Abstract
Background : Chronic lower back pain caused by degenerative disc disease (DDD) is a significant cause of morbidity and mortality, which is currently treated by surgical means such as fusion, or mechanical means such as artificial discs. Only a minority of patients with DDD are eligible for these procedures and in numerous cases they require re-intervention. Cell therapy has been utilized for many spine-based procedures with mixed results. Our goal was to evaluate cell therapy in the treatment of non-surgical DDD. Aim : To perform a pilot study using autologous bone marrow aspirate for treatment of non-surgical, chronic lower back pain. Methods : In this prospective, observational cohort study, fifteen patients between 18 and 85 years of age with the diagnosis of non-surgical chronic lower back pain were enrolled to receive paraspinal injections of autologous bone marrow aspirate. In order to qualify, patients must have been diagnosed with degenerative disease in one, two, or three lumbar discs with predominant back pain after having failed conservative treatment including optimized physical therapy for over 6 months. Patients with surgical back pain as determined by a neuro-specialist, spinal deformity, or spinal instability were all disqualified from participating. All enrolled patients underwent outpatient bone marrow aspiration using the Marrow Cellutions kit. The aspirate was then injected into the paraspinal area using image guidance via ultrasound with each patient having an average of six injections. The patients were then followed at 30, 60, 90, 180, 360, and 720 days. The Oswestry Disability Index and Visual Analogue Scale were used to objectively assess for any change in the patient’s mobility and back pain post procedure. Results : Fourteen of the fifteen patients had injections near the L4, L5, and/or S1 region bilaterally. Evaluation of patients at 1, 30, 90, 180, 360, and 720 days revealed significant improvement in mobility using the Oswestry Disability Index and reduction in pain score using the Visual Analogue Scale. The mean pain score changed from 8.9 at baseline to 4.3 at 30 days and sustained to 1.8 at 6 months, 1.3 at 12 months and 2.2 at 24 months with a gradual reduction in overall pain medication utilization guided by their healthcare team. Two patients at the 24-month time point had recurrence of symptoms without significant MRI changes, and opted to be treated with medical therapy. They both had pain scores back up to 5 and an Oswestry score of 30 and 32 respectively. There was some short-term bruising in two patients at the harvest site and no long-term adverse events were reported related to the procedure. Overall, the procedure was safely performed on 15/15 patients (100%) and 13/15 patients (87%) still had durable results at two years. Conclusion : This data supports the further investigation of paraspinal administration of non-manipulated autologous bone marrow aspirate for reducing pain and improving mobility in patients with non-surgical lower back pain.
Key words
bone marrow aspirate, cell, chronic low back pain, degenerative disc disease, lumbar angina, paraspinal injection
Introduction
It is estimated that approximately 632 million people worldwide are affected by lower back pain, with annual costs in the United States estimated to exceed $100 billion [1-3]. Degenerative disc disease (DDD), also known as intervertebral disc (IVD) degeneration, is a condition associated with the progressive and irreversible deterioration of one or more of the discs in the spine. Although not all patients with radiological evidence of DDD have lower back pain, DDD is considered one of the major causes of chronic lower back pain [4-6].
Currently it is recognized that there are multiple possible and complicated causes of DDD, which include genetic, nutritional, and mechanical influences [7-9]. The processes associated with degeneration of the disc includes progressive decline in nucleus pulposus (NP) hydration due to the loss of extracellular matrix (ECM) molecules such as aggrecan and collagen [10,11], which is associated in part with reduced oxygenation and increased acidification [12]. Inflammatory processes such as increased TNF-alpha [13-16], macrophage activation [17], and NF-kappa B translocation [18-22], result in activation of matrix metalloproteases which further cause degradation of ECM. Furthermore, these processes result in apoptosis of cells in the NP which further results in reduction of ECM synthesis [23-25].
This decreased disc hydration results in a loss of mechanical tension in the collagen fibers of the annulus fibrosus and results in abnormal spinal axial loading forces and segmental instability [26]. Eventually, disc degeneration progresses to cause abnormalities of other components of the disc space, like the endplate and facet joint, which can develop into serious conditions such as disc herniation, spondylolisthesis, spinal canal stenosis, or facet joint syndrome [27-29].
Suggestions that atherosclerotic disease may be associated with DDD originally came from studies associating various cardiovascular disease factors with prevalence of DDD. A study of two hundred seventy adults who participated in a health promotion program were observed and analyzed for risk factors that correlated with likelihood of DDD. Amongst previously known risk factors such as aging and obesity, it was found that elevations in cholesterol and the presence of atherosclerosis correlated with DDD [30]. In another study, 300 lumbar arteries (150 lumbar artery pairs of the first to fifth lumbar arteries) were evaluated on consecutive CT-angiography scans. Severity of vascular disease of lumbar arteries was documented as normal, mild, moderate, severe, or occluded. Aortic vascular disease was documented along the posterior wall where the lumbar arteries originate. It was found that lumbar artery and aortic atherosclerosis had a positive relationship with DDD, facet arthritis, and spinal stenosis that was statistically significant [31]. These studies support the possibility that atherosclerosis may contribute to DDD.
Numerous studies have utilized bone marrow mononuclear cells to induce regeneration/angiogenesis of ischemic tissue. The two primary areas that this has been reported in has been limb ischemia and ischemic heart disease.
