Background: Both severities of cardiac surgery and technical features of extracorporeal circulation circuit demand blood transfusion from donors, which involves a number of risks for the patient, especially with the low body weight. “Priming” of the cardiopulmonary bypass circuit with patients' own blood [retrograde autologous priming (RAP)] is a technique used to limit haemodilution and reduce transfusion requirements.
The aim of the study was to investigate what are the conditions for successful management of cardiopulmonary bypass (CPB) in children with the weight less < 20 kg without or with minimal possible amount of transfused blood.
Methods: the study included 250 children (131 boys, 119 girls) with congenital heart disease, operated on heart under CPB, weighing less than 20kg (18.45 ± 2.15) and 3.4 ± 1.7 years average age, who were divided into an experimental (125 children) and a control group (125children). In the control group conventional CPB was performed (supplementing the “priming” with red blood cells), while in experimental one CPB started after RAP via aortic cannula with recuperation till 45 % of crystaloid “priming”. The hematocrit (Hct), lactate (Lac) levels at two perioperative time-points, intraoperative and postoperative blood usage were recorded. There were no significant differences in CPB time, aortic cross-clamp time between the groups.
Results: No hospital lethality occurred in the study and no surgical hemostasis was performed. Blood loss accounted for 6.2 ml/kg/24h in whole study. Among children who received transfusion on pump, the number of packed red blood cells was less in the RAP group than that in the standard “priming” group intraoperatively and postoperatively (0.54 ± 0.17 vs. 1.48 ± 0.68 units, P = 0.03; 0.94 ± 0.54 vs. 1.69 ± 0.69 units, P = 0.15). 73 children needed perioperative transfusion of homologous blood (erythrocyte mass), that made up only 29, 2% of the whole study group. There were no significant differences in CPB time, aortic clamp, and Lac value between the two groups (P>0.05). Length of ICU and hospital stay was similar.
Conclusions: The team approach management is successful for bloodless or minimally usage of blood infant cardiosurgery. Priming” minimalization and retrograde autologous blood priming (RAP), modified ultrafiltration (MUF) could diminish the necessity of perioperative blood transfusion in infant cardiac surgery.
Blood transfusion may be responsible for transfusion reactions (Table1, Figure 1), transmission of infection is associated with increased postoperative morbidity and mortality, risk of immunosuppression and increased hospitalization costs [1,2]. The use of blood components presents an independent risk factor for postoperative infection [3]. Cardiopulmonary bypass (CPB) may increase the rate of postoperative bleeding [4], thus contributing to the need for blood components administration. CPB-free surgical interventions have a significant reduction in haemorrhagic complications and haematransfusion, respectively [5].
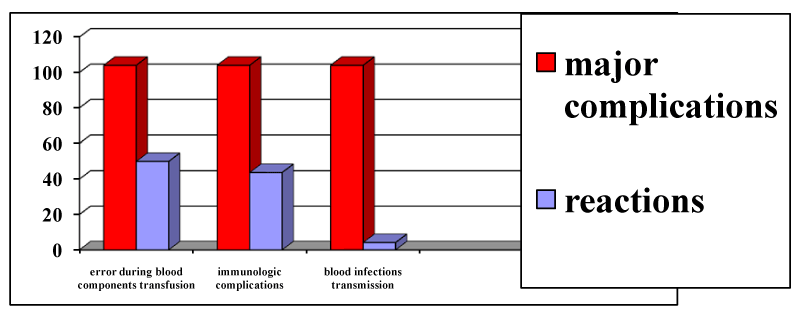
Figure 1. Dutch transfusion complications (2010)
Table 1. Number of post-transfusion complications in Republic of Moldova.
