Abstract
DNA extraction and PCR are among the most fundamental, essential, and inevitable experiments in plant genetics and biotechnology. Despite their long history, these protocols are still improved by researchers to perform more efficient and more successful experiments. In this review, we will try to introduce the key to successful DNA extraction and PCR experiments, especially for beginners of genetic analyses, to avoid making easy mistakes which often result in great waste of time and reagent. These keys are related to how to correctly measure DNA amount/purity, how to extract DNA from ‘difficult’ plant species, and how to successfully amplify target DNA sequence from huge plant genomic DNA.
Introduction
For genetic analysis and molecular breeding of plants, we must extract DNA from the target plants at first, then perform PCR reactions. Model plants such as arabidopsis (Arabidopsis thaliana) and tobacco (Nicotiana tabacum) are relatively easy to extract DNA, but on the other hand DNA is not effectively extracted from many horticultural plants. Without preparation of high-density and high-quality genomic DNA, the quality of genetic studies is lowered, or even are inoperable. DNA extraction kits such as DNeasy (QIAGEN, Venlo, Netherlands) are recommended for extraction of relatively small amount of pure DNA from model plants, but you cannot use these kits for ‘recalcitrant’ (difficult) plants.
We have tried DNA extraction by ourselves from a variety of plants such as arabidopsis, rice, wasabi, locust tree, torenia, petunia, cyclamen, apple, and gentian. Genomic DNA is usually extracted from healthy leaves. People often extract DNA from soft tissues at shoot apices, but such samples are limited both in quality and in seasons of the year. This is why we usually extract DNA from expended leaves. The most recalcitrant plants in my life would be ginkgo, cyclamen, and rose. We especially needed to extract high-density and high-quality DNA from cyclamen in the research project of molecular breeding of flowers. After trials of novel methods, we discovered an effective method which we named ‘Alkaline PVPP method’.
In the present report, we will introduce Alkaline PVPP and other DNA extraction methods, including rapid extraction methods specialized for preparation of PCR templates. We will also introduce easy but important tips/mistakes in general experiments of DNA extraction and PCR reaction. This knowledge will help successful performance of DNA extraction and PCR.
Quantification of genomic DNA
Before describing about DNA extraction, let us check how to quantify genomic DNA extracted from plants. Everyone who has extracted DNA should have measured UV absorbance. DNA concentration (c) in water solution is calculated by absorbance at 260 nm (A260) as:
c = 50 · A260 (ng µL−1)
For example, a solution with the A260 value of 0.500 is estimated to contain 25 ng µL−1 of DNA in it. This measurement is correct for pure DNA samples. Measurement of UV absorbance used to be performed by UV/VIS spectrophotometer, but recently it is going to be replaced by NanoDrop (Thermo Scientific, Waltham, USA), which can measure absorbance with small amount (approx. 4 µL) of DNA solution. It is often believed that the ratios of UV absorbances show quality of DNA: DNA is estimated to be pure if the ratios A260/A280 and A230/A280 are around 1.9. This is not true.
Actually, extracted DNA cannot be quantified by UV absorbance. There are often great gaps between UV-estimated DNA concentrations and true DNA concentrations. DNA is not even contained in the solution, even if the UV absorbance values are good. This will be because there are impurities in plant-extracted DNA, which cause similar UV absorbance to that of DNA in many cases. The UV-estimated DNA concentrations are just estimations based on spectrometry (in spectrometry: ‘is’ hereafter), then the value is expressed as follows:
c = 50 · A260 (ng-is µL−1)
In order to measure the true amount of genomic DNA, you should perform agarose gel electrophoresis. We usually load 300, 100, and 30 ng of λ-DNA as standards in separate wells. These standards are electrophoresed with 300 ng-is of plant-extracted DNA. For example, the purity of extracted DNA is 33% if the band densities of 100-ng λ-DNA and plant extract are the same. Purities of plant DNA extract are usually less than 10% only with the popular treatments with PCI (later described), RNase, and ethanol precipitation. Intensities of DNA bands can be quantified with ImageJ. We should be careful so that band images are not saturated for correct calculations. Figure 1 shows CsCl-purified DNA samples extracted from different horticultural plants.