In limb ischemia, the first robust publication supporting the safety and feasibility of therapeutic angiogenesis using bone marrow mononuclear cells consisted of an initial pilot study in which 25 patients (group A) with unilateral ischemia of the leg were injected with bone marrow-mononuclear cells into the gastrocnemius of the ischemic limb and with saline into the less ischemic limb. The investigators then recruited 22 patients (group B) with bilateral leg ischemia, who were randomly injected with bone marrow-mononuclear cells in one leg and peripheral blood-mononuclear cells in the other as à control. Primary outcomes were safety and feasibility of treatment, based on ankle-brachial index (ABI) and rest pain, and analysis was per protocol. At 4 weeks, in group B patients, the ABI was significantly improved in legs injected with bone marrow-mononuclear cells compared to those injected with peripheral blood-mononuclear cells. Similar improvements were seen for transcutaneous oxygen pressure, rest pain, and pain-free walking time. These improvements were sustained at 24 weeks. Similar improvements were seen in group A patients. Two patients in group A died after myocardial infarction unrelated to treatment [32]. Numerous subsequent studies using bone marrow mononuclear cells have been reported demonstrating safety and efficacy of pain reduction, improved walking distance, and stimulation of angiogenesis [33-39].
Additionally, the use of bone marrow mononuclear cells (BM-MNC) has also been shown effective and safe in the induction of therapeutic angiogenesis in cardiac conditions. One study reported the 5-year outcomes of early (3-6 weeks after acute myocardial infarction [AMI], BM-MNC Early group) and late (3-4 months after AMI, BM-MNC Late group) combined (percutaneous intra-myocardial and intracoronary) delivery of autologous bone marrow mononuclear cells in patients with ejection fractions (EF) between 30-45% post-AMI [40]. Cardiac function improvement was observed, and in some patients, maintained for the 5-year examination period. Other studies using bone marrow mononuclear cells have confirmed therapeutic benefit, which are reviewed in the following meta-analysis reports.
Ye et al. (2012) analyzed 10 clinical trials, with a total of 757 patients, with at least 12-month follow-up after AMI receiving primary percutaneous coronary intervention in addition to intracoronary BM-MNCs administration. The pooled statistics showed intracoronary administration of BM-MNCs significantly improved post-infarction left ventricular ejection fraction (LVEF), attenuated the enlargement of left ventricular end-diastolic volume (LVEDV) as well as demonstrated a reduction in infarct size [41]. In another study, Xu et al. (2014) reviewed nineteen trials, with a total of 886 patients suffering from chronic ischemic heart disease (CIHD). They found that compared with controls, patients who received bone marrow mononuclear cells had significantly improved LVEF and left ventricular end-systolic volume (LVESV). Furthermore, it was revealed in subgroup analysis that significant improvement in LVEDV was observed in patients with lower baseline LVEF. Cell administration was associated with a significant decrease in all-cause death [42]. Other meta-analyses of cardiac trials further supports safety with indication of efficacy [43-46]. Given the safety of bone marrow mononuclear cell administration intramuscularly, we conducted a pilot study to assess whether stimulation of angiogenesis using bone marrow aspirate in patients with DDD may be associated with safety and potential clinical efficacy. Using our proposed technique combined with the Marrow Cellution kit, the procedure is less invasive and has a higher potency than bone marrow mononuclear cells in comparison to other devices [47].
Materials and methods
Patients between 18 and 85 years of age, with the diagnosis of degenerative disc disease were enrolled into the study. The patients had typical discogenic LBP in which the pain is typically in the low lumbar region with or without radiation to the buttock and thigh, and worse with sitting and bending activities. All subjects had conservative treatment including medications, physical therapy, etc. over 6 months prior to enrollment. Patients had a diagnostic MRI to determine the source of the pain. The MRI revealed concordant pain at each injected disc. There was no indication for surgical intervention as determined by a neuro-specialist. All patients gave informed consent prior to the procedure. Any patients with a herniated nucleus pulposus along with the disruption of the outer annulus on MRI were excluded. Patients with a suspected inflammatory or infectious spondylitis with altered blood tests such as CBC, ESR, and CRP were excluded. Additional exclusion criteria included spinal deformity, spinal segmental instability, spinal canal stenosis, congenital anomalies, spondylolysis, Modic III changes on MRI images, and greater than 50% loss of disc height.
Autologous bone marrow was aspirated by placing the patient in a prone position, where they were sterilely prepped and draped. A 1% lidocaine was injected into the epidermis down into the periosteum at the level of the posterior iliac crest. The Marrow Cellutions kit was heparinized using an unfractionated heparin solution of 5000 U/mL with a total of 2 mL used including the syringe(s) used for aspirating the bone marrow. An 11-gauge access trocar was introduced through the cortex of the posterior Iliac crest. A 1 mL aspiration was done to ensure access into marrow space. To promote safety, the blunt stylet was used to access a distal point in the ileum. The black top closed aspiration cannula was then placed and secured tightly to prevent any air leak. The external leverage housing system was unwound until contact was made with the skin. This repositioning allowed for the recovery of stem and progenitor cells from different levels within the marrow with each complete turn of the system. Negative pressure was applied by carefully withdrawing the syringe plunger. Twenty milliliters of bone marrow aspirate was obtained. Only 1-2 mL of marrow is aspirated at each position in the ileum, turning the blue T handle counterclockwise 360° after each 1-2 mL aspiration. This exposed the aspiration cannula tip to a different marrow area, in order to maximize the quality of the bone marrow collection. The black top aspiration cannula was then removed, and the blunt stylet was introduced into the access trocar and secured in locked position. A series of clockwise and counterclockwise rotations were used as the trocar was gently withdrawn. Hemostasis was obtained and a dressing applied. The bone marrow aspirate was not manipulated in anyway and was not allowed to leave the sterile field. A total of 21 mL of bone marrow was harvested ; 20 mL were used in for the paraspinal injections and one mL was sent to lab for CFU-F analysis.
