Abstract
Sphingosine 1-phosphate (S1P) is a lipid mediator involved in various cellular actions. Although, numerous biological functions of S1P have been documented, there have been relatively few studies on the role of S1P in adipocytes. While these studies have consistently shown that S1P suppresses adipogenetic differentiation, others have reached discordant conclusions regarding issues such as the relation between S1pr and adipogenetic differentiation.
Introduction
Obesity-induced insulin resistance has been regarded as the underlying pathogenetic mechanism of diverse metabolic disorders, including type 2 diabetes mellitus, hypertension, dyslipidemia and metabolic syndrome. Understanding of adipocytes, which play the most important role in establishing obesity, is indispensable; but remains incomplete. In this short article, we will review research on S1P and adipocytes or insulin resistance. In addition, we will introduce our recent data on S1P receptors and adipocyte growth and differentiation.
S1P was first recognized as a lipid mediator derived from sphingosine [1]. Subsequent research demonstrated that S1P regulates cell proliferation, differentiation, apoptosis and migration [2]. S1P is produced by sphingosine kinase 1 (Sphk1) and Sphk2. Activated Sphk1 translocates from cytosol to plasma membrane, whereas Sphk2 is located in nuclei and mitochondria [3]. Deletion of Sphk1 and Sphk2 resulted in no apparent abnormalities, whereas simultaneous deletion of Sphk1/Sphk2 was lethal [4]. These facts indicate that although Sphk1 and Sphk2 have their respective roles, they also complement each other. There are 5 classes of G protein coupled receptors for S1P, S1pr1-5 [5-8]. These receptors mediate distinct signals, sometimes acting cooperatively and sometimes adversely.
S1P and adipocytes
Recently, we accessed the role of S1P-S1pr axis in the pathogenesis of obesity-induced insulin resistance in S1pr2-deficient mice (S1pr2-/-) [9]. Our results showed that body weight and fat weight were suppressed in normal diet-fed S1pr2-/- compared with those in wild type (WT) mice. The body weight and fat weight differences between these mice diminished after 4-week feeding with a high-fat diet (HFD). However, HFD-fed S1pr2-/- mice displayed better glucose tolerance and insulin sensitivity compared with HFD-fed WT mice. Epididymal adipocytes in both normal diet-fed and HFD-fed S1pr2-/- mice were smaller than those in WT mice. Accordingly, numbers of crown like structure and expression levels of Cd11c and Nos2 mRNA, hallmarks of adipose tissue inflammation, were reduced in HFD-fed S1pr2-/- mice compared with those in HFD-fed WT mice. Moreover, proliferating cell nuclear antigen (PCNA), a marker of proliferating cells, was elevated in both diet-fed and HFD-fed S1pr2-/- mice. These results suggested that blockade of S1pr2 signal might lead to an increased number of adipocytes, which might prevent adipocyte hypertrophy and subsequent inflammation of adipose tissue. This prompted us to examine the role of S1P-S1prs signals in adipocyte proliferation and differentiation using cultured preadipocytes (3T3-L1 and 3T3-F442A). Our results indicated that S1P enhanced proliferation of preadipocytes and suppressed differentiation into adipocytes. Blockade of S1pr2 signal caused increased preadipocyte proliferation and decreased adipogenesis, whereas blockade of S1pr1/3 led to the opposite results (Figure 1). In addition, oral administration of JTE-013, an inhibitor of S1pr2, for 4 weeks resulted in reduction of body weight and improvement of glucose tolerance in hereditary obese ob/ob mice, but not lean (C57/black) ones. Our new experiment demonstrated that treatment with JTE-013 and an equal amount of SEW2871, an agonist of S1pr1, equally reduced body weight and improved glucose tolerance in ob/ob mice (unpublished data). These results suggested that activation of S1pr1 or inhibition of S1pr2 may be a novel therapeutic candidate for obesity-induced insulin resistance. Our result that S1pr2 signal attenuated adipogenesis was consisted with that of Moon et al, [10]. On the other hand, Jeong et al, despite performing the same experiment as ours to silencing S1pr2 with siRNA in 3T3-L1 adipocytes, obtained the opposite result to ours that adipogenesis was accelerated [11]. We cannot explain this discrepancy now. Anyway, it is more important how the S1P signal works in vivo in the obese state. The fact that the appearance of adipocytes is the same whether adipogenesis is enhanced or suppressed [12], makes analysis in vivo difficult.