Tip of reaction |
Year |
2012 |
2013 |
Total |
52 |
45 |
Pirogenic |
3 |
2 |
Antigenic |
27 |
22 |
Alergic |
22 |
21 |
There are several causes that expose the operated child with congenital heart disease to the development of major bleeding during heart surgery. Among the predictors of increased need for haematransfusions in children are attributed: 1. Cyanotic cardiac disease; 2. Low age and body weight. 3. Low postoperative hematocrit and hemoglobin rate. 4. Comorbidities. 5. Urgency. 6. Interventions with increasing CPB time. In this context, CPB was emphasized as an important precipitating factor in the occurrence of postoperative hemorrhage resulting from the consumption of coagulation factors, hemodilution, hypothermia and, in particular, as a result of the inflammatory response [6]
Several strategies have been proposed to reduce postoperative haemorrhages, thereby minimizing the need for blood transfusions and blood components. These strategies include the following links: 1. Preoperative preparation, administration of erythropoietin, or iron preparations several weeks before cardiac surgery [7,8] and autologous blood collection prior to surgery (rarely used in children) [9]; 2. Strategies applied during cardiac surgery, such as the reduction of priming volume – retrograde autologous blood priming (RAP) and the use of hemoconcentrators [8], Cell Saver use [10,11], moderate hypothermia (32-34 ° C) [8], reinfusion of the whole amount of blood remaining in the CPB tubes and oxigenator [13] and the use of antifibrinolytics such as tranexamic acid, aminocapronic acid [14-24], as well as reinfusion of the blood collected from the mediastinal drainage within the first six hours postoperatively [13].
Finally, it is admitted that despite of the recommendations, the main factor in reducing the use of blood components is the well-thought strategies and maneuvers of the operating team. The team approach is first of all the condition for successful child cardiac operation with CPB without or minimally use of homologous blood.
Retrograde autologous priming (RAP) has been routinely applied in cardiac adult and paediatric CPB [2]. However, this technique is performed in pediatric patients weighing more than 20 kg, and research about its application in pediatric patients weighing less than 20 kg is still disputable. This study touched upon the clinical application of RAP in CPB in infant patients undergoing cardiac surgery. With the increasing number of performed cardiac surgeries, priming technique in cardiopulmonary bypass (CPB) has become an important area of research. Complex cardiovascular surgery will often requires a large amount of banked blood or blood products, which are commonly limited, and may cause immune response problems, virus dissemination, and others. This encourages physicians to explore blood conservation measures that can reduce the need for allogeneic blood transfusion. At the same time, priming of conventional crystal solution in CPB will inevitably cause serious haemodilution and reduction of plasma colloid osmotic pressure, which will produce adverse effects [25-26]. It has been demonstrated that the applications of retrograde autologous priming (RAP) in adult rheumatic heart disease and cardiac surgeries for coronary heart diseases can improve the hematocrit (Hct) level, reduce the postoperative chest drainage volume and allogeneic blood transfusion, indicating that RAP is a safe and cost-effective blood conservation technique [27]. The application of RAP in pediatric CPB reduces priming volume, and keeps a high Hct level during bypass [28]. In infants and young children the blood volume is small, and therefore the effect of RAP on hemodynamic is greater than in adults. Therefore, the application of RAP to infants and young children is limited.
We have been successfully applying RAP to children <20 kg. We believe that, as blood volume of infants is less than that of adults, moderately reducing the volume of priming solution could result in an improved outcome in mitigating the haemodilution. In this study, we applied RAP in CPB in pediatric patients with the body weight within 13 and 20 kg, and investigated whether it can reduce the perioperative blood transfusion volume.
The purpose of this study is to evaluate the measures taken by our team to reduce the use of blood transfusion and blood components, starting with the decrease in the incidence of haemorrhagic events, thus reducing morbidity and mortality, and high costs.