The aspirate was injected into the paraspinal space using image guidance via ultrasound. Each patient had an average of six injections.
Results
All patients had 21mL of bone marrow aspirated with CFU-F/mL evaluated for plasticity/potency of the cells. All patients had their entire cell product delivered. There was some short-term bruising in two patients at the harvest site, mild pain in some and no long-term adverse events were reported related to the procedure (Table 1). Fourteen of the fifteen patients had injection near the L4, L5, and/or S1 region bilaterally. Evaluation of patients at 1, 30, 90, 180, 360, and 720 days revealed clinically relevant improvement in mobility using the Oswestry Disability Index (Figure 1) and reduction in pain score using the Visual Analogue Scale (Figure 2). The mean pain changed from 8.9 at baseline to 4.2 at 30 days and sustained to 1.8 at 6 months, 1.4 at 12 months, and 2.3 at 24 months with a gradual reduction in overall pain medication utilization guided by their healthcare team. Two patients at the 24-month time point had recurrence of symptoms without significant MRI changes, and opted to be treated with medical therapy. They both had pain scores back up to 5 and an Oswestry score of 30 and 32 respectively. There was some short-term bruising in two patients at the harvest site and no long-term adverse events were reported related to the procedure. Overall, the procedure was safely performed on 15/15 patients (100%) and 13/15 patients (87%) still had durable results at two years.
Table 1. Patient procedure data
Patient |
Age-
Years |
Gender |
Bone marrow
CFU-f/mL |
Procedure related adverse events |
1 |
46 |
M |
4177 |
Mild pain – gone in 2 days |
2 |
56 |
F |
3452 |
None |
3 |
38 |
M |
2239 |
Mild pain – gone in 1 day |
4 |
51 |
F |
3769 |
None |
5 |
83 |
F |
1790 |
Bruising at harvest site |
6 |
63 |
F |
3988 |
None |
7 |
64 |
F |
3765 |
None |
8 |
56 |
M |
4602 |
None |
9 |
55 |
M |
4120 |
None |
10 |
42 |
F |
4226 |
Mild pain – gone in 3 days |
11 |
67 |
F |
3759 |
Bruising at harvest site |
12 |
71 |
F |
2980 |
None |
13 |
58 |
M |
4556 |
None |
14 |
62 |
M |
4393 |
None |
15 |
68 |
F |
3178 |
None |
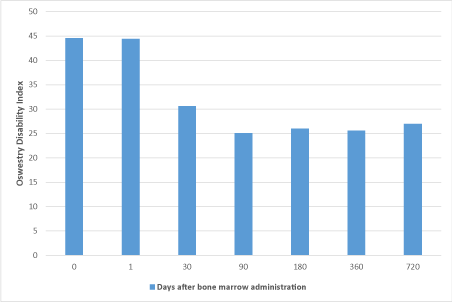
Figure 1. Change in Oswestry disability index
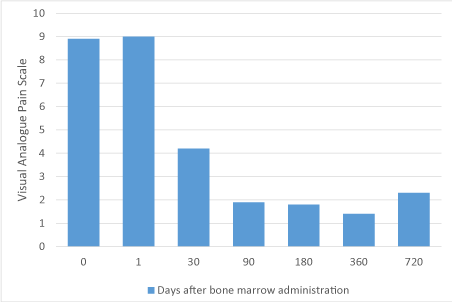
Figure 2. Change in visual analogue pain scale
Discussion
The intervertebral disc contains a jelly-like nucleus pulposus surrounded by a fibrous annulus fibrosus. In humans, the spine is composed of bony structures called vertebrae, separated by intervertebral discs (IVD). One of the main functions of the vertebrae is to provide structural support and protection for the spinal cord. Each vertebrae are comprised of a spinous process, a bony prominence behind the spinal cord, which shields the cord’s nervous tissue on the back side, two bony protrusions on the sides called transverse processes, and a “body” in front of the spinal cord which provides structural support for bearing weight. Of the vertebrae, the lumbar vertebrae are the largest, in part since they are responsible for carrying the majority of body weight. Because of this, the lumbar area is associated with the highest level of degeneration and is believed causative for a wide variety of pain-inducing syndromes [48].
Under an axial load, the nucleus pulposus compresses and radially transfers that load to the annulus fibrosus. The laminated nature of the annulus fibrosus provides it with a high tensile strength and so allows it to expand radially in response to this transferred load. Additionally, it is accepted that in a healthy intervertebral disc, cells within the nucleus pulposus produce an extracellular matrix (ECM) containing a high percentage of proteoglycans. These proteoglycans contain sulfated functional groups that retain water, thereby providing the nucleus pulposus with its cushioning qualities [49]. These nucleus pulposus cells may also secrete small amounts of cytokines as well as matrix metalloproteinases (MMPs). These cytokines and MMPs help regulate the metabolism of the nucleus pulposus cells. In some instances of degenerative disc disease (DDD), gradual degeneration of the intervertebral disc is caused by mechanical instabilities in other portions of the spine [50]. In these instances, increased loads and pressures on the nucleus pulposus cause the cells within the disc (or invading macrophages) to emit larger than normal amounts of the above-mentioned cytokines. In other instances of DDD, genetic factors or apoptosis can also cause the cells within the nucleus pulposus to emit toxic amounts of these cytokines and MMPs. In some instances, the pumping action of the disc may malfunction (due to, for example, a decrease in the proteoglycan concentration within the nucleus pulposus), thereby retarding the flow of nutrients into the disc as well as the flow of waste products out of the disc. This reduced capacity to eliminate waste may result in the accumulation of high levels of toxins that may cause nerve irritation and pain [51].
