Allergen is one of the most important factors in the pathogenesis of allergic inflammation. It can not only sensitize and trigger allergic response but also can induce tolerance through allergen-specific immunotherapy (AIT). Although AIT has been introduced since 1911, and there are many modalities of allergen-based specific AIT been developed since then. Despite rapid progress of the modality, the impacts of allergic diseases remain tremendous. It is estimated that AIT is used in less than 10%. To improve the modality of AIT, the successful elements of AIT including efficacy, safety, convenience and reasonable-cost were reviewed and described. The allergen extract and its response have to be measurable and should include all allergenic components to make sure its effectiveness. For the safety and convenience reason, allergenicity of allergen extract would be better been reduced and could be administrated through non-injection route. Allergen should be reasonable-cost and affordable. If all factors have been taken into consideration, AIT will be more acceptable and allergen-induced tolerance by AIT can be achieved.
The impact of allergic disease
Allergic disease is one of the most common diseases in human, including allergic rhinitis, asthma and atopic dermatitis. The prevalence of allergic rhinitis and asthma remain increasing in the past decade with the incidence of allergic rhinitis around 10-30% in adults and 40% in children in the United States [1]. Similar high incidence was observed in Taiwan with 24-30% in adults and 50% in children [2]. The prevalence of Asthma was 18.3% in US and 13.1% in Taiwan [3].
Since the high co-existing of allergic rhinitis with asthma was observed that there are 38% of allergic rhinitis have coexisting asthma and 58-78% of asthma have allergic rhinitis. These epidemiological studies suggest that these two airway allergic diseases, allergic rhinitis and asthma, have similar disease entity and “one airway one allergic disease” has been considered. Although there are many airborne allergens have been identified and can cause airway allergy, the most important indoor allergen is house dust mite and outdoor allergen is pollen around the world. When chronic persisted allergic airway remain inflammation and been treated unsuccessfully, many comorbidities will be reported including COPD, atopic dermatitis, obstructive sleep apnea, allergic conjunctivitis, otitis media, sinusitis / nasal polyposis. The burden of allergic disease on human health cannot be overlooked [4].
Allergy is a disease cause by allergen-induced reactions
Allergen-induced reaction is initiated by allergen sensitization without inflammation followed by allergic inflammation after re-exposure to the same allergen.
Allergen sensitization: In the allergic rhinitis subjects, when the airway epithelium first exposure to the allergen, the epithelium become sensitized and can be activated to release cytokines IL-25, IL-33 and TSLP (thymus stroma lymphopoietin). TSLP can further stimulate type 2 innate lymphoid cells (ILC2) and basophils to release Th2-driving cytokines (IL-13 and IL-4). Th2 cytokines, in turn, drive B cell-derived plasma cell to produce allergen-specific IgE. These allergen-specific IgE antibodies attach to high-affinity receptors on the surface of tissue-resident mast cells and circulating basophils.
Allergen re-exposure: The allergen binds to IgE on the surface of those cells and cross-links IgE receptors, resulting in mast-cell and basophil activation and the release of neuroactive and vasoactive mediators such as histamine and the cysteinyl leukotrienes. local activation of Th2 lymphocytes by dendritic cells results in the release of chemokines and cytokines that orchestrate the influx of inflammatory cells (eosinophils, basophils, neutrophils, T cells, and B cells) to the mucosa, providing more allergen targets and up-regulating the end organs of the nose (nerves, vasculature, and glands). Th2 inflammation renders the nasal mucosa more sensitive to allergen but also to environmental irritants. In addition, exposure to allergen further stimulates production of IgE. , mediators released by mast cells and basophils can directly activate sensory-nerve endings, blood vessels, and glands through specific receptors.
Typical symptoms of allergic rhinitis are caused by inflammatory mediators: histamine seems to have direct effects on blood vessels (leading to vascular permeability and plasma leakage) and sensory nerves, whereas leukotrienes are more likely to cause vasodilatation. Activation of sensory nerves leads to the generation of pruritus and to various central reflexes. These include a motor reflex leading to sneezing and parasympathetic reflexes that stimulate nasal-gland secretion and produce some vasodilatation. Additionally the sympathetic drive to the erectile venous sinusoids of the nose is suppressed, allowing for vascular engorgement and obstruction of the nasal passages. In the presence of allergic inflammation, these end-organ responses become up-regulated and more pronounced. Sensory-nerve hyper-responsiveness is a common pathophysiological feature of allergic rhinitis [5].
