Abstract
Background: Recent healthcare legislation (i.e., the Affordable Healthcare Act of 2011) emphasizes an increased reliance on primary care providers to coordinate patient’s care and reduce reliance on specialty care, thus increasing the depth and breadth of diagnostic and treatment services expected at the primary care level. The resulting shift toward accountable care organizations (ACOs) and patient-centered medical home models has put increased pressure on primary care providers to be accountable for healthcare spending as well as healthcare quality. In this context of expectation for more in-depth diagnosis and treatment at the primary care level, and increased pressure to control healthcare costs, the shortcomings of PSA-based screening, and costs associated with subsequent overdiagnosis and treatment highlight the need for better diagnostic tools to allow the primary care clinician to more effectively triage only those patients likely to benefit from more costly urology specialty care.
Objective: The performance of a novel, serum-based multiplexed autoantibody assay was correlated with common risk factors in detecting prostate cancer.
Methods: This observational study reviewed the clinical use of Apifiny in 2436 patients during a 12-month period by over 250 primary care physicians across 29 states. 89% of the primary care patients were over age 51, and 31% were over 70. PSA was not reported in ~72% of the patients consistent with recommendations from the U.S. Preventive Services Task Force.
Results: The mean (± SD deviation) age, PSA, and Apifiny score of the study population was 65 ± 11 years, 4.3 ± 3.6 ng/mL, and 43 ± 30, respectively. 45% of African Americans and 30% of Caucasians were classified as high risk. Interestingly, 31% of patients with PSA less than or equal to 4.0 ng/ml were classified as high risk. Indeed, among men with known PSA values, there was little correlation with Apifiny and PSA values. Typically, patients who were classified as high risk were referred to a urologist whereas low-risk men were not.
Conclusion: The novel autoantibody test has previously been shown to be useful for identifying men at high risk of prostate cancer. In this study, we found that the biomarker assay is being using by primary care doctors to screen for prostate cancer without PSA testing. The immune-based blood test correlated with some known risk factors for prostate cancer such as African American race and positive DRE results, but not others such as PSA. This technique is promising and may lead to smarter prostate cancer screening (fewer false positives and negatives) alone or in combination with PSA. Further studies are warranted to validate the performance of this assay.
Introduction
Prostate cancer is the most common nonskin cancer diagnosed in men, with an estimated 161,360 new cases diagnosed and 26,730 deaths in 2017 [1]. Approximately 1 in 7 men will be diagnosed with prostate cancer during his lifetime [2]. It is the second leading cause of cancer deaths in men after lung cancer [1], and is a heterogeneous disease in severity, ranging from slow-growing indolent tumors to rapidly progressing, highly aggressive carcinomas associated with significant morbidity and mortality [3].
Typically, prostate cancer develops slowly, with a long preclinical phase such that most men with prostate cancer die of other causes before their disease becomes symptomatic [4]. The probability of survival in the next 5 years is near 100% for patients with localized or regional disease, and increases with incremental prior years of survival (Figure 1) [5]. The lifetime risk of dying of prostate cancer is less than 3% [6], with about 2% of all prostate cancer deaths occurring before age 55 years, 29% occurring between age 55 and 74 years, and 69% at age 75 years and older (Figure 2) [5].
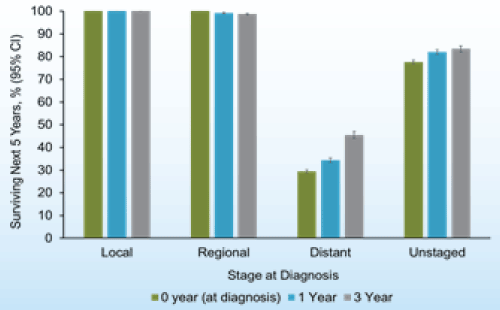
Figure 1. Probability of surviving the next 5 years given survival 0,1 or 3 years after prostate cancer by stage at diagnosis.
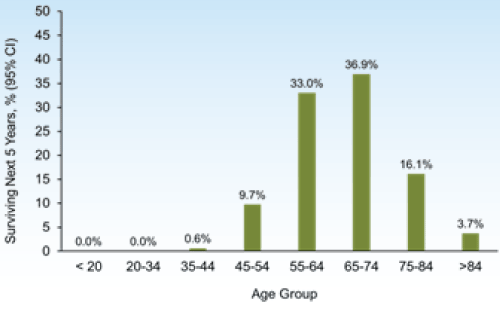
Figure 2. New cases of prostate cancer in the United States in 2008-2012 by age group. The number of new cases of prostate cancer was 138 per 100,000 men/year; the median age at diagnosis was 66 years.