This study was approved by the Bioethics Committee of the Moldavian Republican Hospital and written informed consent was obtained from the parents or their representatives. Between October 2011 and October 2016 250 children (131 boys, 119 girls) with congenital heart disease, operated on heart under CPB, weighing less than 20 kg (18.45 ± 3.15) and 3.4 ± 1.7 years average age, were divided into 2 groups experimental (125 children) and control (125children). Inclusion criteria were: the body weight of 13-20 kg; preoperative hemoglobin (HB) level higher than, or equal to 100 g/L; and were elective for CPB intracardiac correction. Exclusion criteria were: CPB longer than 80 min and any difficulties impermissible during surgery. For this single-blind experiment, the patients were divided into the control group (n=125) and the experimental group (n=125) using the random number table technique. Among the patients of the control group, 48 were submitted to auricular septal defect repairing (ASDR), 68 patients were submitted to ventricular septal defect repairing (VSDR), and 9 patients had tetralogy of Fallot (TOF) repair. In the experimental group, 64 patients had VSDR, 44 patients had ASDR, and 17 patients had ASDR with the correction of anomalous drainage of one, two, or three pulmonary veins (ADPV) (Table 2). All the patients in the control group completed the surgery, while 1 patient from the experimental group was excluded because the CPB time was longer than 80 min. All the patients were operated on by one operating team (surgeon, anesthesiologist and perfusionist).
Table 2. Diagnoses and operations.
Diagnosis |
VSD with pulmonary
hypertension (HPA) |
VSD + Artery pulmonary
stenosis (AP stenosis) |
ASD or foramen ovale (FO) |
ASD+ ADPV |
Atrioventricular canal
partial (without VSD) |
Tetralogy Fallot |
Nr. patients |
131(68exp.+63 control) |
1(control group) |
91(43exp+48) |
17(10exp.+7 control |
1exp. |
9(5exp.+4 control) |
Operaţia |
Suture or plastie |
Plastie + Comisuro tomie of
pulmonary Artery Valve |
Suture or plastie |
Plastie + Redresarea ADPV |
Radical correction |
Radical correction |
Surgical methods
A longitudinal incision was performed at the sternum median, and the heart was exposed. The ascending aorta and the right atrium were isolated, and the aortic cannulae 12-14 Fr and venous cannulas 16-18 Fr were inserted, after standart heparinization ( 3mg per kg) respectively, to connect CPB circuit (standard for infants; composed of ¼ × 1/4 inch tubing lines; total volume of priming 320 mL). The infants membrane oxygenators (“Capiox 05”- “Terumo”, “Affinity Pixie”-“Medtronic”, “Sorin Kids” -“Sorin Group”) were used intraoperatively. After the body temperature was cooled to 34°C, the ascending aorta was clamped, and the antegrade perfusion with pump potassium 40ml/hour , blood cardioplegia (20 mL/kg) was performed, followed by the second intraoperative perfusion after 20-30 min interval (the weight of the left ventricle,(g) x 2 = ml of pump blood cardioplegia). The aortic clamp time was 20-70 min. The CPB continued to operate after the ascending aorta was declamped, for duration of not less than 1/4 of the aortic blocking time. After surgery, the patients were monitored and treated in the intensive care unit (ICU).
RAP method
All the children were actively supplemented with crystalloid or colloid solution before surgery to avoid lack of circulating blood volume due to fasting. For the experimental group a sodium chloride compound priming solution was used to pre-fill the circulation tubes and debubbling air. After the patients were heparinized, the aortic cannulation was connected, and the inner loop and connecting tubes were opened, so that the blood inside the arterial tube could slowly return and replace the same amount of priming solution (stored in a spare bag). When the tube from the oxygenator to the aortic cannulation site was completely filled with blood, the arterial tube was clamped. The vena cava cannulation was connected, and the occlusion clamp of venous drainage tube was slowly opened. The venous blood was used to completely replace the liquid inside the venous tube (antegrade autologous blood priming). Meanwhile, the same amount of liquid was pumped and stored in a spare bag. During surgery, the blood pressure, electrocardiogram and blood oxygen saturation of patients were closely monitored. If necessary, vasoactive agents were used to reduce the adverse effect of RAP on hemodynamics. If the systolic blood pressure dropped to <60 mmHg, 4-10 µg norepinephrine was immediately injected intravenously to elevate blood pressure. If no reaction on blood pressure was achieved after norepinephrine injection, the RAP was immediately interrupted, and the priming with suspended red blood cells and albumin was performed.
