Abstract
Objectives: This study aims to assess the chemical interaction between a Resin-modified Glass Ionomer (RMGI) cement and a 3 mol% yttria-stabilized (3Y-TZP) zirconia, notably to evaluate the interface between the 2 biomaterials using solid-state nuclear magnetic resonance (NMR).
Methods and materials: The RMGI cement alone then the interface RMGI cement /zirconia are analyzed from T=one hour to 12 days with 1H, 13C, 31P and 19F MAS, and 13C and 29Si CPMAS NMR.
Results: The 1H spectrum of the RMGI cement /zirconia mixture slowly evolves. The final spectrum obtained after 12 days indicates a slow rigidification of the RMGI components at the interface with the zirconia particles. A strong signal for the polyacrylic acid signal detected in the 13C CPMAS experiment in presence of zirconia in contrast to RMGI cement alone, indicates a strong interaction of the polyacrylic acid with the zirconia. In addition, observation of sharp acrylate 13C CPMAS signals may be partly related to a physisorbtion of acrylates on zirconia.
Conclusion: With zirconia, the polymerization reaction takes several days to be complete, demonstrating a progressive and evolving interaction with the zirconia.
There is a progressive chemical interaction between RMGI and zirconia that contribute to the understanding of the efficiency of the assembly of dental ceramics.
Key words
Zirconia, Resin-modified glass ionomer cement, Solid state NMR, Chemical organization
Clinical relevance statement
The assembly of prosthetic restorations occupies an essential place for the longevity of these restorations. Glass ionomer cements modified by the addition of resin (RMGI) are commonly used to assemble zirconia restorations. Interface study between RMGI and zirconia with MAS-NMR spectroscopy could identify the nature of interactions.
Introduction
The improvement of the mechanical and optical properties of ceramic materials makes it possible to successfully consider their use in multiple clinical situations. Monolithic zirconia restorations can be considered as a reliable treatment possibility for the realization of unitary crowns [1]. The assembly of prosthetic restorations occupies an essential place for the longevity of these restorations. Among the possibilities sealing cements have a place and in particular glass ionomer cements modified by the addition of resin [2]. Resin-modified Glass Ionomer (RMGI) are a hybrid of glass ionomer cements and composite resin, and thus contain acid-base and polymerizable components. RMGI are usually formulated from fluoro-aluminosilicate glasses, photo-initiators, polyacrylic acid, water, and a water-soluble methacrylate monomer, such as hydroxyethyl methacrylate (HEMA). We know their adhesion to dentin, polycarboxylic acids included in the RMGI electrostatically interacted with hydroxyapatite around collagen. There is micro-mechanical interlocking for those RMGI that additionally hybridize dentin [3]. In the desired assembly between the dental tissues and the reconstitution materials the adhesion with the biological tissue is therefore achieved. If we assemble a zirconia prosthesis we want to be sure of a real adhesion as well.
Interface studies between RMGI cement and different types of zirconia give interesting results but mainly use mechanical tests that give comparative values between several cements tested. They don’t demonstrate reality of interactions of biomaterials to be assembled [4-6].
Magic Angle Spinning (MAS) solid-state Nuclear Magnetic Resonance (NMR) spectroscopy in collecting information about molecular organization has been demonstrated to be a very powerful technique in this field. This investigative technique can resolve key atomic structural details within these materials and has emerged as a crucial tool in characterising cement structure and properties [7]. Commercial glass ionomer were studied using different NMR techniques. NMR spectroscopy was used to characterise the glasses and their chemical structure [8-13]. It can also potentially provide information at the molecular level on the zirconia / RGMI cement interface. In particular, multinuclear MAS NMR should be of specific interest in determining the chemical nature of the species at the interface and studying their own organization and mobility. The objectives of previous studies on ionomer glasses by NMR have been to characterize the glass compositions by 27Al, 29Si, 31P and 19F MAS–NMR spectroscopy and understand the role of their different constituents in their setting reactions [6-12].
The aim of the present study is the NMR characterization of a RMGI cement (ZirCAD) and the study of its interaction with a 3 mol% yttria-stabilized (3Y-TZP) zirconia, notably to determine the interface between the 2 biomaterials. A chemical analysis of the reactivity of the RMGI material and the zirconia at the interface can contribute to the understanding of the efficiency of the assembly of dental ceramics.