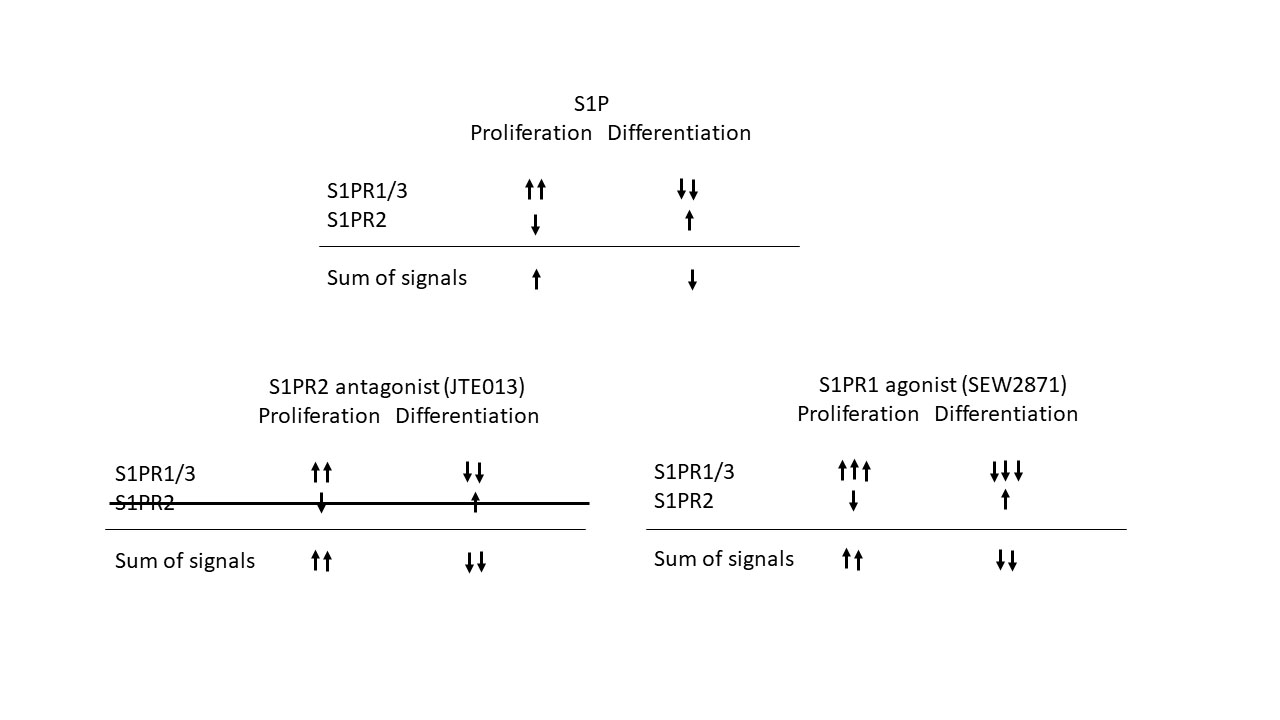
Figure 1. Blockade of S1pr2 signal
HFD-fed Sphk1 deficient mice displayed increased adipose tissue weight, reduced adipocyte size, improved glucose tolerance/insulin sensitivity, improved levels of inflammation markers of adipose tissue and increased adipocyte specific genes compared with the HFD-fed WT mice [13]. The authors also showed that pharmacological inhibition of Sphk1 by 5c ameliorated glucose tolerance/insulin sensitivity and inflammation markers in adipose tissue. Furthermore, an in vitro study using mesenchymal stem cells showed that S1P-induced suppression of adipogenesis was caused by decreased expression of C/EBPb [14].