Prostate cancer screening-controversy and shortcomings
Preventive services, such as prostate cancer screening, fall largely on the shoulders of primary care clinicians [7]. In the United States, early detection of prostate cancer is driven by prostate- specific antigen (PSA)–based screening, followed by prostate biopsy for diagnostic confirmation [8]. Approximately 19 million men undergo PSA screening annually, resulting in approximately 4.7 million abnormal findings on tests (based on a PSA > 4.0 ng/mL) leading to approximately 1.3 million biopsy procedures performed [9].
Deaths in the United States from prostate cancer have decreased approximately 4% per year since 1992 (5 years after the introduction of PSA testing) [10]; however, there are conflicting data that fail to convincingly demonstrate a significant decrease in prostate cancer–specific mortality attributable directly to PSA screening [11-14].
The vast majority of men with prostate cancer have clinically localized disease at a potentially more curable stage, which is attributable to widespread use of PSA screening [3,15]. However, the risks associated with prostate cancer screening with PSA are considerable, and must be weighed against the potential advantage of the still-debated reduction in cancer- specific mortality [16]. Risks include a high rate of false-positive results, complications associated with prostate biopsy, and the serious consequences of prostate cancer treatment [16].
The widespread use of PSA screening has led to an increase in the rate of negative results on biopsies (ie, false-positive PSA test results), as well as a high rate of overdetection or overdiagnosis of prostate cancer
PSA screening has resulted in substantial overtreatment (with attendant adverse effects) of potentially indolent tumors that would have remained asymptomatic and not required treatment for the remainder of a man’s life had tumors not been detected via PSA screening and subsequent biopsy (Figure 3) [3,15,17]. Over diagnosis is generally defined in this context as detection of a prostate cancer that would have remained asymptomatic and undetected during an individual’s lifetime in the absence of screening [3,8]. Estimates from the 2 largest prostate cancer screening trials suggest over diagnosis rates, based on PSA screening, of 17% to 50%3. The rate of over diagnosis and subsequent overtreatment secondary to PSA- based prostate cancer screening appears to be greater than that for other cancers for which routine screening currently occurs (e.g., breast, colorectal, or cervical cancers) [18]. According to a national registry of men diagnosed with prostate cancer in community-based urology practices in the United States, surgery rates for low-, intermediate-, and high-risk cancer were approximately 50%, 70%, and 50%, respectively between 2010 and 2013 [19]. Rate of radiation therapy among those same groups was approximately 10%, 20%, and 20%, respectively, and androgen deprivation therapy use was approximately 0%, 5%, and 25%, respectively [19]
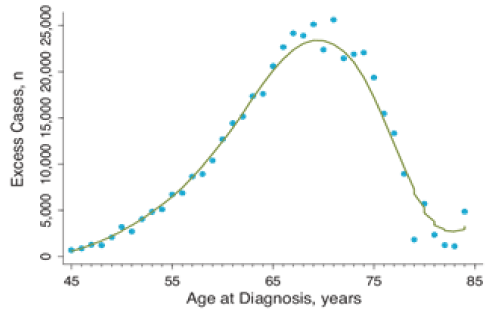
Figure 3. Number of excess prostate cancer cases by age at diagnosis 1987 to 1996. The 95% confidence interval is extremely narrow and is not shown here. ©vickers et al. licensee BioMed Central Ltd.
Positive PSA test results of 3 to 10 ng/mL have an approximate 70% chance of being false positive [16]. Even after multiple screening tests, there is still a 12% to 13% risk of a false-positive test result [16]. The common PSA threshold for biopsy of greater than 4.0 ng/mL is associated with a positive predictive value of about 30% in men aged 50 years or older, and a negative predictive value of about 85% in men with a median age of 69 years at biopsy [20]. At 9 years of follow-up from the large European Randomized Study of Screening for Prostate Cancer (ERSPC) in 182,000 men, 75.9% of men who underwent biopsy after elevated results on PSA (cutoff varied by country between 3.0 and 4.0 ng/mL) had a false- positive result [18].