Materials and methods
The zirconia powder that was used in this study is a 3Y-TZP zirconia (Ivoclar Vivadent, Schaan, Liechtenstein), lot XY 308 573 F. The zirconia used here is based on zirconium oxide but is also composed of aluminum oxide and yttrium oxide (3% by molar weight of yttrium oxide in order to stabilize the zirconia at room temperature). This percentage is equivalent to 4.5-6% by weight of yttrium oxide. The size of the grains is 0.50 μm. We previously analyzed it by NMR spectroscopy [14].
The resin-modified glass ionomer cement of the study is ZirCAD Cement (Ivoclar Vivadent, Schaan, Liechtenstein) lot 2109614. Is a paste-paste formula presented in an automix syringue.
The composition of the cement (from scientific manual) is: fluoro-aluminosilicate glasses, polyacrylic acid, water, HEMA - water-soluble methacrylate monomer-, stabilizer and photo-initiator.
In this study, are analyzed the 2 different pastes of the commercial cement, their mixture, and finally the mixture put in contact with the zirconia powder. For the ZirCAD / zirconia sample, 80 mg of zirconia powder was mixed with 10 mg of cement for 5 minutes, leave to harden for 1 hour and then transferred and packed into the rotors for solid state NMR experiments.
Solid state NMR experiments were recorded on a Bruker Avance 400 III HD spectrometer operating at a 9.4 T magnetic field. Samples were packed into 4 mm zirconia rotors. The rotors were spun at 10 kHz at 298 K. 31P MAS and CP (Cross-Polarization)/MAS were done with recycle delays of 10 s and 3 s, respectively and a contact time of 3 ms. 13C MAS and CPMAS have been performed with recycle delays of 3 sec and 2 sec, respectively and a contact time of 2 ms. 29Si CPMAS have been recorded with recycle delay of 2 s and a contact time of 3ms. 1H MAS have been performed with the DEPTH pulse sequence. 27Al and 19F MAS NMR has been recorded with recycle delays of 3 s and 5 s, respectively.
The RMGI cement alone then the interface RMGI cement /zirconia are analyzed from T=one hour to 12 days with 1H, 13C MAS and CPMAS, 31P MAS, 27Al MAS, 19 F MAS, 29Si MAS NMR.
Results and discussion
The study began first with identification of the two ZirCAD pastes separately, then after their mixing, and finally after their mixing in the presence of zirconia powder.
Paste A is mainly composed of fluoro-alumino-silicates, phosphates and ester-acrylates and paste B is mainly composed of alumino-silicates, polyacrylic acid and HEMA.
1H NMR spectra
1H NMR is particularly interesting for studying the surface of inorganic materials. Figure 1 displays the 1H spectra of the 2 pastes (A and B) of ZirCAD and of the mixing of the 2 pastes in absence and in presence of zirconia and this up to 12 days. (Figure 1)
Figure 1. 1H MAS NMR spectra of the two compartements (A and B) of ZirCAD cement and the cement powder as a whole in absence and in presence of zirconia powder in function of time
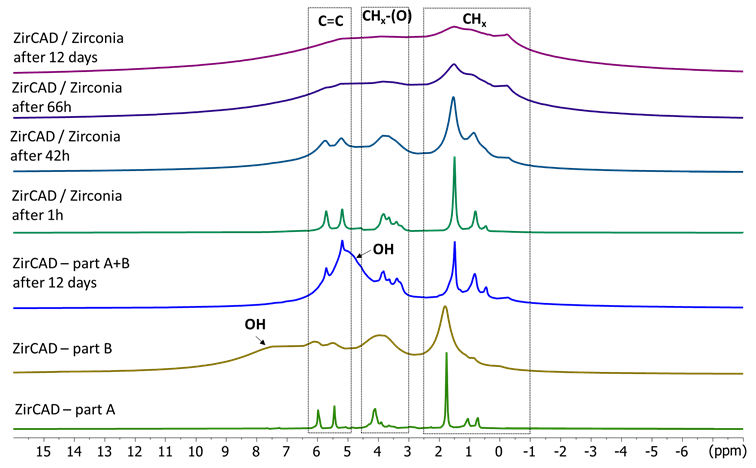
The 1H signals for paste A are very sharp indicating the presence of highly mobile hydrogen (H) species. The signals at 5.4 and 6.0 ppm can be assigned to the alkene groups of acrylate, the ones around 4 ppm to alkoxy groups, and the ones between 0.6 to 1.8 ppm to alkyl groups. The 1H signals for paste B are broader evidencing less mobility in this compartment. The same kind of signals than in paste A are observed with alkene of hydroxyethyl methylacrylate, hydroxyl and aliphatic groups. A broad signal around 7.3 ppm is also detected that can be attributed to hydroxyl H of the polyacrylic acid. After mixing the two pastes of the ZirCAD cement, the material becomes very hard in few minutes. However, surprisingly, the 1H NMR spectrum of the solidified ZirCAD material still evidences sharp signals of mobile species with H signals close to ones of the paste A. Only an additional broad signal around 5 ppm attributable to hydroxyl functions involved in weak hydrogen bonds is observed. This spectrum does not evolve with time (checked up to 12 days).