Remodelling: Airway remodelling is results from persisted airway inflammation. In the asthma subjects, when the symptoms of allergic inflammation persisted and become chronic, the airway remodelling with structure-changed depend on the disease severity and chronicity. In the epithelium, there are loss of ciliated epithelial cells and increased goblet cells and subepithelial fibrosis in the lamina reticularis of basement membrane. In the submucosa, there are increased fibroblast, myofibroblast, muscle myocyte, mucus gland and blood vessels [6].
Allergy determinants - genetic and environment
Allergy is a disease determined by genetic and environmental factors. There are many susceptible genes been identified through the gene association studies. Although GWAS (Genome-Wide Association Studies) can uncover susceptibility genes more than those genes that don’t belong to allergic inflammation pathways, the most consistent finding from 12 GWAS of Asthma is the association of 17q21 locus with asthma and other genes identified in more than one GWAS are IL33, RAD50, IL1RL1 and HLA-DQB1 [7].
Although many genes associated with airway inflammation and expression of disease severity been identified, there are only few studies investigate the promoter genes associated with airway inflammation. Currently our study showed some promoter SNPs of MD2 were associated with not only MD2 expression but also with the expression of Der p2 specific IgE. And the promoter SNPs of FcεR1α were associated with not only the expressions of FcεR1α but also with the expression of Cε. The promoter SNPs of MD2 and FcεR1α were demonstrated closely related to dust mite allergy [8,9].
Many environmental factors have been reported to be related to allergy development, including allergens, respiratory infection, tobacco and pollutants, prenatal maternal influences, prematurity and dietary factors. Many environmental factors can be prevented but some of them are inevitable [10,11]. House dust mite (HDM) is one of the most common indoor allergens in the subtropical area around the world including Southern China, Taiwan, and Hong Kong. Our study showed there were around 80% of allergic asthma were sensitized by HDM. Different ages of asthmatic patients were sensitized by different species of HDM. There are more young allergic subjects sensitized by Dermatophagoides pteronyssinus more elderly allergic subjects sensitized by Tyrophagus putrescentiae [12].
Synergistic effects of genetic and environmental factors have been demonstrated in our previous study [13]. Susceptible genes with MD2 and FcεR1α promotor SNPs are closely related to Dust mite allergy. Protease activity of Der p1/3 and MD2 homologue of Der2 can activate epithelium. Both innate immune response (epithelium/DC cells) and adaptive immune response (Th2 cells) can be induced by dust mite allergen.
Despite many genetic and environmental factors are involved in the pathogenesis of allergic disease. There are only allergens can not only trigger allergen-specific reaction but also induce allergen-specific tolerance. The allergen specific tolerance-induction can be obtained through allergen-specific immunotherapy [14].
Rational of allergen-specific immunotherapy
Airway allergic disease is one of the most common chronic diseases, there are around 30-50% subjects of general population suffered from allergic rhinitis. A high proportion of allergic rhinitis are developed in early childhood and followed by early-onset asthma (25%). Early-onset asthma usually relapse in adulthood and will persisted to adult and adult-onset of asthma also usually persisted and became chronic despite receive treatment. This chronic disease can develop many co-mobility if not treat properly. Current therapy with antihistamine and glucocorticoid (ICS) for allergic inflammation is poor and there is high population of patients are refractory to the current medication. For the improvement of treatment, allergen-specific immunotherapy is one of the alternative choices.
Allergen specific immunotherapy (AIT) was first described in 1911 by Noon L [15] for AR. Its efficacy was documented and confirmed by double blind placebo control study reported in 1999 [16]. The clinic efficacy of AIT for asthma has been demonstrated in our previous report in 1983 [17]. In this study the efficacy of AIT was demonstrated with the reduced-medication and serum allergen-specific IgE, and increased IgG. The large and small airway obstruction were also improved in the group of asthma patients when receive treatment with AIT.
Indication of Allergen-IT
Allergen-IT has been suggested to treat allergy for more than one contrary. It has been recommended to treat allergic asthma, allergic rhinitis and stinging insect hypersensitivity. If allergic patients are not satisfactory to pharmacotherapy and allergen-avoidance, allergen-IT can be suggested.