The nucleus pulposus is located in the center of the avascular IVD around 8 mm apart from the nearest blood supply. Cells in NP tissue receive nutrition from the surrounding blood vessels of the vertebral body by diffusion, which occurs due to concentration gradients set up by cellular metabolism. Small nutrients such as oxygen and glucose are supplied to the disc's cells virtually entirely by diffusion ; convective transport, arising from load-induced fluid movement in and out of the disc, has virtually no direct influence on transport of these nutrients. Consequently, there are steep concentration gradients of oxygen, glucose, and lactic acid across the disc ; oxygen and glucose concentrations are lowest in the center of the nucleus where lactic acid concentrations are greatest. The actual levels of concentration depend on the balance between diffusive transport and cellular demand and can fall to critical levels if the endplate calcifies or nutritional demand increases [52].
Impairment of nutrient transport into IVDs can lead to declined concentration of glucose, pH and oxygen (pO2) that adversely affects the activities as well as the survival of IVD cells, especially NP cells in the middle of the IVDs. Accordingly, nutrient impairment is considered as one of the major factors of IVD degeneration
A noteworthy biochemical modification that occurs with disc degeneration as a result of poor perfusion is the degradation of aggrecan, resulting in the loss of proteoglycan and tissue hydration. This results in the loss of glycosaminoglycans, which in turn results in the decrease of osmotic pressure of the disc matrix. In the degraded state, because of the lack of hydration, the disc’s load-bearing function is altered. These changes in the matrix lead to reduced proteoglycan synthesis, increased collagen synthesis with a switch to fibrillated tissue quality, and an increase in synthesis and activity of matrix degrading enzymes, such as metalloproteinases (MMPs) and A disintegrin and metalloproteinase with thromobosopondin motifs (ADAMTS). Degradative changes in the AF include delamination of the lamellae and increased likelihood for radial fissures. Consequently, degenerated discs have less disc height and aberrant mechanical responses to loading. Though normally avascular, changes in tissue integrity allow for increased vascular and neural in-growth of the disc, which can become a source of peripheral neuropathy producing pain, weakness, and numbness due to nerve damage.
This continued injury and inflammation may be caused by or may lead to further ischemia in the region. Atherosclerosis may also be a contributor to DDD in the aging population. In one study, investigators evaluated whether occlusion of lumbar and middle sacral arteries or serum cholesterol levels are associated with lower back pain and/or with disc degeneration. Atherosclerosis in the wall of the abdominal aorta usually develops at the ostia of branching arteries and the bifurcation, which may obliterate orifices of lumbar and middle sacral arteries. MR aortography and cholesterol blood tests were performed on 51 patients with long-term lower back pain without specific findings (i.e., spinal or nerve root compression) in regular lumbar MR images. It was found that 29 of 37 men and 11 of 14 women showed occluded lumbar and/or middle sacral arteries. The prevalence of occluded arteries was 2.5 times more than in subjects of corresponding age group. Twenty-three men and seven women had significant disc degeneration. Importantly, disc degeneration was associated with occluded lumbar/middle sacral arteries. This study indicated that lumbar and middle sacral arteries are often occluded in patients with nonspecific long-term lower back pain and DDD [53]. These findings support previous studies that occlusion of lumbar/middle sacral arteries is associated with lower back pain and disc degeneration. For example, 56 postmortem lumbar aortograms to study differences between subjects with and without low-back pain in the lumbar and middle sacral arteries. Twenty-two of 25 cases with back pain history had one or more missing arteries, 20 of them had narrow arteries, and 18 had developed collaterals. The cases had on average 2.04 entirely missing and 1.32 narrow (< or = 50% in diameter) arteries, compared with the age-matched controls who had 0.82 missing (p < 0.001 for difference from cases) and 0.59 narrow arteries (p < 0.01). The authors concluded that insufficient arterial blood flow may be an underlying factor for low-back symptoms. Atheromatous lesions in the abdominal aorta or congenital hypoplasia of the arteries may explain the angiographic findings [54].
Another study attempted to evaluate whether calcific lesions in the posterior wall of the abdominal aorta, the source of the feeding for arteries of the lumbar spine, are associated with disc degeneration or back pain, which would suggest that ischemia of the lumbar spine leads to disc degeneration. The presence of radiographic aortic calcification was ascertained in front of each lumbar segment from L1 through L4, and disc degeneration at intervertebral spaces from L1-L2 through L4-L5. The associations between aortic calcification, disc degeneration, and back pain were tested using logistic regression with adjustment for age and sex. At the baseline examination, aortic calcification was significantly associated with general disc degeneration, that is, disc space narrowing or endplate sclerosis at any lumbar level (odds ratio 1.6; 95% confidence interval 1.0-2.5 ; P = 0.034). In longitudinal, level-specific analyses, comparing local aortic calcifications with disc degeneration at the matching level, aortic calcifications predicted disc deterioration, that is, a decrease in disc space or appearance of endplate sclerosis, between the examinations (odds ratio 1.5; 95% confidence interval 1.3-1.8 ; P < 0.001). Furthermore, subjects in whom aortic calcifications developed between the examinations had disc deterioration twice as frequently as those in whom aortic calcifications did not develop (odds ratio 2.0; 96% confidence interval 1.2-3.5 ; P = 0.013). Also, individuals with severe (Grade 3) posterior aortic calcification in front of any lumbar segment were more likely than others to report back pain during adult life (odds ratio 1.6; 95% confidence interval 1.1-2.2 ; P = 0.014). The authors concluded that advanced aortic atherosclerosis, presenting as calcific deposits in the posterior wall of the aorta, increases a person’s risk for development of disc degeneration and is associated with the occurrence of back pain [55].