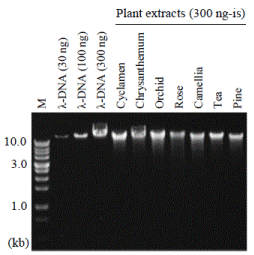
Figure 1. Agarose gel electrophoresis of DNA samples extracted from horticultural plants. M, molecular weight marker. Modified from Kasajima et al. (2013)
Meanwhile, RNA is also extracted together with DNA. RNA is visible in agarose gel as low molecular weight bands. Then, you should not confuse RNA with DNA. It is said that RNA affects enzymatic reactions, so RNA is degraded by RNase enzyme. It also often matters that sticky impurities in crude DNA extracts are lighter than water, and the samples will float out of the well before separation by electrophoresis. In this case, DNA is purified before electrophoresis, or additional loading buffer and electrophoresis buffer are added to samples. Also, rather than staining with ethidium bromide (EtBr) during electrophoresis, gel pictures are clearer when gels are stained with EtBr after electrophoresis. Recently appearing blue-light illuminators are not good. Gels should be illuminated by UV for clear gel images. GelRed (Biotium, Fremont, USA) enjoys good reputation in place of EtBr, although GelRed is expensive. DNA bands are also sharp when electrophoresed at 50 V, instead of 100 V. Polyacrylamide gel electrophoresis also gives sharp band.
Close investigations of DNA purity and quantity will greatly help to judge if the plant-extracted DNA solutions are good enough for the experiments. DNA will be extracted by more competent method, if the quantity is not enough, and DNA will be purified if the quality is not enough.
DNA extraction with modified alkaline PVPP method
Introduction
We previously reported a DNA extraction method ‘Alkaline PVPP method’. This method can extract DNA from any horticultural plants, as far as we tested [1]. The NaCl concentration in the extraction buffer was modified after publication of this report [2,3]. This protocol would be the most competent method from most plant species. The protocol is as follows:
Preparation of reagents
Modified PVPP buffer (Tris-HCl, pH 9.5, 50 mM; EDTA, 10 mM; NaCl, 4 M; CTAB, 1%; PVPP, 0.5%). Also add 1% β-mercaptoethanol immediately before use. PVPP is a white powder and insoluble in water, but PVPP becomes sticky in this solution. Then the buffer becomes white, half-transparent, and sticky. Store at root temperature. ‘Tris’ represents tris(hydroxymethyl) aminomethane, ‘EDTA’ represents ethylenediaminetetraacetic acid, ‘CTAB’ represents cetyltrimethylammonium bromide, and PVPP represents polyvinylpolypyrrolidone.
PCI (phenol-chloroform-isoamyl alcohol = 25:25:1). Phenol-chloroform without isoamyl alcohol is also often used in sample treatments, but isoamyl alcohol helps clear separation of the solid middle phase after centrifugation. Phenol is saturated with water or TE buffer (Tris-HCl, pH 8.0, 10 mM; EDTA, 1 mM) before preparation of PCI, and stored at 4°C as a liquid form.
Weighing plant samples
You must be careful about the ratio between the weight of plant samples and the volume of extraction buffer, whichever protocol you follow. The most standard buffer ratio is 5 times more than leaf: 5 mL of buffer per 1 g of leaf. This ratio is increased to 10 when you extract DNA from difficult samples. The maximum leaf weight is 1 g when you extract with ordinary mortar and pestle, together with liquid nitrogen: larger amount of leaf causes insufficient grinding. The degree of grinding is in fact one of the most important factors for successful DNA extraction. Leaves should be ground to fine powder like Japanese Matcha. Alternatively, samples are frozen at −80°C and crushed by using Micro Smash (Tomy, Tokyo, Japan), together with two metal balls in 2-mL plastic tubes. Micro Smash may cause slight degradation of DNA. There are many similar machines of this type, but crushing may not complete with many of the other machines.
DNA extraction
Cool mortar and pestle with liquid nitrogen, add frozen leaves, and crush immediately to fine powder. Hard leaves such as rice and camellia are cut to 1-mm width beforehand, and siliceous sand is added. Siliceous sand accelerates DNA fragmentation but should be added when grinding hard leaves.
Add modified PVPP buffer and mix with leaf powder. Place at room temperature until the solution starts to thaw. Grind well again.