Working mechanism of AIT
AIT is a type of therapy with relative-safe and can obtain long-lasting efficacy for the treatment of allergic asthma/ rhinitis and stinging venom-induced anaphylaxis. Based on the review by Akdis CA in 2015, the mechanisms of AIT include: Early decreased in mast cell / basophil degranulation and decreased tendency for anaphylaxis after the first administration with a native-like structure allergen. Induction of tolerance with increased Treg cell to suppress Th2 and Th1 cells. An early increase and late decrease in specific IgE in associated with increased IgG4. Decreased in type 1 skin test reactivity [14] (Figure 1).
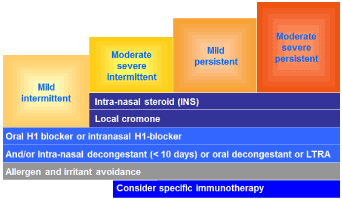
Figure 1. Therapeutic guideline for allergic rhinitis (ARIA treatment outline (2014)
Allergen can be delivered through injection and non-injection for IT based on the route of allergen administration. There are 2 types of injection IT (subcutaneous IT and lymph node IT) and 2 types of non-injection IT (sublingual IT and local nasal IT). Three common conventional allergen IT are: subcutaneous IT (SCIT), sublingual IT (SLIT) and local nasal IT (LNIT).
SCIT for treatment of AR
Guidelines for the treatment of AR and asthma have been published in 1998 in a WHO [18] position paper and refined by ARIA in 2001 [19]. Based on the ARIA, SCIT is indicated for AR with mild persistent symptoms or moderate /severe intermittent symptoms and fail to improve after allergen/irritant avoidance and medications with oral antihistamine and intranasal corticosteroid ICS.
Standard procedure of SCIT
SCIT is a modality for allergen administration subcutaneously with clinically relevant allergens. SCIT is conducted with allergen injection to ameliorate the allergic symptoms can be based on the end point titration through allergen prick test. Once the lowest positive dose has been chosen, it can be subcutaneously injection from the lowest dose followed by build-up phase weekly. The allergens dose was gradually increased to the fixed high maintenance dose. The standardized procedure of SCIT has been reported and its relatively safe following the standard procedure.
SCIT: complications and side effects
Although the standardized procedure of SCIT is relative safe, it has been reported to have complications and side effects ranging from the mild local reaction of urticaria to severe systemic reactions of anaphylactic shock.
SCIT disadvantages
Despite the clinical efficacy there are some disadvantages of SCIT: SCIT need to be performed in the clinic and under supervision for 30 minutes with emergency equipment and facility to treat the allergic side effects and severe allergic reactions. SCIT administration require build-up phase and gradually increase the dose till the high tolerated maintenance dose (usually take months to years). Require monitor the skin reaction and clinical effects while allergen administration.
BCG protect against atopy development
Epidemiological studied suggested either environmental exposure or immunization with Mycobacterium bovis-Bacillus Calmett-Guerin (BCG) provide protection against the development of atopy and asthma in Japanese school children [20]. Since airway allergic inflammation is characterized by Th2 cell predominant infiltration in the airway and BCG vaccination has been reported to induce Th1 cell activation. Whether this imbalance condition can be corrected still under debate and the effect of BCG on the treatment of allergic asthma remain unclarified.
BCG not benefit for allergen SCIT
Since the subjects who received BCG vaccination and have positive tuberculin reactions tend to have low incidence of asthma. We investigate the effect of BCG in conjunction with SCIT on the treatment of asthma. Although IT/BCG with allergen can potentiate and prolong the effects of SCIT to those asthmatic patients who had already received full dose of allergen IT. Our results showed that the airway hyper-responsiveness to methacholine and serum level of Der p specific IgE remained the same [21].
(or Table) Pros and Cons of SCIT
Pros: efficacy is evidence-based.
Patients can tolerate specific allergen and its effects can be monitored with allergen specific IgE after SCIT.
Cons: adverse reactions cannot avoid.
Patients have the risk of allergic reaction while in escalating/build-up phase of allergen administration and require observation in the clinic.
Differences of local nasal immunotherapy (LNIT) and Sublingual immunotherapy (SLIT)
LNIT was first reported in 1972. There are many double-blind placebo control trials prove its efficacy. LNIT has been showed to have the clinical efficacy comparable to SCIT and a measurable reduction of oral medications and specific allergen challenge were reported in many clinical trials.
SLIT was first proved efficacy in a DBPC trial in 1986, the most recent published meta-analysis in 2013 found strong evidence supporting the use of SLIT in improving asthma symptoms and medications use in conjunctivitis, rhinitis, and asthma.
