Abstract
Objective: To perform a diagnostic concordance test for wheezing identification through thoracic auscultation with traditional stethoscope compared with the wheezing sensor device WheezeScan in pediatric patients.
Design: Observational, cross-sectional, diagnostic test study to evaluate diagnostic concordance, sensitivity and specificity in wheezing detection in pediatric patients 4 months to 7 years of age.
Methods: Potential patients were identified during routine hospital visits, if a patient was eligible, the researcher invited the parent or tutor and presented those who agreed to participate, with an informed consent form.
Results and conclusions: Concordance for all patients was 98.9% and the kappa index was 0.98 (0.88, 0.99), identifying the concordance strength as good and very good. Sensitivity was at 100% and a specificity of 99% (92,100). Two incidents were reported during the study: a patient in the wheezing and nursing group (2.2% and another in the control and preschool group showed signs of localized erythema that did not require treatment and were resolved minutes after being in contact with the sensor. No adverse events classified as related to the device were reported.
Key words
Auscultation, concordance, pediatrics, wheeze detector, WheezeScan.
Background
Respiratory diseases constitute the first cause of outpatient visits in the pediatric population with over 50% of visits in the first contact level and mainly in the younger age groups. Viral infections of the upper respiratory tract (URTVI) are infectious diseases that affect the respiratory tract above the epiglottis for at least 15 days and represent an important public health issue in the pediatric population [1].
10% of all respiratory diseases affect the lower airways and are characterized by dyspnea and wheezing, constituting the first cause of hospital admission through the emergency ward, with patients younger than 3 years old being the most affected and admitted more frequently [2].
The main causes related to respiratory diseases that present with wheezing in pediatric patients from 1 to 9 years of age in 2019 were: acute respiratory infections, asthma, pneumonia, and bronchopneumonia. Asthma is the most common chronic disease in childhood and the main cause of academic absenteeism, emergency pediatric consultation, emergency department visits, and hospitalization. At Mexico’s Pediatric Hospital Federico Gómez, out of the 5200 yearly consultations of 2007 to the allergy department, 80% were asthma related [3].
Recurring wheezing in nursing and preschool patients are a frequent consultation reason in first contact clinics that usually requires at-home monitoring, which can pose a challenge to the unexperienced parent or caretaker. The delay on treatment initiation can have an impact on the patient’s long-term health. Objectively detecting wheezing and differentiating them from other respiratory sounds isn’t always simple and patients a healthcare professionals can have different definitions of wheezing. Additionally, thorax auscultation requires a certain technique subtlety and wheezing can elude detection, considering the limited testing options for their diagnosis, a more meticulous auscultation is required for their detection and follow-up [4-6].
When parents aren’t able to identify wheezing correctly, they can underestimate the child’s condition or otherwise worry excessively, this disconnection between the perceived condition of the patient and the reality can pose a danger to their health by delaying care or cause excessive and unnecessary consultations [7].
Omron’s Healthcare WheezeScan device is the first automatic device for domestic use clinically validated to detect wheezing in children from ages 4 months and up to 7 years. It provides an objective evaluation through a screen that reports the presence or absence of wheezing in 30 seconds, eliminating conjectures or doubts in the parents or caregivers [7].
The WheezeScan device is already available in the United Kingdom, Germany, Italy, Sweden, Holland, Luxemburg, Hungary, Slovakia, the Czech Republic and Romania with the labeling of Conformitè Europëenne (CE) that indicates the device conforms to European directives, safety and performance normativity in Europe and is apt for the stated purpose without endangering life or its properties.8 At the moment of the present article, no reports of adverse events while using the WheezeScan device during clinical trials or marketing have been reported. The device is not yet available in Mexico as it is yet to obtain the required sanitary registry with local authorities. The present study intends to verify the usefulness of the device to identify wheezing in the pediatric Mexican population.
Objectives
To perform tests of diagnostic concordance, identify sensitivity, specificity and detection concordance for wheezing through traditional pulmonary auscultation in comparison with the WheezeScan device and to document any techno vigilance incidents during the use of the WheezeScan device in pediatric patients that visited the emergency services due to wheezing and control subjects without diagnosis of respiratory diseases.
Methods
An observational, cross-sectional diagnostic test study was performed to determine diagnostic concordance, sensitivity and specificity in patients aged 4 months to 7 years old that visited the emergency services due to wheezing and control patients without diagnosis of a respiratory disease. The study was approved by the Ethics and Research Committee at Medica Sur.