In a supportive study, computed tomographic images of 29 patients with low back pain, who had been evaluated with computed tomographic discography for diagnostic purposes, were evaluated for the quantity of atherosclerotic calcifications visible on computed tomographic scans of the abdominal aorta. A similar evaluation was performed in an age- and sex-matched control group of 52 patients without low back pain selected from among the patients referred for abdominal computed tomography. Sixteen (55%) of the 29 patients with low back pain had atherosclerotic calcifications visible on computed tomographic scans, whereas 11 (21%) of the 52 age-matched patients without low back pain were found to have aortic calcifications. Eleven (48%) patients with low back pain who were 50 years of age or less (n = 23) had aortic calcifications, whereas only 3 (8%) of the 36 control patients aged less than 50 years had aortic calcifications. There was no correlation between the amount of calcifications and the degree of disc degeneration assessed by computed tomographic discography. A significant association is indicated between atheromatous lesions in the abdominal aorta and low back pain [56]. It is believed that occlusion of the arteries feeding the lumbar area is due to atherosclerosis [57,58]. Epidemiologic and post mortem studies indicate that atheromatous lesions in the abdominal aorta may be related to disc degeneration and long-term back symptoms [54-56,59,60].
The blood supply of the lumbar spine is derived from the aorta through the lumbar and middle sacral arteries. The upper four segments of the lumbar spine receive their blood supply from the four pairs of the lumbar arteries, which arise in the posterior wall of the abdominal aorta. The fifth lumbar segment is supplied partly by the middle sacral artery (arising in the bifurcation) and partly by branches of the iliolumbar arteries (arising from the internal iliac arteries) [61].
Nutrition of the avascular intervertebral disc occurs by diffusion through the vertebral endplates from the blood vessels in the vertebral bodies above and below the disc [63,64]. Cholesterol plaques in the wall of the aorta obliterate orifices of lumbar and middle sacral arteries and decrease blood supply of the lumbar spine and its surrounding structures. As a result, structures with precarious nutrient supply, such as the intervertebral discs, gradually degenerate [57,65,66]. Reduced blood flow causes hypoxia and tissue dysfunction. It also hampers removal of waste products, such as lactic acid. These changes in turn may irritate nociceptive nerve endings, causing pain, as well as lead to deterioration and atrophy of the structures involved [8,67-69]. Pre-clinical studies have also been performed that help better understand the role of atherosclerosis in DDD and help determine whether specific events are causal or associative. Data suggests that predisposition to atherosclerosis, in part by induction of this process by knockout of APOE4, leads to accelerated DDD.
In one study, it was examined whether APOE-knockout promoted IVD degeneration in rabbits is associated with imbalanced inflammatory catabolic activities, as the underlying problem of biological deterioration that mimic the symptoms of advanced IVD degeneration in humans. The study analyzed the lumbar nucleus pulposus (NP) of APOE-knockout rabbits, cell viabilities, and the intracellular levels of inflammatory, catabolic, anti-catabolic and anabolic proteins derogating IVD matrix. NP cells of APOE-knockout and wild-type rabbits showed significantly different in vivo cell population densities (p<0.0001) and similar in vitro proliferation rates. Furthermore, they showed differences in overexpression of selective inflammatory and catabolic proteins (p<0.0001) similar to those found in human NP cells of different disc degeneration grades, such as IL-1β, TNF-α, ADAMTS-4, ADAMTS-5 and MMP-3. This study showed that premature IVD degeneration in APOE-knockout rabbits was promoted by the accumulation of selective inflammatory catabolic factors that enhanced imbalances between catabolic and anabolic factors mimicking the symptoms of advanced IVD degeneration in humans [70].
In a similar study, scientists analyzed IVD degeneration in the lumbar spines of ten homozygous APOE-knockout and four wild-type New Zealand White rabbits of matching age to prove accelerated IVD degeneration in APOE-knockout rabbits, since APOE-knockout rabbits could be a beneficial model for therapeutic approaches of degenerative IVD disorders. Experiments were performed using T1/T2-weighted magnetic resonance imaging, 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide assay, glucose-oxidase assay, enzyme-linked immunosorbent assay, quantitative reverse transcription PCR and western blot. APOE-knockout lumbar spines showed more advanced IVD degeneration, obstructed lumbar arteries and lower enhancement of contrast agent in IVDs. Moreover, lower concentration of glucose, lower number of viable cells and lower concentrations of aggrecan, collagen II and higher concentration of collagen I were detected in APOE-knockout IVDs (p < 0.0001). APOE-knockout in rabbits could induce structurally deteriorating premature IVD degeneration that mimics the symptoms of accelerated IVD degeneration in humans. APOE-knockout rabbits could be used as beneficial model, as they can bypass the standard surgical interventions that are commonly applied in research animals for the induction of enhanced IVD degeneration [71].
Our study demonstrates that using a minimally invasive technique for bone marrow aspiration yielding high quality functional bone marrow as we have previously described by Scarpone et al. (2019) while still maintaining patient safety [47]. The procedure is performed at the bedside using sterile technique and ultrasound guided delivery in an outpatient setting. The marrow aspirate never leaves the sterile field or the patient. There is no manipulation or filtering of the marrow aspirate. The high number of CFU-f in our study compared to traditional centrifuged marrow products may lead to better outcomes but needs to be further investigated in appropriately controlled studies. This pilot study did demonstrate that post procedure patients has improvement mobility and a reduction in pain which was durable.
Conclusion
This data demonstrates safety and supports the further investigation of ultrasound guided paraspinal administration of non-manipulated autologous bone marrow aspirate, a procedure we termed “StemSpine”, as a means of reducing pain and improving mobility in patients with non-surgical lower back pain.