The allergens extracts used in LNIT and SLIT were different, the original material-used for LNIT were powder or solution, while the material used for SLIT was solution/drops. The advanced materials used for LNIT was allergen-coated strips while the material used for SLIT was soluble tablet. The differences of these two modalities are listed in Table 1.
Table 1. Differences of agents used in LNIT and SLIT
LNIT |
Original :solution/ powder |
Advanced : allergen-coated strips |
SLIT |
Original :solution/drops |
Advanced : soluble tablets |
SLIT
SLIT was first proved efficacy in a double-blind placebo control (DBPC) trial in 1986, the most recent published meta-analysis in 2013 found strong evidence supporting the use of SLIT in improving asthma symptoms and medications use in conjunctivitis, rhinitis, and asthma. SLIT can be easily applied and can be self- managed at home, but allergen extract required to be held under the tongue to allow absorption. It is recommended that pollen immunotherapy is commenced a number of months before season and continuously for perennial indoor allergen related allergy patients. Mild gastro-intestinal upset and oral pruritis side effects are common with aeroallergen. The doses of SLIT require a buildup phase and maintenance phase. Currently SLIT was suggested for the treatment of asthma in the GINA guideline 2017.
SLIT -Efficacy and Safety: The EAACl and AAAAI consensus reports concluded that SLIT can prevent development of asthma and new sensitization. Although SLIT is a widely reported as being safe, there have been case reports of anaphylaxis outside of clinical trial. The most recent report showed that anaphylaxis followed by in-season switchover of sublingual immunotherapy formation [22]. It will be of importance to escalating the dose of allergen extract or reducing the allergenicity to avoid side effects. SLIT had been conducted to treat asthma in Taiwan, the results showed that there is significant improvement of symptoms in the group of SLIT with allergen in comparison with placebo. However, when the symptoms of asthma were compared before and after treatment, there are no statistically significant differences before and after SLIT with allergen [23].
LNIT
LNIT has been demonstrated effective in treating allergic rhinitis. It can not only reduce nasal symptoms but also reduce the allergic inflammation both local and systemically. The modulation of systemic immunologic response has been demonstrated after LNIT [24-28]. Since the allergen-used for LNIT have to be sprayed into the nostril during vocalization. This technical limitation causes many patients cannot maintain therapy with LNIT. Currently this drawback has been solved by using allergen-coated paper strips to improve patient’s adherence.
Advanced-LNIT with paper strips
Allergen-coated strips have been shown to improve patients’ compliance and can be self-administrated without any difficulties. LNIT using allergen-coated strips has been demonstrated in a clinical study. In this study, Der p- coated strips was used to treat Der p allergic AR, the results showed that nasal symptoms of allergic rhinitis were improved in association with the reduction of inflammatory mediators secretion in the nasal mucosa and decreased Der p specific IgE in the serum. The study demonstrated that advanced-LNIT can be self-administered at home, improve compliance for treatment adherence and no systemic adverse reaction [28].
Safety assurance of LNIT with paper strips
To make sure the safety of LNIT with paper strips, Dp-coated paper disc was administrated to the nose to make sure it did not cause lower airway response. When patients received Dp-coated paper strips for 10 min, there were significantly increase in nasal resistance after allergen exposure both immediately and 7 hours later. However, there are no significant changes in FEV1 and FMF25%-75% in both allergic rhinitis and asthmatic patients. There were also no significant changes of in bronchial hyper-responsiveness to methacholine both immediately and 7 hours after Der p-coated disc application [29] (Figure 2).

Figure 2. Effect of nasal challenge with Dp-coated strips on airway hyperreactivity. There is no significant change in FVC (a), FEV1(b), FEF25-75%(c), methacholine(d) at each time point (29)
LNIT with Dp-coated strips was used to prove the concepts: A total of 35 allergic rhinitis patients were recruited (24 patients received Dp-coated strips and 11 patients received normal saline-coated strips) to investigate the effect of LNIT using Dp-coated strips. A fixed-dose of Dp-coated strip was administrated to the nostril weekly for 3 months. The results showed that nasal symptoms were reduced significantly after therapy and no unpleasant symptoms and no severe adverse reaction was found. The inflammatory mediators in the nasal discharge and the immunoglobulin E in the serum were also reduced after therapy. However, there were 21.8% of patients withdraw [28]. In comparison with the previous reports LNIT with nasal drops the rate of unpleasant nasal symptoms was 56 % and withdrawal rate was 43.9 % (Table 2 and 3) [30].