When the A and B pastes of ZirCAD are mixed in presence of the zirconia powder, the first H spectrum obtained at one hour after the mixing is very similar to the one of part A indicating the presence of many mobile species. The 1H spectrum of the ZirCAD/zirconia mixture slowly evolves with mainly the observation of a broadening of the resonances. The final spectrum is obtained after 12 days. This result indicates a slow rigidification of the ZirCAD components at the interface with the zirconia particles.
This demonstrates that it is a glass ionomer modified by the addition of resin and not a compomer type material. This is a very important result because we have kinetic characteristics of setting and diffusion possibility of the ions, more interesting for the interaction and the adhesion [15].
13C NMR spectra
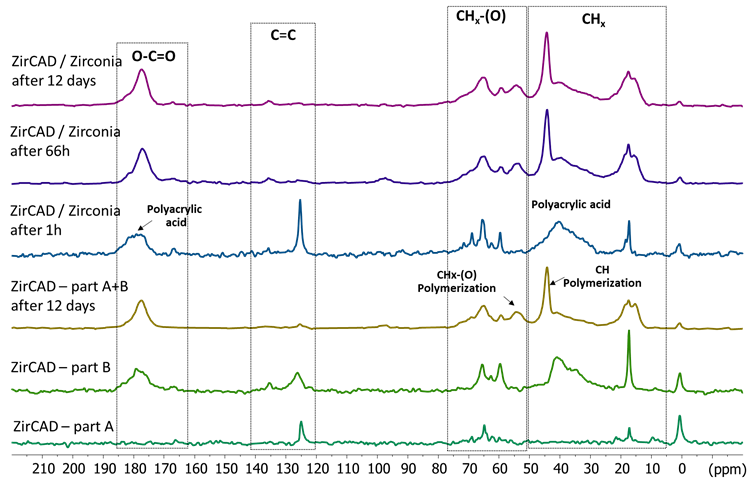
The 13C MAS and CPMAS of the 2 pastes of ZirCAD cement alone, and after their mixing without and with zirconia are shown in Figure 2 and 3. The CPMAS experiment evidence mostly the rigid components of the material while the MAS one performed with a short relaxation delay of 5 s display mostly the mobile components. The 13C results confirm the 1H NMR ones: the presence of mobile species in paste A and of less mobile species in paste B. The signals in the 165-185 ppm, 120-140 ppm, 60-75 ppm and 10-45 ppm areas belong to carboxylate, alkene, alkoxy and alkyl groups. After mixing of the two pastes without zirconia, two new signals at 44 and 54 ppm can be observed that are due to the polymerization reaction of the acrylates. However, even after 12 days, some sharp signals, notably of alkene groups, can still be detected indicating probably that some mobile species are trapped in the rigidified cement. In the presence of zirconia powder, one hour after the mixing, sharp signals similar to the ones of the paste A of ZirCAD are seen. However a strong signal for the polyacrylic acid is detected in the CPMAS experiment in presence of zirconia in contrast to ZirCAD alone. This indicates a strong interaction of the polyacrylic acid with the zirconia with a rigidification of the acid. Also some sharp acrylate signals are observed in the