PSA-driven false-positive results, over diagnosis, and overtreatment of prostate cancer are associated with a number of potentially harmful sequelae that appear to greatly outweigh the modest, at best, benefits of PSA screening (Table 1) [21]. Based on an interpretation of the 2 major trials of PSA screening (the US Prostate, Lung, Colorectal, and Ovarian [PLCO] Cancer Screening Trial and the ERSPC), the US Preventive Services Task Force (USPSTF) determined that the benefit of PSA screening and early treatment ranges from 0 to 1 cancer deaths avoided per 1000 men screened [3].
Table 1: Harmful outcomes associated with PSA Screening and subsequent overdiagnosis and treatment
Harmful Outcome |
Approximate Risk |
References |
Harms of Screening |
Patient anxiety about developing prostate cancer, for up to 1 year after screening (men with negative prostate biopsy after suspicious PSA screening test) |
26% |
Fowler et al. 2006 [23] |
Additional PSA test biopsy, and/or urologist visit over the next year (vs patients with initial
negative PSA result [< 2.5 ng/mL]) |
73% vs 42%;
15%vs1%:
71%vs13%,
respectively |
Fowler et al. 2006 [23] |
Lead time |
5.4 to 6.9years |
Draisma et al. 2009 [42] |
Harms of Biopsy |
Moderate or major bothersome symptoms, including pain; fever: blood in urine, semen, or stool: infection; and temporary urinary difficulties |
Upto32% |
Rosario et al. 2016 [26] |
Hospitalization |
1% to 6.9% |
Moyer et al. 2012 [3], Loeb et al. 2011 [25] |
Complications of Treatment |
|
|
Serious CV event due to treatment DVT or PE due to treatment |
2 in 1000 men, 1 in 1000 men |
Moyer et al. 2012 [3], Moyer et al. 2012 [3] |
Erectile dysfunction due to treatment |
29 in 1000 men;
up to 19% to 27% |
Moyer et al. 2012 [3], Wolf et al. 2010 [22] |
Urinary incontinence due to treatment |
18 in 1000 men; up to 12% to 16% long term |
Moyer et al. 2012 [3], Wolf et al. 2010 [22] |
Death due to Treatment |
<1 in 1000 men;
0.1% to 0.2% within 30days |
Moyer et al. 2012 [3], Wolf et al. 2010 [22] |
CV: Cardiovascular; DVT: Deep vein thrombosis; PE: Pulmonary embolism.
*Average time by screening advances in diagnosis of prostate cancer among patients who have been diagnosed with prostate cancer during their in the screening
The most frequently reported direct harms associated with prostate cancer screening relate to anxiety [22]. Two well- designed surveys indicate that men with false-positive PSA results have greater short-term and long-term prostate cancer anxiety than do men with true-negative results (26% vs 6% after 1 year [P<0.001] in 1 study), and that men with false-positive results have more subsequent tests/visits compared with men who have true-negative results [23,24]. This is one of several reasons why most clinical practice guidelines regarding PSA screening now encourage shared decision making between clinician and patient, with the patient being informed of the potential benefits and harms of screening [7,20]. Such guidance, however, places primary care clinicians in a somewhat untenable position, given the lack of additional options to help guide primary care clinicians in determining when referral to a urologist for further evaluation is warranted (Table 2).
Table 2. Comparison of Apifiny Score with PSA Level in 2,436 Patients in the Primary Care Setting [43].
PSA cutoff |
Patients (n) |
Apifiny Higher Risk |
Apifiny Lower Risk |
≤4 ng/mL |
302 |
94 (31%) |
208 (69%) |
4.1-10 ng/mL |
323 |
112 (35%) |
211 (65%) |
>10 ng/mL |
66 |
22 (33%) |
44 (67%) |
Unknown |
1745 |
542 (31%) |
1203 (69%) |
Men who are referred for biopsy based on PSA test results face prostate biopsy–related risks such as bleeding, infection, and hospitalization due to complications [22,25]. Estimated incidence of hematuria is approximately 6% to 13%, but the risk of serious bleeding requiring transfusion is low. The estimated rate of urinary tract infection is 0.3% to 4%, and that of serious infection is <2% [22]. There is a 2.65-fold increased risk (95% confidence interval [CI], 2.47-2.84; P<0.0001) of hospitalization within 30-days of the procedure owing to infectious or noninfectious complications compared with a control population, based on analysis of a Medicare database (6.9% vs 2.7%) [25]. Interim results of an ongoing, randomized trial reported that 32% of men experienced 1 or more moderate/major adverse events after prostate biopsy that required clinician follow-up, including pain; fever; blood in urine, semen, or stool; infection; transient urinary difficulties; or other issues [26].