In the control group, the volume of banked blood was calculated as follows: Volume of donors’ blood = 320 × Hct target - blood volume × (Hct preoperative – Hct target)/Hct banked blood. The fresh (<5 days age) blood, and 25ml of 20% albumin were added to CPB circuit to replace the exact volume of the priming solution.
CPB management
The surgeries were performed using CPB machine “Perfusion system 1” (“Terumo”). During CPB operation, the exogenous liquid input was reduced, and the conditions maintained as follows: the colloid osmotic pressure >275 mosm/l, the body temperature 32-34°C, the average blood pressure >40 mmHg, CPB flow 120-150 mL/kg. When the average blood pressure dropped to <40 mmHg, 4-10 µg norepinephrine was immediately intravenously injected to elevate the blood pressure. When BE achieves the ratio -4-6 mmol/l, 20-30 mL of sodium chloride compound solution was intravenously injected. If, the RAP was immediately interrupted, the priming with fresh red blood cells, and albumin was performed. After CPB, the modified ultrafiltration and transfusion of blood products were used according to the patient Hct level (target Hct >0.40) and normal colloid osmotic pressure.
CPB time, AORTIC CLAMP time, blood transfusion
Hct and lactate (Lac) levels were recorded before surgery. During surgery, the CPB time, aortic clamp time and the intraoperative blood transfusion were recorded. In addition, Hct and Lac values 15 min after the beginning (T2) and at the end (T3) of CPB were recorded. After surgery, the mechanical ventilation time, ICU time, hospitalization duration and postoperative blood transfusion were recorded. In addition, the Hct value at 2 h after surgery (T4) was recorded.
Statistical analysis was carried out using the SPSS 14.0 software (SPSS Inc., USA). Data are reported as means ±SD. Comparisons between the two groups were performed using the two-sample t-test. P<0.05 was considered to be statistically significant.
One case in the experimental group was excluded because the CPB time was longer than 80 min. All the patients of the experimental group completed RAP, and only 12 patients were administrated norepinephrine for unstable blood pressure.
The experimental group significantly reduced priming amount, and 101 patients had no allogeneic blood transfusion perioperatively, while 76 patients of the control group have not received allogeneic blood transfusion. Perioperative and postoperative transfusion of homologous blood (red blood cells) needed 73 (24 children from experimental group and 49 from control group), that make up only 29.2% of the whole study group. All the patients were discharged successfully, and exhibited no blood transfusion-induced complications during hospitalization.
General information
There were no significant differences in gender, age, body weight or other general information between the two groups (P>0.05). Furthermore, the preoperative Lac, creatinine, urea and Hct levels between the two groups showed no significant difference (P>0.05 Table 3.).
Table 3. Comparison of intraoperative and postoperative indicators.
Index |
Experimental group (125) |
Control group (125) |
P |
Age (months) |
39.91 ± 19.1 |
40.02 ± 20.5 |
0.96 |
boys |
66 |
65 |
|
girls |
55 |
56 |
|
Body weight (kg) |
18.47 ± 2.7 |
18.1 ± 1.91 |
0.23 |
Preoperative urea, mmol/l |
3.96 ± 0.59 |
4.14 ± 0.6 |
0.37 |
Preoperative creatinine, mmol/L |
28.91 ± 4.71 |
29.87 ± 4.75 |
0.94 |
Pre CPB hematocrite, % |
34.73 ± 2.19 |
36.97 ± 2.71 |
0.72 |
PreCPB lactate,mmol/l |
1.07 ± 0.27 |
0.94 ± 0.27 |
0.24 |
There were no significant differences of CPB time, aortic clamp time, T2-Lac or T3-Lac between the two groups (P>0.05). (Table.4). However, the T2-Hct and T3-Hct values, and the intraoperative blood transfusion exhibited significant differences between the two groups (P<0.05; Table 2.). Hct levels in the experimental group were lower than those in the control group, but still maintained at >0.25 (except for twelwe cases), which met the requirements for intraoperative blood management (added blood to 12 children to target Hct >0.25). In addition, the blood gas results were normal, and there was no difference in oxygen metabolism between the two groups, indicating that hemodynamics was stable during CPB in both groups. In order to further improve the Hct level (the target being >0.40), the modified ultrafiltration was performed in both groups. According to the target hematocrite and the return all the blood to the patient, the volume of the modified ultrafiltration was set as 320-450 mL (till 20 minutes).