CPMAS experiment at one hour. Our hypothesis is that acrylates may be partly physisorbed on zirconia (or silica or polyacrylic acid) but this interaction is significantly weaker than the effect observed on polyacrylic acid. The monitoring over time indicates a much slower polymerization reaction in the presence than in the absence of zirconia particles. With zirconia, the polymerization reaction takes several days to be complete, demonstrating a progressive and evolving interaction with the zirconia. (Figure 2,3)
Figure 2. 13C MAS NMR spectra of the two compartements (A and B) of ZirCAD cement and the cement powder as a whole in absence and in presence of zirconia powder in function of time
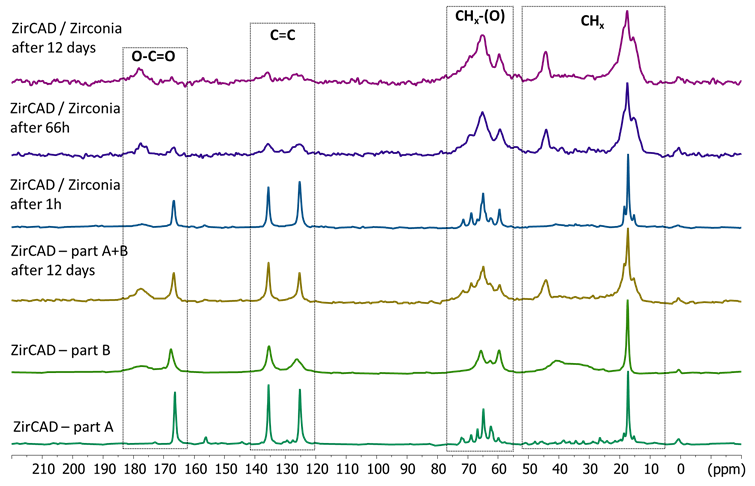
Figure 3. 13C CPMAS NMR spectra of the two compartements (A and B) of ZirCAD cement and the cement powder as a whole in absence and in presence of zirconia powder in function of time
27Al MAS–NMR spectra
Aluminium sites are generally differentiated by their coordination number. Tetrahedral Al sites typically resonate within the regions about 50 ppm, while resonances assigned to five coordinated Al and highly distorted tetrahedral Al environments have been observed in the region 50-20 ppm [7]. For previous studies all glass ionomer cements had mainly coordinated Al (IV) between about 50 and 60 ppm with very small amounts of coordination states Al(V) and Al (VI) also present [9,11,12].
Figure 4 shows the 27Al MAS NMR spectra of the ZirCAD powder studied. The Al (IV) and Al (VI) peaks are well resolved at 44 ppm and -6 ppm. The peak that corresponds to Al (V) is more poorly resolved and appeared as a broad shoulder at about 20 ppm. (Figure 4)
Figure 4. 27Al MAS NMR spectra of ZirCAD cement powder in absence (black) and in presence (red) zirconia
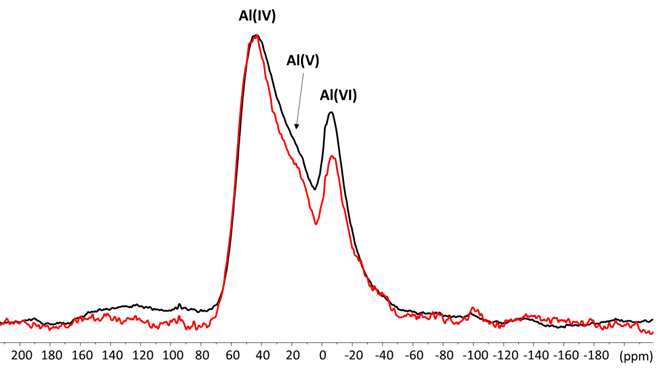
The main difference detected in the presence or the absence of zirconia is a notable decrease of the amount of Al (VI) and (V) with zirconia. The results of 27Al MAS NMR were decisive for Zainuddin which demonstrates that the setting reaction of glass ionomer cement’s can be followed by 27Al MAS-NMR spectroscopy discovering the conversion of Al (IV) to Al (VI) [11-12].