Patients who are treated for potentially asymptomatic prostate cancer are at risk for the adverse events associated with such treatment. Radical prostatectomy is associated with a 20% increased absolute risk for urinary incontinence and a 30% increased absolute risk for erectile dysfunction compared with watchful waiting after 1 to 10 years; perioperative deaths or cardiovascular events occur in approximately 0.5% or 0.6% to 3% of patients, respectively [3]. Radiation therapy is associated with a 17% absolute increased risk for erectile dysfunction and an increased risk for bowel dysfunction compared with watchful waiting after 1 to 10 years [3].
Attendant with the increased over diagnosis and overtreatment associated with PSA screening are the associated costs. Approximately $1.86 billion is spent annually on PSA tests alone [9], and the estimated national expenditure for care of men with prostate cancer in 2014 in the United States was $13.4 billion, according to the National Cancer Institute [27].
Recent healthcare legislation (i.e., the Affordable Healthcare Act of 2011) emphasizes an increased reliance on primary care providers to coordinate patient’s care and reduce reliance on specialty care, thus increasing the depth and breadth of diagnostic and treatment services expected at the primary care level [28]. The resulting shift toward accountable care organizations (ACOs) and patient-centered medical home models has put increased pressure on primary care providers to be accountable for healthcare spending as well as healthcare quality [29]. In this context of expectation for more in- depth diagnosis and treatment at the primary care level, and increased pressure to control healthcare costs, the shortcomings of PSA-based screening, and costs associated with subsequent over diagnosis and treatment highlight the need for better diagnostic tools to allow the primary care clinician to more effectively triage only those patients likely to benefit from more costly urology specialty care.
Prostate cancer screening-guidelines
In 2012, the USPSTF published guidelines recommending against PSA-based screening for prostate cancer in the general population, citing convincing evidence that PSA-based screening results in over diagnosis of asymptomatic cancer that
would likely have remained asymptomatic for the man’s lifetime, resulting in increased biopsies and treatment with little to no demonstrated reduction in prostate cancer mortality [3]. Recently, the USPSTF has amended its stance in a draft statement, determining that the decision about whether to be screened should be an individual one. The new recommendation –which changed the grade for PSA screening from D to C – states the potential benefits and harms of PSA-based screening are closely balanced in men aged 55 to 69 years. As a result, the USPSTF recommends clinicians evaluate a person’s risk because of family history and race as well as competing causes of morbidity and mortality [30].
Clinical practice guidelines of major medical societies regarding screening with PSA conflict with those of the USPSTF, with most recommending shared decision-making between clinician and patient, consideration of risk factors, and recommendations regarding screening intervals [20]. For example, the American College of Physicians (ACP) recommends screening with shared decision making for men aged 50 to 69 years with a life expectancy of greater than 10 to 15 years [31]. ACP also recommends screening at 45 years of age for men at higher risk for prostate cancer (patients who are black, and those patients who have a first-degree relative who was diagnosed with prostate cancer before 65 years of age), and at 40 years of age for men with multiple family members diagnosed with prostate cancer before 65 years of age [31]. Intervals longer than 1 year between screening PSAs are recommended [31]. The ACP guidelines are largely similar to those promulgated by the American Urological Association (AUA) and the American Cancer Society (ACS) [8,22]. ACS guidelines further recommend a PSA level of ≥ 4.0 ng/mL as the cutoff for referral to a urologist for further evaluation or biopsy for men at average risk for prostate cancer, and a PSA level of 2.5 ng/mL to 4.0 ng/mL as the range in which to consider an individualized risk assessment incorporating other risk factors that may be used to recommend a biopsy [22].
Despite conflicting guidelines, and the shortcomings and risks associated with PSA screening, it continues to be used as a primary screening tool by many primary care clinicians, and is then repeated by a urologist on patient referral because of the elevated PSA levels identified by the primary care clinician at the outset. Continued use of PSA screening may be due to a clinician’s fear of missing a serious, potentially lethal cancer or for potential liability concerns, or screening may be performed at a patient’s request [32-35].