Table 2. Difference of Der p-specific Immunoglobulins in the Sera---between groups (28)
|
Group |
No. of cases |
Month 4-Month 1 |
Power |
p-value |
Serum IgE |
Der P |
19 |
-0.13±0.03a |
76% |
<0.001b* |
NS |
9 |
0.05±0.05 |
Serum Ig1 |
Der P |
19 |
-0.09±0.02 |
72% |
0.001b* |
NS |
9 |
0.10±0.08 |
Serum Ig4 |
Der P |
19 |
0.49±0.09 |
92% |
<0.001b* |
NS |
9 |
-0.11±0.07 |
a: Changes of Igs in the sera. Data represented as mean ± SEM; b: Mann-Whitney U-test; *: p<0.01
Table 3. Differences of histamine and ECP Secretion in Nasal Discharge-between groups (28)
|
Group |
No. of cases |
Month 4-Month 1 |
p-value |
Histamine (ng/ml) |
Der P |
11 |
-48.63 ± 7.47a |
0.011m* |
NS |
3 |
-9.67 ± 2.20 |
ECP |
Der P |
11 |
-0.08 ± 0.01 |
0.038m* |
NS |
3 |
-0.005 ± 0.01 |
m: Mann-Whitney U-test; *: p<0.01; a: Changes of month 1 to 4. Data represented as mean ± SEM
A steady dose of Dp-coated strip is effective for mite-induced allergic rhinitis. Dp-coated strip can be self-administered at home without any severe adverse reactions. Mild local nasal symptoms are observed in the initial 4 weeks and can be relieved by oral antihistamine. Both local and systemic immune responses were observed after LNIT [28].
These serum samples from the patients, who had received LNIT with Dp-coated strip, were analyzed for the specific IgE to Dp and Tp. Results showed: Both specific IgE to Dp and Tp were decreased simultaneously. Both specific IgG to Dp and Tp were increased. Therefore, LNIT with Dp-coated strips might protect patients from Tp induced allergic reaction [31].
Based on this study, LNIT with paper strips may serve as a good alternative therapeutic modality for LNIT
Allergoid (i.e reduced allergenicity) immunotherapy
Reduced allergenic antigen can be produced through chemical manipulation. Several chemically modified allergens were prepared to avoid IgE-mediated allergic reaction. The most common allergoid antigen preparation was urea denature. Although they are less potent, but its immunogenicity remains preserved. Since urea treatment of the allergen can separate allergen peptides chains held together by non-covalent forces. Removal of urea by dialysis allows some re-aggregation of chains but it become non or less allergenic (Figure 3) [32].
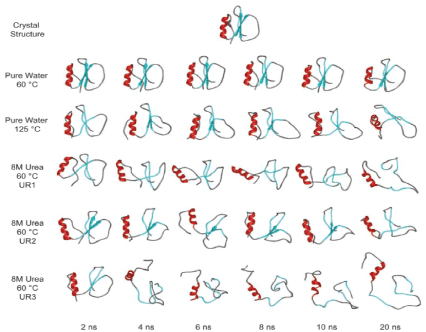
Figure 3. Structural changes of CI2 as a function of time and environment. The positions of native secondary structure are colored in red (helix) and cyan (β-strands)
Allergoid immunotherapy
Mechanisms: Larger dose of urea denatured antigen might have more effect in reducing suppression IgE synthesis it might cause local and systemic allergic reactions. Suppression of seasonal IgE response in human and mice treated with either unaltered extract or urea-denatured antigen. Antigen-specific suppressor T cells can be induced by injection of a large dose of either unaltered extract or urea-denatured antigen.
In the animal studies of allergoid-prepared by urea denatured (SCIT)
Guinea pigs could be sensitized by repeated exposure to OVA but not DN-OVA. DN-OVA could abrogate oral induction of airway hypersensitivity and IgG1/ IgG2 antibody responses. In the OVA sensitized guinea pigs, airway resistance was significantly increased in both early (30min) and late (6h) phase after OVA sensitization. OVA-induced airway response could be modulated by Immunotherapy with subcutaneously administration of DN-OVA. Airway hyperreactivity to methacholine in OVA sensitized GP can be modulated by DN-OVA.