29Si CPMAS
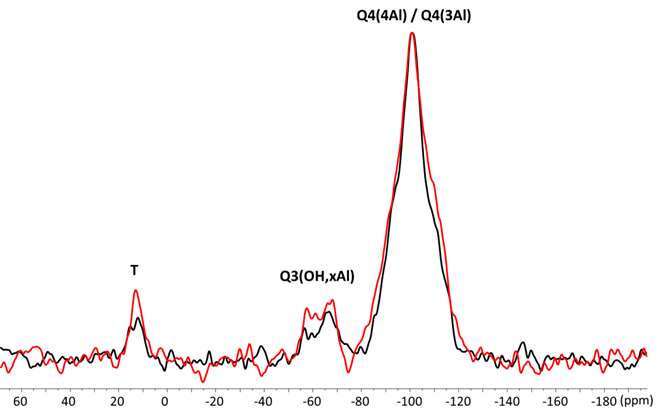
29Si CPMAS of ZirCAD cement (Figure 5) shows a net peak at about -100.6 ppm, a characteristic aluminosilicate signal of 29Si of Q4(4Al) and Q4(3Al) type. A broad signal at ca. -63 ppm is also observed that can be assigned to Q3(OH,xAl) species. A small signal 13 ppm can be potentially attributed to 29Si of type T((OSi/Al)3Si-C) [16]. No clear difference were detected with or without zirconia. (Figure 5)
Figure 5. 29Si CPMAS NMR spectra of ZirCAD cement powder in absence (black) and in presence (red) zirconia
31P MAS-RMN spectra
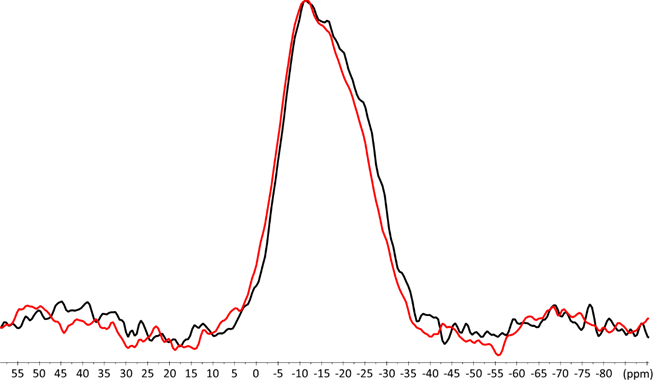
For ZirCAD, a broad and poorly defined signal is seen in the -10 to -30 ppm area (Figure 6). This signal has been assigned to phosphorus in a Q1 environment of Alx-PO4-Y, x=0 a 4; Y=cation Ca2+, Sr2+ [17]. No big difference between the 31P MAS spectra of ZirCAD alone and ZirCAD mixed with zirconia powder could be observed. (Figure 6)
Figure 6: 31P MAS NMR spectra of ZirCAD cement powder in absence (black) and in presence (red) zirconia
19F MAS–NMR spectra
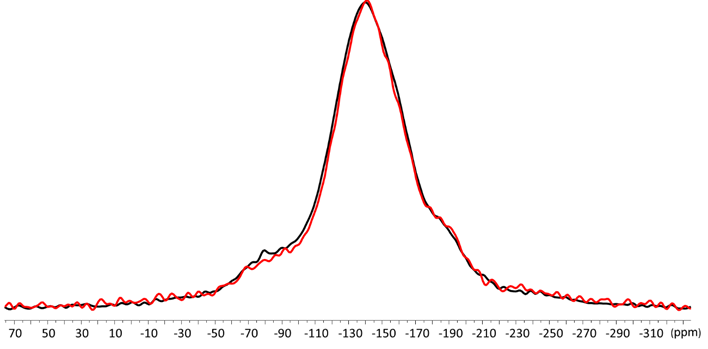
A broad peak at -140 ppm was observed for the ZirCAD cement (Figure 7) that has been assigned to Si-F-Ca or Si-F-Sr by Q. Zeng in the 19F spectra of the glass ionomer cement [18]. Again no difference was observed between the 19F MAS-NMR spectra of ZirCAD alone and ZirCAD mixed with zirconia powder. (Figure 7)
Figure 7. 19F MAS NMR spectra of ZirCAD cement powder in absence (black) and in presence (red) zirconia
The choice of luting cement is essential in the success of the assembly of zirconia crowns [19]. In a previous study we have demonstrated the reactivity of zinc oxyphosphate cement and zirconia at the interface [14]. Our study demonstrate that here is a strong interaction between RMGI and zirconia and confirms the relevance of the assembly of zirconia crowns by the RMGI studied. However, considering the progressive chemical interaction between the 2 materials, it can be recommended not to solicit the assembly immediately.
This study of the chemical interaction between a RMGI cement and a 3Y-TZP zirconia using solid-state nuclear magnetic resonance (NMR) demonstrates a clear difference between the 1H/13C spectra of ZirCAD in the presence and absence of zirconia. There is a strong and stable interaction of the polyacrylic acid with the zirconia through the carboxylic acid functions. With zirconia, the polymerization reaction of RMGI cement takes several days to be complete in contact with zirconia, demonstrating a progressive and evolving chemical interaction between the 2 biomaterials.
Acknowledgments
The authors thank the manufacturer for the kind support of the materials used in the study.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
- Mazza LC, Lemos CAA, Pesqueira AA, Pellizzer EP (2021) Survival and complications of monolithic ceramic for tooth- supported fixed dental prostheses: A systematic review and meta-analysis. J Prosthet Dent.
