatography and determination of relative stabilities of complexes. Carbohydr Res 97: 181-188.
- Trachootham D, Chinqsuwanrote P, Yoosadiang P, Mekkriangkrai D, Rathawong T, et al. (2017) Partilal substitution of glucose with xylitol suppressed the glycolysis and selectively inhibited the proliferation of oral cancer cells. Nutr Cancer 18: 1-11.