Contributorship
JT and JP were involved in design, treating patients, analysis of data, and writing of manuscript. NT was involved in data collection, data analysis, and writing of manuscript. CB and TI were involved with analysis and writing of the manuscript. All authors have read and approved the final copy of the manuscript for submission.
Acknowledgments
Creative Medical Technology Holdings, Inc. for donating the devices.
Funding information
Creative Medical Technology Holdings, Inc. provided the devices for the clinical program and was involved in study design and data collection.
Competing interest
TI and CB are consultants for Creative Medical Technology Holdings. Creative Medical Technology Holdings, Inc. is the owner of the US Patent 9598735 under which TI is a co-inventor.
References
- Andersson G.B (1999) Epidemiological features of chronic low-back pain. Lancet 354(9178): 581-585. [Crossref]
- Katz JN (2006) Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am 88(Suppl 2): 21-24. [Crossref]
- Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859): 2163-2196. [Crossref]
- Raj PP (2008) Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract 8(1): 18-44. [Crossref]
- Smith LJ, Nerurkar NL, Choi KS, Harfe BD, Elliott DM (2011) Degeneration and regeneration of the intervertebral disc: lessons from development. Dis Model Mech 4(1): 31-41. [Crossref]
- Chou R (2014) In the clinic. Low back pain. Ann Intern Med 160(11): ITC6-1. [Crossref]
- Bartels EM, Fairbank JC, Winlove CP, Urban JP (1998) Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine 23(1): 1-7. [Crossref]
- Bibby SR, Urban JP (2004) Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J 13(8): 695-701. [Crossref]
- Grunhagen T, Wilde G, Soukane DM, Shirazi-Adl SA, Urban JP (2006) Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am 88(Suppl 2): 30-35. [Crossref]
- Buckwalter JA (1995) Aging and degeneration of the human intervertebral disc. Spine 20(11): 1307-1314. [Crossref]
- Costi JJ, Stokes IA, Gardner-Morse MG, Iatridis JC (2008) Frequency-dependent behavior of the intervertebral disc in response to each of six degree of freedom dynamic loading: solid phase and fluid phase contributions. Spine 33(16): 1731-1738. [Crossref]
- Fu J, Yu W, Jiang D (2018) Acidic pH promotes nucleus pulposus cell senescence through activating the p38 MAPK pathway. Biosci Rep 38(6). [Crossref]
- Abdollahzade S, Hanaei S, Sadr M, Mirbolouk MH, Fattahi E, Rezaei N, et al (2018) Significant association of TNF-alpha, but not other pro-inflammatory cytokines, single nucleotide polymorphisms with intervertebral disc degeneration in Iranian population. Clin Neurol Neurosurg 173: 77-83. [Crossref]
- Evashwick-Rogler TW, Lai A, Watanabe H, Salandra JM, Winkelstein BA, Cho SK, et al (2018) Inhibiting tumor necrosis factor-alpha at time of induced intervertebral disc injury limits long-term pain and degeneration in a rat model. JOR Spine 1(2): e1014. [Crossref]
- Liu H, Kang H, Song C, Lei Z, Li L, Guo J, et al (2018) Urolithin A Inhibits the Catabolic Effect of TNFalpha on Nucleus Pulposus Cell and Alleviates Intervertebral Disc Degeneration in vivo. Front Pharmacol 9: 1043. [Crossref]
- Wang C, Han X, Li Y, Zhang B (2018) Impact of bone marrow mononuclear cells therapy on left ventricular function in patients with ST-elevated myocardial infarction: A meta-analysis. Medicine 97(16): e0359. [Crossref]
- Hamamoto H, Miyamoto H, Doita M, Takada T, Nishida K, Kurosaka M (2012) Capability of nondegenerated and degenerated discs in producing inflammatory agents with or without macrophage interaction. Spine 37(3): 161-167. [Crossref]
- Liang H, Yang X, Liu C, Sun Z, Wang X (2018) Effect of NF-kB signaling pathway on the expression of MIF, TNF-alpha, IL-6 in the regulation of intervertebral disc degeneration. J Musculoskelet Neuronal Interact 18(4): 551-556. [Crossref]
- Luo L, Gao Y, Yang C, Shao Z, Wu X, Li S, et al (2018) Halofuginone attenuates intervertebral discs degeneration by suppressing collagen I production and inactivating TGFbeta and NF-small ka, CyrillicB pathway. Biomed Pharmacother 101: 745-753. [Crossref]
- Sun J, Hong J, Sun S, Wang X, Peng Y, Zhou J, et al (2018). Transcription factor 7-like 2 controls matrix degradation through nuclear factor kappaB signaling and is repressed by microRNA-155 in nucleus pulposus cells. Biomed Pharmacother 108: 646-655. [Crossref]
- Wu X, Liu Y, Guo X, Zhou W, Wang L, Shi J, et al (2018) Prolactin inhibits the progression of intervertebral disc degeneration through inactivation of the NF-kappaB pathway in rats. Cell Death Dis 9(2): 98. [Crossref]
- Yang H, Tian W, Wang S, Liu X, Wang Z, Hou L, et al (2018) TSG-6 secreted by bone marrow mesenchymal stem cells attenuates intervertebral disc degeneration by inhibiting the TLR2/NF-kappaB signaling pathway. Lab Invest 98(6): 755-772. [Crossref]