Sample size
We took into consideration published literature that proposes the need to estimate sensitivity of a diagnostic test, defined as the probability of a positive test when the subject is truly sick; specificity relates to the probability of a negative test in patients that are healthy; the Likelihood Ratio (LR), understood as the ratio between the possibility of a result amongst patients with the disease versus the possibility of a result in patients without the disease [9]. We defined α significance at 0.05 and power 1-β at 80, considering a sample size estimation to detect a difference in proportions which is assumed for an equal proportion adjusted to the prevalence of the disease of up to 70% of emergency visits with 12 per group.
Based on the results published by Habukawa et al on the sensitivity, specificity, positive and negative predictive values obtained with the WheezeScan (HWZ-1000T) device of 96.6%, 98.5%, 98,3% and 97.0% respectively, we estimated the sample size for the present study [8]. The resulting sample size for each group with the G* Power V 3.1.9.2 software determined that considering the estimate of 45 patients per group and 2 additional subjects for each strata to ensure the statistical assumptions in case of consent withdrawal, we should include 96 patients. The resulting distribution was: 48 patients with pulmonary disease and wheezing and 48 controls (without pulmonary disease and matching the number by age strata). We included pediatric patients of 4 months to 7 years of age considering the following age groups (Table 1).
Table 1.Age group allocation.
Pediatric age groups |
Wheezing group |
Control Group |
Nursing 120 days to -1 year |
24 |
24 |
Preschool 1-5 years |
12 |
12 |
School age 5- 8 years* |
12 |
12 |
*Only up to 7 years old
Statistical analysis
A primary statistical analysis was performed through bilateral tests with a confidence interval of 95%. We performed a global evaluation of a diagnostic test (true positives and true negatives) to determine sensitivity, specificity and diagnostic concordance in wheezing detection in pediatric patients through traditional thorax auscultation that is considered the gold standard, in comparison with the wheezing sensor WheezeScan amongst a control group vs the wheezing group; considering a p=0.05, with confidence interval at 95% and a concordance level superior to 80%
Population
We included patients aged 4 months to 7 years old with a diagnosis of asthma, bronchitis, bronchiolitis, bronchial hyperactivity, cystic fibrosis or viral pneumonia that were clinically stable or those with an acute onset of respiratory symptoms and/or wheezing without a previous respiratory disease diagnosis as well as control patients without pulmonary disease. We excluded patients with thoracic dermatitis, crying patients, those requiring ventilatory support, patients with intrathoracic devices and those whose parents or caregivers decide to rescind consent. We also excluded patients that present adverse events that, based on the clinician judgement, justify removing them from the study, however; these would still be considered for the concordance and safety analysis.
Procedures
Information was collected through medical records, height and weight evaluation, vital signs measurements and a conference with parents or caregivers to explain the purpose and procedures of the study, presentation of the informed consent form and signature requests.
Diagnostic maneuvers
The patient was situated either sitting or lying on their back based on convenience, uncovering the right side of the thorax. The thoracic auscultation and WheezeScan testing were performed at the same time by different researchers, placing both instruments below the right clavicle for a maximum of 30 seconds. The skin in area where the WheezeScan was placed was examined 30 minutes later to document the presence of any signs of adverse events on the skin.
Results
A total of 96 patients were included during the period of the 22nd of April to the 22nd of July of 2022, 48 patients were in the wheezing group and 48 in the control group. During the diagnostic manouvers, two patients in the wheezing group were removed from the study due to crying (Figure 1).
Figure 1. Study patient flow.
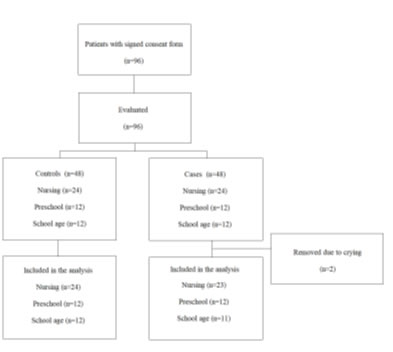
The demographic and clinical characteristics of the analyzed patients are shown in Table 2. Theaverage age was 2.66 years and 53.2% (n=50) were male. At the moment of the diagnostic manouvers, the most common respiratory diseases were: Bronchial hyperreactivity 18 (19.1%), bronchitis 9 (9.6%), bronchiolitis 9 (9.6%) and viral pneumonia 9 (9.6%).