Add samples to 15-mL or 50-mL plastic tubes, and heat in pre-heated heating block at 60°C for 30 min. Longer heating will cause DNA degradation. Heating at 80°C will slightly increase DNA yield. Invert tubes 2-3 times during heating.
Cool samples to room temperature. Centrifuge and recover supernatant, when there are much solid substance in the solution.
Add half volume (of the solution) of PCI, vortex well, and centrifuge at the maximum speed (such as 14000 rpm) for 5 min. Recover water (upper) phase to new tubes.
Repeat the same step (PCI treatment) again. Dilute the sample with the same volume of distilled water.
Add twice the volume of ethanol and mix well by inverting. Place at −80°C for at least 15 min. Thaw samples and centrifuge at the maximum speed for 60 min at 4°C.
Discard supernatant and centrifuge briefly. Remove any solution by using pipette. Air-dry or more conveniently, dry by using hair dryer, to some extent (not completely dry). Dissolve in distilled water (typically 500 µL of distilled water per 1 g of leaf sample). Add 2 µg of RNase A, place at 37°C or room temperature (25°C) for 15 min. Store at −20°C for several months. DNA samples will be more stably stored in ethanol for longer period.
Other DNA extraction buffers
Introduction
The modified PVPP buffer needs relatively more experimental steps, then other DNA extractions buffers are usually used. ‘CTAB buffer’ suffer from low capacity of DNA extraction, but it also extracts lower concentration of impurities. ‘SDS buffer’ can extract DNA more efficiently that CTAB buffer. The protocol of DNA extraction with these buffers is the same as the protocol with modified PVPP buffer, except that PCI treatment do not need to be repeated, and the solution do not have to be diluted after PCI treatment.
Reagents
CTAB buffer (Tris-HCl, pH 8.0, 50 mM; EDTA, 10 mM; NaCl, 0.7 M; CTAB, 1%). Also add 1% of β-mercaptoethanol immediately before use.
SDS buffer (Tris-HCl, pH 7.5, 200 mM; EDTA, 25 mM; NaCl, 250 mM; SDS, 0.5%). Also add 1% of β-mercaptoethanol immediately before use. SDS represents sodium dodecyl sulfate. When SDS precipitate at low temperature (e.g. in winter), warm the stock solution to completely dissolve SDS and mix well to homogeneity before experiments.
DNA purification by isopropanol precipitation
Introduction
Isopropanol precipitation is a simple method for DNA purification. Here, a protocol which I adopted in my former experiments is introduced. Alternatively, DNA-containing solution can be mixed with the same volume of isopropanol, then treated just like the protocol for ethanol precipitation. We have also tried DNA purification by ‘CTAB precipitation’ method, but this trial was far from successful [1].
Reagent
High-Salt Solution for Precipitation (Takara, Kusatsu, Japan).
Protocol
Be careful so as not to directly touch isopropanol with your skin.
Add half volume (of DNA solution) of High-Salt Solution for Precipitation and mix.
Add the same volume (as High-Salt Solution for Precipitation) of isopropanol and mix. Place at room temperature for 10 min. Centrifuge at the maximum speed for 10 min at 4°C. Discard supernatant.
Add 1 mL of 70% ethanol. Place at −80°C for 15 min.
Thaw out sample and immediately proceed to centrifugation at the maximum speed for 5 min at 4°C. Discard all supernatant and dry.
DNA purification by CsCl ultracentrifugation
Introduction
Ultracentrifugation with CsCl needs relatively long time of experiments, but DNA is highly purified by this treatment, and RNA is also removed. Ethidium bromide is often used to stain DNA in CsCl solution, but usage of GelRed instead of ethidium bromide halves the time of ultracentrifugation. GelRed is also only weakly carcinogenic, then you can keep safe environment. It seems that almost 100% of genomic DNA is recovered in CsCl ultracentrifugation [1,4]. In addition to CsCl ultracentrifugation, we have also recently noticed that silica monolith column (MonoFas, GL Sciences, Tokyo, Japan) could be used for high purification of genomic DNA within quite short time.
Reagent
TE buffer (Tris-HCl, pH 8.0, 10 mM; EDTA, 1 mM).
Protocol
Ethidium bromide is highly carcinogenic. Wear rubber gloves and perform experiments carefully.