- Merceron C, Mangiavini L, Robling A, Wilson TL, Giaccia AJ, Shapiro IM, et al (2014) Loss of HIF-1alpha in the notochord results in cell death and complete disappearance of the nucleus pulposus. PLoS One 9(10): e110768. [Crossref]
- Wang W, Deng G, Qiu Y, Huang X, Xi Y, Yu J, et al (2018) Transplantation of allogenic nucleus pulposus cells attenuates intervertebral disc degeneration by inhibiting apoptosis and increasing migration. Int J Mol Med 41(5): 2553-2564. [Crossref]
- Zhang J, Wang X, Liu H, Li Z, Chen F, Wang H, et al (2019) TNF-alpha enhances apoptosis by promoting chop expression in nucleus pulposus cells: role of the MAPK and NF-kappaB pathways. J Orthop Res. 37(3): 697-705. [Crossref]
- Pennicooke B, Moriguchi Y, Hussain I, Bonssar L, Hartl R (2016) Biological Treatment Approaches for Degenerative Disc Disease: A Review of Clinical Trials and Future Directions. Cureus 8(11): e892. [Crossref]
- Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, et al (1996) The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest 98(4): 996-1003. [Crossref]
- Martin MD, Boxell CM, Malone DG (2002) Pathophysiology of lumbar disc degeneration: a review of the literature. Neurosurg Focus 13(2): E1. [Crossref]
- Buser Z, Chung AS, Abedi A, Wang JC (2019) The future of disc surgery and regeneration. Int Orthop 43(4): 995-1002. [Crossref]
- Hangai M, Kaneoka K, Kuno S, Hinotsu S, Sakane M, Mamizuka N, et al (2008) Factors associated with lumbar intervertebral disc degeneration in the elderly. Spine J 8(5): 732-740. [Crossref]
- Beckworth WJ, Holbrook JF, Foster LG, Ward LA, Welle JR (2018) Atherosclerotic Disease and its Relationship to Lumbar Degenerative Disk Disease, Facet Arthritis, and Stenosis with Computed Tomography Angiography. PM R 10(4): 331-337. [Crossref]
- Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, et al (2002) Therapeutic Angiogenesis using Cell Transplantation Study. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet 360(9331): 427-435. [Crossref]
- Ruiz-Salmeron R, de la Cuesta-Diaz A, Constantino-Bermejo M, Perez-Camacho I, Marcos-Sanchez F, Hmadcha A, et al (2011) Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transplant 20(10): 1629-1639. [Crossref]
- Li M, Zhou H, Jin X, Wang M, Zhang S, Xu L (2013) Autologous bone marrow mononuclear cells transplant in patients with critical leg ischemia: preliminary clinical results. Exp Clin Transplant 11(5): 435-439. [Crossref]
- Maione C, Botti C, Coppola CA, Silvestroni C, Lillo S, Schiavone V, et al (2013) Effect of autologous transplantation of bone marrow cells concentrated with the MarrowXpress system in patients with critical limb ischemia. Transplant Proc 45(1): 402-406. [Crossref]
- Wang ZX, Li D, Cao JX, Liu YS, Wang M, Zhang XY, et al (2014) Efficacy of autologous bone marrow mononuclear cell therapy in patients with peripheral arterial disease. J Atheroscler Thromb 21(11): 1183-1196. [Crossref]
- Molavi B, Zafarghandi MR, Aminizadeh E, Hosseini SE, Mirzayi H, Arab L, et al (2016) Safety and Efficacy of Repeated Bone Marrow Mononuclear Cell Therapy in Patients with Critical Limb Ischemia in a Pilot Randomized Controlled Trial. Arch Iran Med 19(6): 388-396. [Crossref]
- Madaric J, Valachovicova M, Paulis L, Pribojova J, Mateova R, Sebekova K, et al (2017) Improvement in asymmetric dimethylarginine and oxidative stress in patients with limb salvage after autologous mononuclear stem cell application for critical limb ischemia. Stem Cell Res Ther 8(1): 165. [Crossref]
- Pignon B, Sevestre MA, Kanagaratnam L, Pernod G, Stephan D, Emmerich J, et al (2017) Autologous Bone Marrow Mononuclear Cell Implantation and Its Impact on the Outcome of Patients with Critical Limb Ischemia- Results of a Randomized, Double-Blind, Placebo-Controlled Trial. Circ J 81(11): 1713-1720. [Crossref]
- Gyongyosi M, Giurgea GA, Syeda B, Charwat S, Marzluf B, Mascherbauer J, et al (2016) Long-Term Outcome of Combined (Percutaneous Intramyocardial and Intracoronary) Application of Autologous Bone Marrow Mononuclear Cells Post Myocardial Infarction: The 5-Year MYSTAR Study. PLoS One 11(10): e0164908. [Crossref]
- Ye Z, Zhang BL, Zhao XX, Qin YW, Wu X, Cao J, et al (2012) Intracoronary infusion of bone marrow-derived mononuclear cells contributes to longstanding improvements of left ventricular performance and remodelling after acute myocardial infarction: a meta-analysis. Heart Lung Circ 21(11): 725-733. [Crossref]
- Xu R, Ding S, Zhao Y, Pu J, He B (2014) Autologous transplantation of bone marrow/blood-derived cells for chronic ischemic heart disease: a systematic review and meta-analysis. Can J Cardiol 30(11): 1370-1377. [Crossref]
- Afzal MR, Samanta A, Shah ZI, Jeevanantham V, Abdel-Latif A, Zuba-Surma EK, et al (2015) Adult Bone Marrow Cell Therapy for Ischemic Heart Disease: Evidence and Insights from Randomized Controlled Trials. Circ Res 117(6): 558-575. [Crossref]