- Maroulakos G, Thompson GA, Kontogiorgos ED (2019) Effect of cement type on the clinical performance and complications of zirconia and lithium disilicate tooth-supported crowns: A systematic review. Report of the Committee on Research in Fixed Prosthodontics of the American Academy of Fixed Prosthodontics. J Prosthet Dent 121: 754-765. [Crossref]
- Coutinho E, Yoshida Y, Inoue S, Fukuda R, Snauwaert J, et al. (2007) Gel phase formation at resin-modified glass-ionomer/tooth interfaces. J Dent Res 86: 656-661. [Crossref]
- De Angelis F, D'Arcangelo C, Buonvivere CM, Rondoni GD, Vadini M (2020) Shear bond strength of glass ionomer and resin-based cements to different types of zirconia. J Esthet Rest Dent 32: 806-814. [Crossref]
- Comino-Garayoa R, Peláez J, Tobar C, Rodríguez V, Suárez MJ (2021) Adhesion to zirconia: a systematic review of surface pretreatments and resin cements. Materials (Basel)14: 2751. [Crossref]
- Özcan M, Bernasconi M (2015) Adhesion to zirconia used for dental restorations: a systematic review and meta-analysis. J Adhes Dent 17: 7-26. [Crossref]
- Walkley B, Provis JL (2019) Solid-state nuclear magnetic resonance spectroscopy of cements. Materials Today Advances 1: 1-47
- Stamboulis A, Law RV, Hill RG (2004) Characterisation of commercial ionomer glasses using magic angle nuclear magnetic resonance(MAS-NMR). Biomaterials 25: 3907-3913. [Crossref]
- Stamboulis A, Hill RG, Law RV (2005) Structural characterization of fluorine containing glasses by 19F, 27Al, 29Si and 31P MAS–NMR spectroscopy. Journal of Non-Crystalline Solids 351: 3289-3295.
- Stamboulis A, Matsuya S, Hill RG, Law RV, Udoh K, et al. (2006) MAS-NMR spectroscopy studies in the setting reaction of glass ionomer cements. J Dent 34: 574-581. [Crossref]
- Zainuddin N, Karpukhina N, Law RV, Hill RG (2012) Characterisation of remineralising Glass Carbomer® ionomer cement by MAS-NMR spectroscopy. Dent Mater 28: 1051-1058. [Crossref]
- Zainuddin N, Karpukhina N, Hill RG, Law RV (2009) A long-term study on the setting reaction of glass ionomer cements by 27Al MAS-NMR spectroscopy. Dent Mater 3: 290-295. [Crossref]
- Pires R, Nunes TG, Abrahams I, Hawkes GE, Morais CM, et al. (2004) Stray-field imaging and multinuclear magnetic resonance spectroscopy studies on the setting of a commercial glass-ionomer cement. J Mater Sci Mater Med 15: 201-208. [Crossref]
- Tavernier B, Coppel Y, Prigent Y, Grégoire G (2021) Zirconia interaction with zinc phosphate cement studied by solid-state NMR spectroscopy. Oral Health Care 6: 1-5.
- Couret H, Grégoire G, Fontan F, De Parseval PH, Armand S (2004) Electron microprobe analysis into interactions of resin-modified glass-ionomer cement and a modified composite with human dentin in vitro. CR Biologies 327: 21-28. [Crossref]
- Tang HM, Shahid S, Karpukhina N, Law RV, Hill RG (2015) Glass polyalkenoate cements based on simple CaO–Al2O3–SiO2 glasses. Mater Sci Tech 31: 197-202.
- Kirkpatrick RJ, Brow RK (1995) Nuclear magnetic resonance investigation of the structures of phosphate and phosphate-containing glasses: a review. Solid State Nuclear Magnetic Resonance 5: 9-21.
- Stebbins JF, Zeng Q (2000) Cation ordering at fluoride sites in silicate glasses: a high-resolution 19F NMR study. J Non-Crystalline Solids 262: 1-5.
- Alfadhli R, Alshammari Y, Baig MR, Omar R (2022) Clinical outcomes of single crown and 3-unit bi-layered zirconia-based fixed dental prostheses: An up to 6- year retrospective clinical study; Clinical outcomes of zirconia FDPs. J Dent 127: 104321. [Crossref]