Dissolve DNA-containing sample in 3.0 mL of TE buffer. Add 3.0 g of CsCl and mix to dissolve.
Add 120 µL of ethidium bromide solution (10 mg mL−1) or 20 µL of GelRed solution (×10000 concentration) and mix.
Apply 3.8 mL of the solution to 5-mL centrifuge tubes.
Ultracentrifuge at 50000 rpm for 48 h at room temperature. Orange DNA band will form near the middle layer of the tube (Figure 2).

Figure 2. Cyclamen DNA separated by CsCl ultracentrifugation. Modified from Kasajima et al. (2013)
Remove the above layers and recover the layer containing DNA (approx. 0.4 mL) to new tubes.
Rinse with n-butanol for 5 times or more to remove ethidium bromide.
Perform ethanol precipitation, after diluting DNA solution with water (see the protocol of DNA extraction).
Note
It will depend on experimental conditions, but the ultracentrifugation for long period (such as 48 h) was only successful with a swing rotor and was not successful with an angle rotor. We used Optima MAX Ultracentrifuge (Beckman-Coulter, Brea, USA), but other ultracentrifuge machine may not be successful. The reason for the failure of purification seems that the air inside the rotor leaks to the outside vacuum during ultracentrifugation, then selection of air-tight rotor will be the key to success.
Rapid DNA extraction from arabidopsis
Introduction
DNA can be quite easily extracted from arabidopsis, then there exist very simple methods of DNA extraction from arabidopsis. DNA extract with these rapid methods contain impurity and the concentration of DNA is low, but this solution was enough for stable amplification of DNA by PCR [5].
Reagent
SDS-D buffer (The SDS 2021 Copyright OAT. All rights reservh TE buffer by 10 times. β-mercaptoethanol is not added).
Protocol
Arabidopsis leaf (around 5 mg) is sampled into a 1.5-mL plastic tube.
Add 200 µL of SDS-D buffer to the tube.
Crush several times with plastic rod until the solution becomes pale green.
Store sample at −20°C until use. Apply 1 µL of this DNA solution to a total of 20 µL of PCR reaction.
Rapid DNA extraction from torenia
Introduction
The rapid DNA extraction method from arabidopsis above was tested in transgenic (GM) torenia but failed to amplify T-DNA sequence. Then, a special protocol for torenia was developed [6].
Reagent
SDS-R buffer (5 µg mL−1 of RNase A is added to SDS buffer, without addition of β-mercaptoethanol).
Protocol
Leaf disc of torenia (approx. 5-mm square) is sampled into a 1.5-mL plastic tube.
Add 200 µL of SDS-R buffer.
Crush with plastic rod for 5 times or more.
Heat in heating block at 55°C for 5 min. The solution becomes pale green.
Store samples at −20°C. When performing PCR analysis, dilute 10 times with sterilized distilled water. Apply 1 µL of this diluted solution to a total of 20 µL of PCR reaction.
Summary of DNA extraction methods
A DNA extraction method suitable to a plant species does not necessarily function in the other species. Such situations are often encountered and fluctuating as well. These problems are sometimes overcome by improving sample manipulations such as vigorously crushing frozen leaves to fine Matcha powder, and heating in heat block at suitable temperature and for suitable time. The protocol of DNA extraction is simple but consists of many important factors. In the present report we described all imaginable keys for successful DNA extraction from plants, based on our long experience. We would say that DNA can be more or less extracted from any plant species, if the protocols are faithfully followed. Alkaline PVPP method needs relatively many manipulations, then is not applicable to an analysis of hundreds of plants. The rapid methods for arabidopsis and torenia are the solutions to this problem. Finally, there is close relationship between the protocol of DNA extraction and the protocol of PCR analysis. However clean DNA be prepared, incorrect PCR conditions will fail to amplify target DNA. On the contrary, the target DNA can be amplified even from low-concentration template DNA, when the PCR condition is correct. Thus, following sentences will explain how to successfully perform PCR amplification by using plant genomic DNA as the template.