- Gyongyosi M, Haller PM, Blake DJ, Rendon EM (2018) Meta-Analysis of Cell Therapy Studies in Heart Failure and Acute Myocardial Infarction. Circ Res 123(2): 301-308. [Crossref]
- Wang H, He P, Pan H, Long J, Wang J, Li Z, et al (2018) Circular RNA circ-4099 is induced by TNF-alpha and regulates ECM synthesis by blocking miR-616-5p inhibition of Sox9 in intervertebral disc degeneration. Exp Mol Med 50(4): 1-14. [Crossref]
- Wen Y, Ding J, Zhang B, Gao Q (2018) Bone marrow-derived mononuclear cell therapy for nonischaemic dilated cardiomyopathy-A meta-analysis. Eur J Clin Invest 48(4). [Crossref]
- Scarpone M, Kuebler D, Chambers A, De Filippo CM, Amatuzio M, Ichim TE, et al (2019) Isolation of clinically relevant concentrations of bone marrow mesenchymal stem cells without centrifugation. J Translational Med 17(1): 10. [Crossref]
- Zhao L, Manchikanti L, Kaye AD, Abd-Elsayed A (2019) Treatment of Discogenic Low Back Pain: Current Treatment Strategies and Future Options-a Literature Review. Curr Pain Headache Rep 23(11): 86. [Crossref]
- Inoue N, Espinoza Orias AA (2011) Biomechanics of intervertebral disk degeneration. Orthop Clin North Am 42(4): 487-499. [Crossref]
- Podichetty VK (2007) The aging spine: the role of inflammatory mediators in intervertebral disc degeneration. Cell Mol Biol 53(5): 4-18. [Crossref]
- Smith LJ, Fazzalari NL (2009) The elastic fibre network of the human lumbar anulus fibrosus: architecture, mechanical function and potential role in the progression of intervertebral disc degeneration. Eur Spine J 18(4): 439-448. [Crossref]
- Urban JP, Smith S, Fairbank JC (2004) Nutrition of the intervertebral disc. Spine 29(23): 2700-2709. [Crossref]
- Kauppila LI, Mikkonen R, Mankinen P, Pelto-Vasenius K, Maenpaa I (2004) MR aortography and serum cholesterol levels in patients with long-term nonspecific lower back pain. Spine 29(19): 2147-2152. [Crossref]
- Kauppila LI, Tallroth K (1993) Postmortem angiographic findings for arteries supplying the lumbar spine: their relationship to low-back symptoms. J Spinal Disord 6(2): 124-129. [Crossref]
- Kauppila LI, McAlindon T, Evans S, Wilson PW, Kiel D, Felson DT (1997) Disc degeneration/back pain and calcification of the abdominal aorta. A 25-year follow-up study in Framingham. Spine 22(14): 1642-1647. [Crossref]
- Kurunlahti M, Tervonen O, Vanharanta H, Ilkko E, Suramo I (1999) Association of atherosclerosis with low back pain and the degree of disc degeneration. Spine 24(20): 2080-2084. [Crossref]
- Cluroe AD, Fitzjohn TP, Stehbens WE (1992) Combined pathological and radiological study of the effect of atherosclerosis on the ostia of segmental branches of the abdominal aorta. Pathology 24(3): 140-145. [Crossref]
- Kauppila LI, Penttila A, Karhunen PJ, Lalu K, Hannikainen P (1994) Lumbar disc degeneration and atherosclerosis of the abdominal aorta. Spine 19(8): 923-929. [Crossref]
- Kauppila LI (1995) Can low-back pain be due to lumbar-artery disease? Lancet 346(8979): 888-889. [Crossref]
- Boggild H (2006) Ischemia and low-back pain--is it time to include lumbar angina as a cardiovascular disease? Scand J Work Environ Health 32(1): 20-21. [Crossref]
- Crock HV, Yoshizawa H (1997) Veins of the spinal cord. Springer 3(4): 55-63.
- Kauppila LI (1994) Blood supply of the lower thoracic and lumbosacral regions. Postmortem aortography in 38 young adults. Acta Radiol 35(6): 541-544. [Crossref]
- Urban JP, Smith S, Fairbank JC (2004) Nutrition of the intervertebral disc. Spine 29(23): 2700-2709.
- Walker MH, Anderson DG (2004) Molecular basis of intervertebral disc degeneration. Spine J 4(6 Suppl): 158S-166S. [Crossref]
- Ross R (1998) Atherosclerosis. In: Wyngaarden JB, S. L., eds. Cecil's Textbook of Medicine. Philadelphia: Saunders, 1988: 318-23.
- Mitchel JR, Adams JH (1977) Aortic size and aortic calcification. A necropsy Study. Atherosclerosis 27(4): 437-446. [Crossref]
- Ohshima H, Urban JP (1992) The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine 17(9): 1079-1082. [Crossref]
- Iwabuchi M, Rydevik B, Kikuchi S, Olmarker K (2001) Effects of anulus fibrosus and experimentally degenerated nucleus pulposus on nerve root conduction velocity: relevance of previous experimental investigations using normal nucleus pulposus. Spine 26(15): 1651-1655. [Crossref]
- Naves LA, McCleskey EW (2005) An acid-sensing ion channel that detects ischemic pain. Braz J Med Biol Res 38(11): 1561-1569. [Crossref]
- Beierfuss A, Hunjadi M, Ritsch A, Kremser C, Thome C, Mern DS (2019) APOE-knockout in rabbits causes loss of cells in nucleus pulposus and enhances the levels of inflammatory catabolic cytokines damaging the intervertebral disc matrix. PLoS One 14(11): e0225527. [Crossref]
- Beierfuss A, Dietrich H, Kremser C, Hunjadi M, Ritsch A, Rulicke T, et al (2017) Knockout of Apolipoprotein E in rabbit promotes premature intervertebral disc degeneration: A new in vivo model for therapeutic approaches of spinal disc disorders. PLoS One 12(11): e0187564. [Crossref]