Table 4. Comparison of intraoperative and postoperative indicators.
Index |
Experimental group (125) |
Control group (125) |
P |
CPB time,min. |
51.07 ± 16.71 |
52.05 ± 18.95 |
0.81 |
Aortic clamp time,min |
24.91 ± 12.27 |
24.48 ± 12.41 |
0.64 |
T2 Lac mmol/l (during CPB) |
1.34 ± 0.97 |
1.047 ± 0.69 |
0.32 |
T2 Ht %(during CPB) |
25.07 ± 0.27 |
27.82 ± 0.54 |
0.01 |
T3 Lac mmol/l(end of surgery) |
1.91 ± 0.14 |
2.05 ± 0.27 |
0.24 |
T3 Ht %(during CPB) |
40.91 ± 4.5 |
41.85 ± 1.92 |
0.003 |
T4 Ht% after 2 hour of surgery(in ICU) |
41.85 ± 0.27 |
40.97 ± 0.47 |
0.31 |
Mechanical ventilation time, min |
270.22 ± 9.51 |
279.11 |
0.84 |
ICU time, days |
2,1 ± 0,45 |
1,91 ± 0,34 |
0,51 |
Perioperator blood transfusion |
12(intraoperator)+12postoperator |
49(29 intraoperator +20 postoperator) |
P<0.05 |
There was no significant difference in T4-Hct value, mechanical ventilation time, ICU time, hospitalization duration. At 2h postoperative, Hct levels in experimental group were higher than the control group, but the difference was not significant.
There exist various degrees of hemodilution in CPB, which may exhibit advantages such as reduced peripheral vascular resistance, improved microcirculation perfusion, and reduced blood destruction. Excessive hemodilution may lead to kidney damages and affect other organs perfusion. Therefore, moderate hemodilution is an important part of CPB management [29]. Blood conservation has already been vastly studied in CPB research, which includes preoperative autologous preservation, intraoperative hemodilution, and autologous transfusion [9]. Many years of clinical trials, as well as the improvement of membrane oxygenators and CPB tube lines, resulted in the progress of adult CPB, advancing from blood priming to almost bloodless priming, currently. In pediatric CPB, the priming amount should be relatively larger. Therefore, the need for allogeneic blood priming still cannot be avoided in small children. However, apart from having a high risk for immune response problems and disease transmission, banked blood may have shortcomings such as decreased erythrocyte deformability, hemolysis, acidosis, abnormal inflammatory responses of white blood cells, and others. Therefore, in recent years, studies are aimed at reducing allogeneic blood priming in pediatric patients, and important progress has been achieved in children and infants with enough body weight (>20 kg).
This study targeted pediatric patients with body weight within 13-20 kg. Our results indicated that the experimental group, which did not use banked blood, obtained outcomes similar to the control group. Patients with body weight <20 kg have less blood volume then necessary for RAP, which would likely affect hemodynamic stability. Therefore, to perform RAP, blood volume should be positively supplemented before surgery, thus avoiding inadequate circulating blood volume, caused by fasting. During this operation, the patient’s blood pressure, echocardiogram and oxygen saturation should be closely monitored, and anesthesiologists, surgeons and CPB physicians should cooperate closely. Vasoactive drugs should be administered when necessary to reduce the adverse effects of RAP towards hemodynamics. As for patients who show poor heart functions, or signs of intolerance for the RAP technique, the operation should be interrupted promptly. Furthermore, this technique must take into consideration the overall condition of the patients, and a combination with other blood conservation methods, such as modified ultrafiltration, might be considered to achieve the best blood-protective effects and improve prognosis.