Tips of PCR reactions
Amount of DNA samples in PCR reaction
Of course, certain amount of template DNA must be added to the reaction mix, but excessive amount of DNA will inhibit PCR amplification. Typically, 5 ng-is of template DNA is quite enough for amplification. The concentrations of DNA stocks are often quite high. Except for low-density DNA such as the solution prepared by rapid extraction method of arabidopsis, DNA stocks are usually diluted by 100 to 1000 times with sterilized distilled water before added to the reaction. It is also important to compare a series of DNA dilutions (e.g. 1/1, 1/10, 1/100, 1/1000, and 1/10000) to determine the best dilution scale. In the case of cDNA, which are prepared by reverse transcription of RNA, samples should be diluted by 2, 5, or 10 times before PCR reaction. This is because the components of reverse transcription reaction partly inhibit PCR reaction.
Utilization of high-fidelity PCR polymerase
High-fidelity type DNA polymerase would be more successful (on average) than the ordinary type, when amplifying target DNA sequence from the genome of horticultural plants. PCR seems to be equally successful with the ordinary polymerase in arabidopsis with relatively small genome, but PCR was more successful with high-fidelity polymerase in rice in our experience. The relative frequency (concentration) of the target sequence is very low in the large genomes of horticultural plants, thus higher selectivity of PCR amplification will be needed in many horticultural plants. We usually use KOD Plus Neo (Toyobo, Osaka, Japan) in crop plants. Many other polymerases are also released from many companies.
Setting the best condition of the reaction
Usage of high-fidelity polymerase is not enough for success of PCR. After designing suitable primers, PCR is tested with the gradient annealing temperature from 46°C to 68°C, with 2°C or 4°C intervals. If the target is not amplified at any temperature, primers should be re-designed. Primer3 software is nice for primer design. Adenine residues are not added to DNA ends when amplified with high-fidelity polymerases. Sequences are TA-cloned after A attachment by using an enzyme kit.
High-throughput manipulations
Manipulations for PCR reaction also need a lot of time, when large number of samples are analyzed. For the sake of shortening the time of manipulation, PCR reactions are not needed to be prepared on ice. A key of PCR reaction is to homogenize PCR mixes well before thermal cycles. Vortexing all samples on a tube rack greatly shortens the time of mixing, instead of mixing one by one by using pipette. The number of PCR cycles are not enough for 30 cycles in many cases, then PCR is usually performed for 40 cycles. Agarose gel is prepared in 200-mL PYREX medium bottle, by heating with microwave at 400W. Heating is repeated for several times: the first heating is 1 min, then the following heating are 30 s. After completely melting the agarose powder, bottle is cooled by tap water and agarose solution is applied to gel tray. Keeping moisture with aluminum sheet, gel is completely solidified at room temperature for 1 h.
Conclusion
The essential keys to the success of DNA extraction and PCR reaction described in the present report will help all beginners and all researchers of plant genes. However highly genetic technologies are developed by researchers, the most fundamental experiments, that are DNA extraction and PCR reaction, are inevitable. Thus, knowledge on successful protocols will benefit any kind of genetic analyses. The present report is an English version of a review written in Japanese [2], with modifications in part. Japanese readers are also recommended to read the Japanese version.
Conflicts of interest
The author declares no conflict of interests.
References
- Kasajima I, Sasaki K, Tanaka Y, Terakawa T, Ohtsubo N (2013) Large-scale extraction of pure DNA from mature leaves of Cyclamen persicum Mill. and other recalcitrant plants with alkaline polyvinylpolypyrrolidone (PVPP). Sci Hortic 164: 65-72.
- Kasajima I (2016) DNA extraction from horticultural plants. Nougyou-Oyobi-Engei 91: 729-734.
- Kasajima I (2017) Protocol for DNA extraction from any plant species (alkaline PVPP method). Protoc Exch.
- Kasajima I, Ohtsubo N, Sasaki K (2014) Faster, safer, and better DNA purification by ultracentrifugation using GelRed stain and development of mismatch oligo DNA for genome walking. Biosci Biotechnol Biochem 78: 1902-1905. [Crossref]
- Kasajima I, Ide Y, Ohkama-Ohtsu N, Yoneyama T, Fujiwara T (2004) A protocol for rapid DNA extraction from Arabidopsis thaliana for PCR analysis. Plant Mol Biol Rep 22: 49-52.
- Kasajima I, Sasaki K (2016) A chimeric repressor of petunia PH4 R2R3-MYB family transcription factor generates margined flowers in torenia. Plant Signal Behav 11: e1177693. [Crossref]