S1P and insulin resistance
FTY-720 is known as a potent agonist of S1prs except S1Pr2. Especially, it binds to and activates S1pr1, followed by downregulation of S1pr1. Therefore, FTY-720 acts as an S1pr1 inhibitor in lymphocytes, although whether it acts as an agonist or antagonist depends on the cell type [15]. Treatment with FTY-720 was reported to improve systemic and muscle insulin sensitivity in HFD-fed mice [16]. On the other hand, treatment with JTE-013 prevented hyperglycemia and hepatic insulin resistance [17] in HFD-fed mice. Numerous investigations have studied the associations between S1P and another lipid mediator, ceramide [18].
Conclusion
At present, FTY-720 is the only clinically applicable immune modulator compound. However, the present data taken together suggest that an agonist or antagonist of S1P signal might be effective as a therapeutic agent against obesity and diabetes mellitus in the future.
References
- Zhang H, Desai NN, Olivera A, Seki T, Brooker G, et al. (1991) Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol 114:155-167. [Crossref]
- Spiegel S, Milstien S (2003) Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4: 397-407. [Crossref]
- Maceyka M, Harikumar KB, Milstien S, Spiegel S (2012) Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol 22: 50-60. [Crossref]
- Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, et al. (2005) Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol 25: 11113-11121. [Crossref]
- Kihara Y, Maceyka M, Spiegel S, Chun J (2014) Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br J Pharmacol 171: 3575-3594. [Crossref]
- Wang F, Van Brocklyn JR, Hobson JP, Movafagh S, Zukowska-Grojec Z, et al. (1999) Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem 274: 35343-35350. [Crossref]
- Ishii I, Fukushima N, Ye X, Chun J (2004) Lysophospholipid receptors: signaling and biology. Annu Rev Biochem 73: 321-354. [Crossref]
- Takuwa Y, Okamoto Y, Yoshioka K, Takuwa N (2012) Sphingosine-1-phosphate signaling in physiology and diseases. Biofactors 38: 329-337. [Crossref]
- Kitada Y, Kajita K, Taguchi K, Mori I, Yamauchi M, et al. (2016) Blockade of Sphingosine 1-phosphate receptor 2 signaling attenuates high-fat diet-induced adipocyte hypertrophy and systemic glucose intolerance in mice. Endocrinology 157: 1839-1851. [Crossref]
- Moon MH, Jeong JK, Park SY (2015) Activation of S1P2 receptor, a possible mechanism of inhibition of adipogenic differentiation by sphingosine 1-phosphate. Mol Med Rep 11: 1031-1036. [Crossref]
- Jeong JK, Moon MH, Park SY (2015) Modulation of the expression of sphingosine 1-phosphate 2 receptors regulates the differentiation of pre-adipocytes. Mol Med Rep 12: 7496-7502. [Crossref]
- Yamauchi T, Waki H, Kamon J, Murakami K, Motojima K, et al. (2001) Inhibition of RXR and PPARgamma ameliorates diet-induced obesity and type 2 diabetes. J Clin Invest 108: 1001-1013. [Crossref]
- Wang J, Badeanlou L, Bielawski J, Ciaraldi TP, Samad F (2014) Sphingosine kinase 1 regulates adipose proinflammatory responses. Am J Physiol Endocrinol Metab 306: E756-E768. [Crossref]
- Hashimoto Y, Matsuzaki E, Higashi K, Takahashi-Yanaga F, Takano A, et al. (2015) Sphingosine-1-phosphate inhibits differentiation of C3H10T1/2 cells into adipocyte. Mol Cell Biochem 401: 39-47. [Crossref]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, et al. (2004) Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor. Nature 427: 355-360. [Crossref]
- Bruce CR, Risis S, Babb JR, Yang C, Lee-Young RS, et al. (2013) The sphingosine-1-phosphate analog FTY720 reduces muscle ceramide content and improves glucose tolerance in high fat-fed mice. Endocrinology 154: 65-76. [Crossref]
- Fayyaz S, Henkel J, Japtok L, Kramer S, Damm G, et al. (2014) Involvement of sphingosine 1-phosphate in palmitate-induced insulin resistance of hepatocytes via the S1P2 receptor subtype. Diabetologia 57: 373-382. [Crossref]
- Fayyaz S, Japtok L, Kleuser B (2014) Divergent role of sphingosine 1-phosphate on insulin resistance. Cell Physiol Biochem 34: 134-147. [Crossref]