Table 2. Demographic and clinical characteristics.
|
Total |
Wheezing |
Controls |
p |
|
(N = 94) |
(N = 46) |
(N = 48) |
|
Age (avg (SD)) |
2.66 (2.31) |
2.59 (2.28) |
2.72 (2.35) |
0.789 |
Gender (male) n (%) |
50 (53.2) |
25 (54.3) |
25 (52.1) |
0.989 |
Disease History |
|
|
|
|
Allergy/Immunologic n (%) |
5 (5.3) |
4 (8.7) |
1 (2.1) |
0.333 |
Endocrine n (%) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
- |
Pulmonology n (%) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
- |
Psychiatric n (%) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
- |
Other n (%) |
14 (14.9) |
11 (23.9) |
3 (6.3) |
0.035 |
Disease Presenting with Wheezing |
|
|
|
|
Asthma n (%) |
2 (2.1) |
2 (4.3) |
0 (0.0) |
0.456 |
Bronchitis n (%) |
9 (9.6) |
9 (19.6) |
0 (0.0) |
0.004 |
Bronchiolitis n (%) |
9 (9.6) |
9 (19.6) |
0 (0.0) |
0.004 |
Bronchial Hyperactivity n (%) |
18 (19.1) |
18 (39.1) |
0 (0.0) |
<0.001 |
Cystic fibrosis n (%) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
- |
Viral Pneumonia n (%) |
9 (9.6) |
9 (19.6) |
0 (0.0) |
0.004 |
Other n (%) |
7 (7.5) |
7 (15.2) |
0 (0.0) |
0.016 |
No pulmonary disease n (%) |
48 (51.1) |
0 (0.0) |
48 (100.0) |
<0.001 |
Probable Adverse Event |
|
|
|
|
Rash, itching, redness or cutaneous lesion = YES n (%) |
2 (2.1) |
1 (2.2) |
1 (2.1) |
0.983 |
Required treatment = NO n (%) |
2 (2.1) |
1 (2.2) |
1 (2.1) |
0.983 |
Lesion resolved = YES n (%) |
2 (2.1) |
1 (2.2) |
1 (2.1) |
0.983 |
N: number; avg: average; SD: Standard Deviation
Two patients in the nursing and preschooler age group presented localized erythema that required no treatment and resolved minutes after contact with the wheezing sensor, both of these incidents were classified as not related to the use of the devise based on definitions on the norm NOM-240-SSA1-2012 [10].
Concordance values and diagnostic test obtained by groups are shown on Table 3, where the concordance for the total population was 98.9%, 97.8% in nursing age and 100% for preschool and school age patients. The Kappa index by age groups was 0.95 (0.77, 0.99), 1 (0.71,1) and 1 (0.70, 1) each and 0.98 (0.88, 0.99) for the total population with a good and very good concordance strength based on the qualitative index that classifies them as insignificant (0.00 to 0.20), low (0.21 to 0.40), moderate (0.41 to 0.60), good (0.61 to 0.80), and very good for values above 1 [11].
Table 3. Concordance and Diagnostic Test evaluations
|
Total
(N=94) |
Nursing age
(N=47) |
Preschool age
(N=24) |
School age
(N=23) |
Concordance(%) |
98.9 |
97.8 |
100 |
100 |
kappa (CI 95%) |
0.98 (0.88, 0.99) |
0.95 (0.77, 0.99) |
1 (0.72, 1) |
1 (0.70, 1) |
Sensitivity % (CI 95%) |
100 (86, 100) |
100 (72, 100) |
100 (63, 100) |
100 (48, 100) |
Specificity % (CI 95%) |
99 (92, 100) |
97 (85, 100) |
100 (79, 100) |
100 (81, 100) |
Positive Predictive Value % (CI 95%) |
96 (80, 100) |
92 (62, 100) |
100 (63, 100) |
100 (48, 100) |
Negative Predictive Value % (CI 95%) |
100 (95, 100) |
100 (90, 100) |
100 (79, 100) |
100 (81, 100) |
LR+ % (CI 95%)* |
70 (10, 490) |
36 (5.21, 248.66) |
- |
- |
LR- % (CI 95%)* |
0 (0, NaN) |
0 (0, NaN) |
0 (0, NaN) |
0 (0, NaN) |
False positive % (CI 95%) |
1 (0, 8) |
3 (0, 15) |
0 (0, 21) |
0 (0, 19) |
False negatives % (CI 95%) |
0 (0, 14) |
0 (0, 28) |
0 (0, 37) |
0 (0, 52) |
Precision (TP+TN) / NTotal % (CI 95%) |
4 (0, 20) |
8 (0, 38) |
0 (0, 37) |
0 (0, 52) |
Concordance (%) |
0 (0, 5) |
0 (0, 10) |
0 (0, 21) |
0 (0, 19) |
kappa (CI 95%) |
99 (94, 100) |
98 (89, 100) |
100 (86, 100) |
100 (85, 100) |
SD: Standard Deviation; N: number; %: Percentage; CI: Confidence Interval; LR+: Positive Likelihood Ratio; LR- Negative Likelihood Ratio; NaN: Not a Number. *Values are omitted in LR+ and LR- where at the moment of estimation, we obtained non-defined expressions (divided by zero) due to the fact of having sensitivity and/or specificity at 100%
Sensitivity was 100% for the total population vs the traditional method. Specificity values were 97% (85,100) for the nursing age group, 100% (79, 100) for preschoolers,100% (81, 100) in school-age patients and 99% (92, 100) for the entire sample. The Positive Predictive Value (PPV) and Negative Predictive Value (NPV) were 96% (80, 100) and 100% (95, 100) for the entire population. (Table 3). Only one false positive was identified and it was amongst the nursing age group (Table 2).