In cardiac surgery, the probability of using allogeneic blood in infants and young children is relatively higher than in adults. At present, blood source is relatively limited; therefore using less or not using any banked blood can be an advantage. The successful application of RAP in children with body weight <20 kg can result in satisfactory Hct levels in CPB, and maintain stable hemodynamics. This can effectively ease the situation with the lack of banked blood, and avoid the risk of various complications and infectious diseases related to blood transfusion. In conclusion, RAP can effectively reduce the hemodilution in CPB when using less or not using any banked blood, while meeting the intraoperative perfusion conditions, and decreasing the perioperative blood transfusion volume in pediatric patients. In conclusion RAP could effectively reduce haemodilution in CPB for patients weighting 13-20 kg, while using a minimum of allogenic blood, meeting securely perfusion conditions . As a result fewer (only 24) children required red blood transfusion in experimental group.
- Alghamdi AA, Davis A, Brister S, Corey P, Logan A (2006) Development and validation of Transfusion Risk Understanding Scoring Tool (TRUST) to stratify cardiac surgery patients according to their blood transfusion needs. Transfusion 46: 1120-1129. [Crossref]
- Ferraris VA, Ferrar2021 Copyright OAT. All rights reservperative blood transfusion after cardiac procedures: a review. Tex Heart Inst J 22: 216-230. [Crossref]
- Souza HJ, Moitinho RF (2008) [Strategies to reduce the use of blood components in cardiovascular surgery]. Rev Bras Cir Cardiovasc 23: 53-59. [Crossref]
- Banbury MK, Brizzio ME, Rajeswaran J, Lytle BW, Blackstone EH (2006) Transfusion increases the risk of postoperative infection after cardiovascular surgery. J Am Coll Surg 202: 131-138. [Crossref]
- Hall TS, Brevetti GR, Skoultchi AJ, Sines JC, Gregory P, et al. (2001) Re-exploration for hemorrhage following open heart surgery differentiation on the causes of bleeding and the impact on patient outcomes. Ann Thorac Cardiovasc Surg 7: 352-357. [Crossref]
- Niranjan G, Asimakopoulos G, Karagounis A, Cockerill G,Thompson M, et al. (2006) Effects of cell saver autologous blood transfusion on blood loss and homologous blood tranfusion requirements in patients undergoing cardiac surgery on-versus off-cardiopulmonary bypass: a randomised trial. Eur J Cardiothorac Surg 30: 271-277. [Crossref]
- Inada E (1990) Blood coagulation and autologous blood transfusion in cardiac surgery. J Clin Anesth 2: 393-406. [Crossref]
- Alghamdi AA, Albanna MJ, Guru V, Brister SJ (2006) Does the use of erythropoietin reduce the risk of exposure to allogeneic blood transfusion in cardiac surgery? A systematic review and meta-analysis. J Card Surg 21: 320-326. [Crossref]
- Van der Linden P, De Hert S, Daper A, Trenchant A, Jacobs D, et al. (2001) A standardized multidisciplinaryapproach reduces the use of allogeneic blood products in patients undergoing cardiac surgery. Can J Anesth 48: 894-901. [Crossref]
- Dietrich W, Busley R, Kriner M (2006) Preoperative autologous blood donation in cardiac surgery. Reduction of allogeneic blood requirements. Anaesthesist 55: 753-759.