New Developments in Screening and Diagnosis of Prostate Cancer
The focus of early detection has shifted from efforts to diagnose any and all prostate cancers to an effort to diagnose clinically significant prostate cancers at an early stage [36].This is reflected in a trend toward a decreased number of initial biopsy procedures performed (from 24% to 16%), increased use of repeat PSA testing (from 72% to 82%), and increased use of prostate cancer antigen-3 (PCA3) testing (from 11% to 27%) by urologists for those patients referred to their offices from primary care practices because of previous elevated PSA screening results [33]. Furthermore, several recent studies report decreased frequency in PSA screening among primary care clinicians subsequent to the May 2012 USPSTF recommendation [33,37,38], possibly reflecting an increased selectivity in screening practices on the part of clinicians based on patients’ age, prior PSA level (if previously screened), or other risk factors for prostate cancer.
Few additional options are currently available to guide primary care clinicians in determining when referral to a urologist for further evaluation is warranted, or to guide urologists in determining whether a first or follow-up biopsy is warranted. Fear of occult prostate cancer leads to additional procedures; therefore, many men receive second, third, and fourth repeat biopsy procedures to rule out the presence of cancer [39]. These shortcomings have led many researchers to investigate ways to optimize the use of PSA and develop novel serum and tissue biomarkers to address the need for more accurate, dependable screening tools. The goal is to identify patients more likely to benefit from referral and further evaluation, biopsy, and potentially from treatment for early prostate cancer, while reducing inaccurate readings, unnecessary invasive testing in healthy men, and attendant excess healthcare costs.
Laboratory tests account for only approximately 2.3 cents of every dollar spent on healthcare, but their results affect between 70% and 80% of clinical decisions made [40]. Therefore, more accurate diagnostic laboratory tests to screen for prostate cancer are needed to help primary care clinicians more effectively coordinate patient care and triage appropriate patients to specialty care for further workup and treatment.
Several other serum biomarkers are available to aid primary care clinicians in prostate cancer diagnosis, but most, unlike the multiplexed autoantibody assay described herein (APIFINY®), are based on PSA. The biomarker test measures 8 signature autoantibodies in the blood stream that are released by the immune system in response to the presence of prostate cancer [41]. The scores from the developed algorithm can be used to indicate a relative high or low risk of the presence of autoantibodies known to be associated with an immune response to prostate cancer, particularly for patients with intermediate (4.0 to 10 ng/mL) PSA levels that are associated with a high rate of false-positive results due to a lack of sensitivity and specificity in this range [42]. Measurement of these cancer-specific biological markers may be used in men with an elevated PSA to help provide additional insight to support a more informed clinical decision about when to refer to a urologist for further evaluation. Potential benefits of utilizing this autoantibody assay to aid in diagnostic decisions may include earlier detection of cancer and, therefore, improved survival rates, as well as a reduction of unnecessary biopsies, with a consequent reduction in associated morbidity and healthcare costs related to over diagnosis and overtreatment.
Clinical updates on the use of a novel autoantibody test to determine the risk of prostate cancer by community physicians
In an observational study [43] of the clinical use of Apifiny in 2,436 patients in the primary care setting, Apifiny score was correlated with known risk factors for prostate cancer including African American race and positive digital rectal examination (DRE) findings, but showed little correlation with PSA values [11]. In fact, nearly one-third (31%) of patients with PSA levels ≤4 ng/mL were classified as higher risk based on Apifiny scores of >59 (Table 3). Those patients classified as higher risk on Apifiny testing were typically referred to a urologist while those classified as lower risk were not [44]. In most cases, PSA level was not available, consistent with the USPSTF recommendations. In another observational study comparing clinical use of Apifiny in over 5,000 patients treated by primary care physicians (n=2,436) or urologists (n=2,707), researchers found that higher and lower risk measurements were consistent with known patterns of prostate cancer risk, and appeared to be independent of PSA levels [12]. Approximately one third of patients ≥40 years were considered at higher risk for prostate cancer (Apifiny score >59), regardless of PSA level. African American men had higher Apifiny scores compared with Caucasian and Hispanic men, and men with abnormal digital rectal exams had slightly higher Apifiny scores (Table 3).
Table 3. Apifiny scores Reflect Racial and DRE Patterns of Prostate Cancer Risk in Primary Care and Urology Settings [44].