In the human studies of allergoid-prepared by urea-denatured (SCIT)
Human with ragweed allergic rhinitis were under taken to receive treatment with urea denarurd-antigen E (DNE). A total 10 cases received DNE treatment for 18 months. Local and systemic allergic reactions limit the dose to 4-75 ug per injection. Five of 8 patients who completed the study had the evidence of suppression of IgE responses by existing a 20% or less increase of IgE antibody to ragweed on nature exposure. Patients’ symptoms still exist next season 10 months after the last injection. There was no clinical evidence of improvement. However, allergen-specific IgE suppressor T cells can be reduced by a non- allergic material, with the resulting amelioration of allergic symptoms [33].
In the animal studies of allergoid-prepared by urea denature (LNIT)
Balb/c mice sensitized by Dp and treated with DN-Dp. The serum levels of allergen-specific IgE were significantly decreased and the expressions of IL-4 were reduced in the lung tissue. LNIT can also down-regulated IL-1beta, IL-6 and TNF-alfa expression and then decrease Dp-induced airway inflammation. The airway inflammation was improved with decreased hypersensitivity to methacholine in the DN-Dp treated mice (Table 4) [34].
Table 4. Effects of Dp/DN-Dp on cytokine expression in CD4+ cells (34)
| |
Medium |
Dp |
DN-Dp |
| |
mean±S.E.M. |
mean±S.E.M. |
mean±S.E.M. |
CD4+IFNγ/CD4+ |
|
|
|
(n=7) |
5.71±1.19 |
4.40±1.31 |
4.80±1.15 |
CD4+IL-4/CD4+ |
|
|
|
(n=7) |
9.98±2.05 |
12.87±2.55* |
9.49±2.00 |
On listing trial of Peptide-based immunotherapy: Peptide with adjuvant for IT
FIP has been demonstrated asTh1 stimuli. Adding or conjugating adjuvants to allergens may increase desired (Th1) response. CpG (oligodeoxynucleotides) can enhance Th1 response and has been added or conjugating to allergen. Both adjuvants have been listed on clinical trial.
On listing biological agents: Three listing biological immune response modifier for IT
Cytokine and receptors: IL-1beta, IL-2, IL-4, IL-5, IL-9, IL-13, IL- 17, IL-17RA, IL-25, IL-31, IL-33, TNF-alfa. Chemokine and mediators: CCR3, CCR4, CXCR2, DP1, CRTH2, TSLP, LFA-1, OX-40L. Cell surface molecules and receptors: CD20 and CTLA4. Pro and con of immunotherapy: Knowledge of biologics in the pathology of tissue inflammation should be clarified. Phenotype of patients and biomarker of inflammation should be target [35].
Drawback of allergen immunotherapy
The major problem of AIT is safety and inconvenience. Since SCIT might cause allergen-induced reaction and have to be administered subcutaneously in the clinics under supervision. The minor problems are AIT require dose escalation and long-term therapy [36].
Currently a retrospective review of electronic health records of patients with allergic rhinitis from January 2005 to June 2011 showed only 36.2 % initiated AIT and 39.6% of AIT discontinued before completed at 3 years because of lack of efficacy, financial burden, adverse events and time consumption [37]. Successful elements of AIT should target to patients with all allergen been identified and should all allergens/allergenic components have been included in the therapy at the same time. Efficacy can be accessed and monitored and be decided when/how to start, follow-up and stop. AIT should achieve clinical success in a relative short-period and obtain long-term allergen tolerance.
Pros of immunotherapy
Knowledge of most allergen and its cellular mechanisms of allergenic components were well elucidated. Phenotype of patients and allergen specific biomarker of inflammation can be easily identified and monitoring.
Successful elements of AIT
To obtain the efficacy of AIT, several important elements have to be taken into consideration including: Convenience: easy administration; Safety: reduced allergenicity; Allergen components and doses: allergen doses and components are fixed and all-included; Therapeutic duration: quick and long-lasting effects; Affordable price: cost reasonable
Design a new biological product
To design a new biological agent, require four considerations as followings: Treat target organ: Nasal inflammation is better treated the nose using local nasal immunotherapy. Include all allergenic components in the extract (allergoid from crude extract). Reduced allergenicity and preserved immunogenicity (allergoid). Easily administration with fixed-dose of allergen by nasal paper strips (Table 5).