- Parrot D, Lançon JP, Merle JP, Rerolle A, Bernard A, et al. (1991) Blood salvage in cardiac surgery. J Cardiothorac Vasc Anesth 5: 454-456. [Crossref]
- Sellevold OF, Berg TM, Rein KA, Levang OW, Iversen OJ, et al. (1994) Heparin-coated circuit during cardiopulmonary bypass. A clinical study using closed circuit, centrifugal pump and reduced heparinization. Acta Anaesthesiol Scand 38: 372-379. [Crossref]
- Paiva P, Ferreira E, Antunes M (2005) Bloodless open heart surgery: simple and safe. Rev Port Cardiol 24: 647-654. [Crossref]
- Penta de Peppo A, Pierri MD, Scafuri A, De Paulis R, Colantuono G, et al. (1995) Intraoperative antifibrinolysis and bloodsaving techniques in cardiac surgery. Prospective trial of 3 antifibrinolytic drugs. Tex Heart Inst J 22: 231-236. [Crossref]
- Landymore RW, Murphy JT, Lummis H, Carter C (1997) The use of low-dose aprotinin, epsilon-aminocaproic acid or tranexamic acid for prevention of mediastinal bleeding in patients receiving aspirin before coronary artery bypass operations. Eur J Cardiothorac Surg 11: 798-800. [Crossref]
- Slaughter TF, Faghih F, Greenberg CS, Leslie JB, Sladen RN (1997) The effects of epsilon-aminocaproic acid on fibrinolysis and thrombin generation during cardiac surgery. Anesth Analg 85: 1221-1226. [Crossref]
- Greilich PE, Brouse CF, Whitten CW, Chi L, Dimaio JM, et al. (2003) Antifibrinolytic therapy during cardiopulmonary bypass reduces proinflammatory cytokine levels: a randomized, double-blind, placebo-controlled study of epsilonaminocaproic acid and aprotinin. J Thorac Cardiovasc Surg 126: 1498-1503. [Crossref]
- Kikura M, Levy JH, Tanaka KA, Ramsay JG (2006) A double-blind, placebo-controlled trial of epsilon-aminocaproic acid for reducing blood loss in coronary artery bypass grafting surgery. J Am Coll Surg 202: 216-222. [Crossref]
- Levi M, Cromheecke ME, de Jonge E, Prins MH, de Mol BJ, et al. (1999) Pharmacological strategies to decrease excessive blood loss in cardiac surgery: a meta-analysis of clinically relevant endpoints. Lancet 354: 1940-1947. [Crossref]
- Bick RL (1976) Alterations of hemostasis associated with cardiopulmonary bypass: pathophysiology, prevention, diagnosis, and management. Semin Thromb Hemost 3: 59-82. [Crossref]
- Tuman KJ, McCarthy RJ, O’Connor CJ, McCarthy WE,Ivankovich AD (1995) Aspirin does not increase allogeneic blood transfusion in reoperative coronary artery surgery. Anesth Analg 83: 1178-1184. [Crossref]
- Alderman EL, Levy JH, Rich JB, Nili M, Vidne B, et al. (1998) Analyses of coronary graft patency after aprotinin use: results from the International Multicenter Aprotinin Graft Patency Experience (IMAGE) trial. J Thorac Cardiovasc Surg 116: 716-730. [Crossref]
- Boldt J, Zickmann B, Czeke A, Herold C, Dapper F, et al. (1991) Blood conservation techniques and platelet function in cardiac surgery. Anesthesiology 75: 426-432. [Crossref]
- Hall RI, Schweiger IM, Finlayson DC (1990) The benefit of the hemonetics cell saver apparatus during cardiac surgery. Can Janaesth 37: 618-623. [Crossref]
- Gurbuz HA, Durukan AB, Tavlasoglu M, Yorgancioglu C (2013) eComment. Is retrograde autologous priming effective on cerebral functions and haematocrit levels? Interact Cardiovasc Thorac Surg 16: 783. [Crossref]
- Sun P, Ji B, Sun Y, Zhu X, Liu J, et al. (2013) Effects of retrograde autologous priming on blood transfusion and clinical outcomes in adults: a metaaanalysis. Perfusion 28: 238–243. [Crossref]
- Robbins S, Ng A, Lakshmanan S, Luckraz H (2013) Assessing the effectiveness of retrograde autologous priming of the cardiopulmonary bypass machine in isolated coronary artery bypass grafts. Ann R Coll Surg Engl 95: 207–210. [Crossref]
- Hou X, Yang F, Liu R, Yang J, Zhao Y, et al. (2009) Retrograde autologous priming of the cardiopulmonary bypass circuit reduces blood transfusion in small adults: a prospective, randomized trial. Eur J Anaesthesiol 26: 1061–1066. [Crossref]