The Likelihood Ratio (LR) values showed a +LR of 36 (5.21, 248.66) for the nursing age group and 70 (10, 490) for the entire sample. No -LR values were obtained. (Table 3). According to Monsreal et al, a positive LR above 10 and a negative LR below 0.1 can indicate a high plausibility that a patient that presents the measured event has a positive result and that a patient without it has a negative result [12] (Table 3).
Discussion
In 2008, a study evaluated the precision of a multisensory device with an automatized technique for wheezing detection. The sensitivity and specificity obtained where 83% and 85%, and the NPV and PPV were 89% and 79% respectively. The authors concluded that the device was easy to use and the detection algorithm was precise, sensible and specific, with good PPV and NPV for the detection of wheezing, similar to the findings in our present study [13].
Chun Yu et al, conducted a study in 2013 with a soft stethoscope with a polymer chamber and a unidirectional microphone; a diaphragm was set to the sound collector chamber to propagate the sound from the body surface to the microphone in order to measure the respiratory sounds of pediatric patients in the emergency room of the pediatrics department in the University Hospital of Taiwan. The results revealed the system provided an 88% sensitivity and 94% specificity in detecting wheezing, concluding that the device could be easily used in young children in a loud environment, hence it could be used at home by parents that wanted to measure and monitor their children’s condition [14].
In 2020, Habukawa et al published a study in 214 pediatric patients between the ages of 2 months and 12 years 11 months that visited the emergency services in Wakayama, Japan for the treatment of recurring qwheezing with cough and dyspnea, finding that wheezing detection was not influenced by age, and they were successfully detected by the algorithm. Sensitivity, specificity, PPV and NPV for the wheezing recognition algorithm were 100%, 95,7%, 90,3% and 100%, respectively [15].
Habukawa et al published another study in 2021 with 374 ambulatory patients aged 4 to 107 months of age in the pediatric services of 10 institutions where wheezing was detected through thoracic auscultation with a stethoscope and registered during 30 seconds using the device of wheezing recognition algorithm WheezeScan. Sensitivity, specificity, PPV and NPV for the device were 96.6%, 98.5%, 98.3% and 97.0%, respectively, concluding that the WheezeScan could be useful in the practical implementation of homebased management of asthma patients and remote medical assistance [16].
Detecting and documenting respiratory sounds objectively and with precision is an essential part of the diagnostic, treatment and follow-up process of respiratory diseases in pediatric patients to ensure timely treatment and avoid exacerbations and asthma attacks, reducing hospital admissions and mortality. Ruling out wheezing can also help by calming parents and caretakers during follow-up and could contribute to improve quality of life [6].
The COVID-19 pandemic brought along new challenges to wheezing detection, particularly in first contact clinics, where telemedicine, infection control measures and social distancing can result in treatment barriers and where the close quarters required for thoracic auscultation becomes both a real and perceived risk of infection [6].
Precise and easy to use devices for wheezing detection can help reduce the element of doubt, increasing objectivity and precise documentation of symptoms, could facilitate agreement and understanding amongst healthcare personnel, parents, and caregivers. Using these devices as monitoring tools can also bring tranquility and help families understand the evolution of symptoms, allowing them to identify the adequate moment to seek medical attention [6].
Conclusions
Concordance for all patients was 98.9% and the kappa index was 0.98 (0.88, 0.99), with a good and very good concordance strength. Sensitivity was 100% and specificity was 99%. 2 incidents of localized erythema were reported but it disappeared after removing the sensor and they were not classified as related to the device.