Table 5. Comparison of successful elements of allergen (all fragment)-based IT
|
|
Efficacy |
Safety |
Convenience |
Cost |
SCIT |
Allergen extract |
5 |
1 |
1 |
High |
|
Allergoid |
3 |
3 |
1 |
Low |
LNIT |
Allergen extract |
5 |
4 |
5 |
High |
|
Allergoid |
4 |
5 |
5 |
Low |
SLIT |
allergen extract |
4 |
3 |
5 |
High |
|
Allergoid |
ND |
ND |
5 |
Low |
IT: Immunotherapy, SCIT: Subcutaneous IT, LNIT: Local nasal IT, SLIT: Sublingual IT
ND: Non-Determined, Grade 1, 2: poor, Grade 3: fair, Grade 4, 5: excellent
Allergen can also induce tolerance
Both genetic factors and environment factors can synergistically contribute to allergic immune response and develop allergic diseases. Repeatedly exposure to allergen may induce chronic airway inflammation and remodelling. Treatment of allergy should be focused on allergen avoidance and anti-inflammation. Allergen tolerance-induction can be achieved by allergen-based immunotherapy with either allergen or allergoid.
- Dykewicz MS (1998) Diagnosis and management of rhinitis: complete guidelines of the Joint Task Force on Practice Parameters in Allergy, Asthma and Immunology. American Academy of Allergy, Asthma, and Immunology. Annals of allergy, asthma & immunology. 81: 478-518.
- Liao PF, Sun HL, Lu KH, Lue KH (2009) Prevalence of childhood allergic diseases in central Taiwan over the past 15 years. Pediatr Neonatol 50: 18-25. [Crossref]
- Pearce N (2007) Worldwide trends in the prevalence of asthma symptoms: phase III of the international study of asthma and allergies in childhood (ISAAC). Thorax 62: 758-766. [Crossref]
- Bousquet PJ, Demoly P, Devillier P, Mesbah K, Bousquet J (2013) Impact of allergic rhinitis symptoms on quality of life in primary care. International archives of allergy and immunology 160: 393-400. [Crossref]
- Wheatley LM, Togias A (2015) Clinical practice. Allergic rhinitis. The New England journal of medicine 372: 456-463.
- Shifren A, Witt C, Christie C, Castro M (2012) Mechanisms of remodeling in asthmatic airways. J Allergy (Cairo) 2012: 316049. [Crossref]
- Akhabir L, Sandford AJ (2011) Genome-wide association studies for discovery of genes involved in asthma. Respirology 16: 396-406. [Crossref]
- Liao EC, Chang CY, Wu CC, Wang GJ, Tsai JJ (2015) Association of single nucleotide polymorphisms in the MD-2 gene promoter region with Der p 2 allergy. Allergy, asthma & immunology research. 7: 249-255.
- Liao EC, Chang CY, Hsieh CW, Yu SJ, Yin SC (2015) An exploratory pilot study of genetic marker for ige-mediated allergic diseases with expressions of fcepsilonr1alpha and cepsilon. International journal of molecular sciences 16: 9504-9519.
- Liao EC, Lin YH, Tsai JJ (2013) Detection of group 2 Dermatophagoides pteronyssinus allergen for environmental monitoring of dust mite infestation. Bioscience trends 7: 82-88.
- Yu SJ, Liao EC, Tsai JJ (2014) House dust mite allergy: environment evaluation and disease prevention. Asia Pacific allergy 4: 241-252.
- Liao EC, Ho CM, Tsai JJ (2010) Prevalence of Tyrophagus putrescentiae hypersensitivity in subjects over 70 years of age in a veterans' nursing home in Taiwan. International archives of allergy and immunology 152: 368-377.
- Yin SC, Liao EC, Chiu CL, Chang CY, Tsai JJ (2015) Der p2 Internalization by Epithelium Synergistically Augments Toll-like Receptor-Mediated Proinflammatory Signaling. Allergy Asthma Immunol Res 7: 393-403. [Crossref]
- Akdis CA, Akdis M (2015) Advances in allergen immunotherapy: aiming for complete tolerance to allergens. Science translational medicine 7: 280ps286.
- Cohen SG, Frankland AW, Dworetzky M (2003) Noon and freeman on prophylactic inoculation against hay fever. The Journal of allergy and clinical immunology 111: 1142-1150.