Author’s contributions
Study concept: RSAV, JJEZ, SGV. Patient evaluation: RSAV, JLPG, AAC, MPCG, APCT, MQC, JDMPU, AICG. Analysis: SGV. Manuscript: RSAV, SGV, KDTM, BPS, HGF. Review and approval: all authors read and approved the final manuscript.
Consent for publication
All authors have read and approved the present manuscript and are in agreement of its publication.
Acknowledgements
The review and publication of this article was financed by Omron HealthCare México S.A de C.V.
Funding
The present study was funded by Omron HealthCare México S.A de C.V.
Competing interests
RSAV, JLPG, AAC, MPCG, APCT, MQC, JDMPU, AICG declare no conflict of interest for the present study.
SGV, KDTM and BPS declare a paid work relationship with HS Pharmacoeconomic Research.
YM is a Sponsor, JJEZ is a medical marketing and VS is a regulatory affairs manager at Omron HealthCare Mexico
- Aldana RSV, Almaraz, Cantu GMC, Ceballos BA, García CA, et al. (2016) Safety and efficacy of portable nebulizer NE-C801KD, for humid application on pediatric patients with non-complicated upper respiratory tract viral infections. Pediatr Dimen 1: 130
- Aguilera ZF, Huerta LJG (2016) Recurrent wheezing and risk factor for asthma development. Alerg Asma Inmunol Pediatr 25: 12-23. [Text in Spanish]
- Mexico’s Epidemiology Department – National Epidemiology Surveillance System (SUIVE) Top twenty national disease causes by age group for 2018. https://epidemiologia.salud.gob.mx/anuario/html/principales_nacional.html [Text in Spanish]
- Sansano MÚ (2017) Treatment of recurrent wheezing. Rev Pediatr Aten Primaria 19: 27-34.
- Ortega Martell JA, Fernandez-Vega M (2009) Asthma Diagnosis. Diagnóstico de asma. Neumología y cirugía de tórax 68: S116-S122. [Text in Spanish]
- 6.Russell R (2021) Detect and Document: Early and Accurate Identification of Wheeze Can Improve Quality of Life in Children with Asthma. EMJ Respir 9: 2-6.
- Jarvis S (2020) Identifying asthmatic symptoms in very young children. OMRON Healthcare Europe.
- Habukawa C, Ohgami N, Arai T, Makata H, Tomikawa M, et al. (2021) Wheeze Recognition Algorithm for Remote Medical Care Device in Children: Validation Study. JMIR Pediatr Parent 17: e28865
- Ward JJ, Wattier BA (2011) Technology for enhancing chest auscultation in clinical simulation. Respir Care 56: 834-845.
- Mexico’s Federal Official Journal NOM-240-SSA1-2012, Establishment and operation of technovigilance systems. October 30th of 2012 Internet. Available at: https://dof.gob.mx/nota_detalle.php?codigo=5275834&fecha=30/10/2012#gsc.tab=0 [Text in Spanish]
- Andalusian Society for Intensive Care Medicine and Coronary Units.Concordance meassurements Cohen’s Kappa.2018 Internet.Available at: https://www.samiuc.es/estadisticas-variables-binarias/medidas-de-concordancia/kappa-de-cohen/ [Text in Spanish]
- Monsreal F, Panti AM, Peraza LES, Uluac MSS (2021) Positive and negative likelihood ratios of two anthropometric indices in the diagnosis of nutritional situations overweight and obesity. South Florida Journal of Development, Miami 2: 1319-1334.
- 13.. Guntupalli KK, Alapat PM, Bandi VD, Kushnir I (2008) Validation of automatic wheeze detection in patients with obstructed airways and in healthy subjects. J Asthma 45: 903-907. [Crossref]
- 14. Yu C, Tsai TH, Huang SI, Lin CW (2013) Soft stethoscope for detecting asthma wheeze in young children. Sensors (Basel) 6: 7399-413.
- 15. Habukawa C, Ohgami N, Matsumoto N, Hashino K, Asai K, et al. (2020) A Wheeze Recognition Algorithm for Practical Implementation in Children. PLoS One 15: e0240048. [Crossref]
- 16. Habukawa C, Ohgami N, Arai T, Makata H, Tomikawa M, et al. (2021) Wheeze Recognition Algorithm for Remote Medical Care Device in Children: Validation Study. JMIR Pediatr Parent 17: e28865. [Crossref]