- Durham SR, Walker SM, Varga EM, Jacobson MR, O'Brien F, et al. (1999) Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med 341: 468-475. [Crossref]
- Tsai JJ, Wang SR (1983) [Appraisal of the efficacy of hyposensitization in extrinsic asthmatics]. Taiwan yi xue hui za zhi 82: 1040-1044.
- Bousquet J, Lockey R, Malling HJ (1998) Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. The Journal of allergy and clinical immunology 102: 558-562.
- Bousquet J, Van Cauwenberge P, Khaltaev N, Aria Workshop G, World Health O (2001) Allergic rhinitis and its impact on asthma. The Journal of allergy and clinical immunology 108: S147-334.
- Shirakawa T, Enomoto T, Shimazu S, Hopkin JM (1997) The inverse association between tuberculin responses and atopic disorder. Science 275: 77-79. [Crossref]
- Tsai JJ, Peng HJ, Shen HD (2002) Therapeutic effect of bacillus calmette-guerin with allergen on human allergic asthmatic patients. Journal of microbiology, immunology, and infection 35: 99-102.
- Hsiao KC, Smart J (2015) Comment on 'Anaphylaxis caused by in-season switchover of sublingual immunotherapy formulation'. Pediatric allergy and immunology 25: 714-715.
- Niu CK, Chen WY, Huang JL, Lue KH, Wang JY (2006) Efficacy of sublingual immunotherapy with high-dose mite extracts in asthma: a multi-center, double-blind, randomized, and placebo-controlled study in Taiwan. Respiratory medicine 100: 1374-1383. [Crossref]
- Georgitis JW, Reisman RE, Clayton WF, Mueller UR, Wypych JI (1983) Local intranasal immunotherapy for grass-allergic rhinitis. The Journal of allergy and clinical immunology 71: 71-76.
- Georgitis JW, Clayton WF, Wypych JI, Barde SH, Reisman RE (1984) Further evaluation of local intranasal immunotherapy with aqueous and allergoid grass extracts. The Journal of allergy and clinical immunology 74: 694-700.
- Andri L, Senna G, Betteli C, Givanni S, Andri (1993) Local nasal immunotherapy for Dermatophagoides-induced rhinitis: efficacy of a powder extract. The Journal of allergy and clinical immunology 91: 987-996.
- Motta G, Passali D, De Vincentiis I, Ottaviani A, Maurizi M, et al. (2000) A multicenter trial of specific local nasal immunotherapy. Laryngoscope 110: 132-139. [Crossref]
- Tsai JJ, Liao EC, Tsai FH, Hsieh CC, Lee MF (2009) The effect of local nasal immunotherapy in allergic rhinitis: using strips of the allergen dermatophagoides pteronyssinus. The Journal of asthma 46: 165-170.
- Tsai JJ, Ho CY, Wang SR (1995) Relationship between nasal resistance and airway hyperreactivity following nasal provocation with Dermatophagoides pteronyssinus in allergic rhinitis. International archives of allergy and immunology 106: 286-290.
- Pajno GB (2005) Children's compliance with allergen immunotherapy according to administration routes. The Journal of allergy and clinical immunology 116: 1380-1381.
- Liao EC, Tsai JJ (2011) Clinical effectiveness of Tyrophagus putrescentiae allergy by local nasal immunotherapy using strips of Dermatophagoides pteronyssinus. The Journal of asthma 48: 957-964.
- Bennion BJ, Daggett V (2003) The molecular basis for the chemical denaturation of proteins by urea. Proceedings of the National Academy of Sciences of the United States of America 100: 5142-5147.
- Norman PS, Ishizaka K, Lichtenstein LM, Adkinson NF (1980) Treatment of ragweed hay fever with urea-denatured antigen E. The Journal of allergy and clinical immunology 66: 336-341. [Crossref]
- Yu SJ, Liao EC, Tsai JJ (2015) Effects of local nasal immunotherapy in allergic airway inflammation: Using urea denatured Dermatophagoides pteronyssinus. Human vaccines & immunotherapeutics 11: 915-921. [Crossref]
- Akdis CA (2012) Therapies for allergic inflammation: refining strategies to induce tolerance. Nature medicine 18: 736-749.
- Burks AW (2013) Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. The Journal of allergy and clinical immunology 131: 1288-1296 e1283.
- Canonica GW, Compalati E (2015) Year in review: allergen immunotherapy. Annals of allergy, asthma & immunology. Official publication of the American College of Allergy, Asthma, & Immunology 114: 173-174.