Variable |
Higher Risk Apifiny Score in Primary Care |
Higher Risk Apifiny Score in Urology |
|
| |
Race |
|
|
|
Caucasian |
30% |
29% |
|
African american |
45% |
45% |
|
Hispanic |
34% |
31% |
|
Asian |
22% |
41% |
|
Other |
33% |
25% |
|
DRE results |
|
|
|
Normal |
30% |
29% |
|
Abnormal |
31% |
|
unknown |
31% |
35% |
|
As in the previous study, more than 70% of patients treated in the primary care setting did not have a PSA score level listed, possibly suggesting that Apifiny was used alone for prostate cancer screening [45]. In the urology setting, 32% of patients did not have a PSA listed. The researchers concluded that “Apifiny may be an effective clinical tool in improving patient management decisions relative to costly specialist referrals and unnecessary biopsies. ”Together, these data show that research into novel biomarkers for prostate cancer is emerging rapidly. These biomarkers have the potential to more accurately identify patients who are most likely to benefit from prostate biopsy and early treatment, while reducing false-positive results, unnecessary biopsies, morbidity and healthcare costs.
The current study has several limitations, including the need to more fully assess the test in all races, as well as to determine how other conditions (such as obesity and its pro-inflammatory state, or steroid use) may affect the assay’s performance. Additional studies and long-term follow-up including prostate cancer death are warranted to verify this as a marker of clinically meaningful prostate cancer.
Conclusion
The degree of potential over diagnosis and associated overtreatment of prostate cancer appears to be greater than that for any other cancer for which routine screening currently occurs and is associated with serious adverse effects. PSA- based screening for prostate cancer, including its limitations, has been well understood by clinicians and reimbursement authorities for over three decades. There is a need to move beyond PSA testing with new biological markers that are cancer specific to improve early detection of cancer. Such markers will more accurately identify patients who are most likely to benefit from referral to a urologist for further evaluation, biopsy and, potentially, treatment for early prostate cancer while reducing inaccurate readings, unnecessary invasive testing in healthy men, and associated morbidity and healthcare costs.
Acknowledgments
We are indebted to Arul Chinnaiyan, M.D., Ph.D. for his continued encouragement of this work, to Dr. Robert Reinhardt for his guidance, and to the entire Armune Bioscience and MagArray Inc staff for their assistance in the laboratory.
Disclosures
MagArray Inc and Armune Bioscience provided all financial support for the work reported in this article. Drs. Allen and Hafron are consultants and speakers for Armune Bioscience.
References
- American Cancer Society. Key Statistics for Prostate Cancer. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
- American Cancer Society. Prostate Cancer. 2014. http://www.cancer.org/acs/groups/cid/documents/webcontent/003134-pdf
- Moyer VA; US. Preventive Services Task Force. (2012) Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 157: 120-134. [Crossref]
- Bell KJ Del Mar C, Wright G, Dickinson J, et al. (2015) Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int J Cancer 137: 1749-1757. [Crossref]
- SEER Stat Fact Sheets: Prostate Cancer. [National Cancer Institute Website]. Available at: http://seer.cancer.gov/statfacts/html/prost.html.
- Lifetime Risk of Developing or Dying from Cancer. http://www.cancer.org/cancer/cancerbasics/lifetime-probability-of-developing-or-dying-from-cancer
- Hoffman RM, Barry MJ, Roberts RG, Sox HC (2012) Reconciling primary care and specialist perspectives on prostate cancer screening. Ann Fam Med 10: 568-571. [Crossref]
- Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, et al. (2013) Early detection of prostate cancer: AUA Guideline. J Urol 190: 419- 426. [Crossref]
- Aubry W, Lieberthal R, Willis A, et al. (2013) Budget impact model: epigenetic assay can help avoid unnecessary repeated prostate biopsies and reduce healthcare spending. Am Health Drug Benefits 6: 15-24.
- Barry MJ (2009) Screening for prostate cancer--the controversy that refuses to die. N Engl J Med 360: 1351-1354. [Crossref]
- Andriole GL, Crawford ED, Grubb RL III, et al. (2012) Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst 104:125-132. [Crossref]
- Heijnsdijk EA, Wever EM, Auvinen A, Hugosson J, Ciatto S, et al. (2012) Quality-of-life effects of prostate-specific antigen screening. N Engl J Med 367: 595-605. [Crossref]
- Ilic D1, Neuberger MM, Djulbegovic M, Dahm P (2013) Screening for prostate cancer. Cochrane Database Syst Rev CD004720. [Crossref]
- Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, et al. (2014) Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 384: 2027- 2035. [Crossref]
- Falzarano SM, Ferro M, Bollito E, Klein EA, Carrieri G, et al. (2015) Novel biomarkers and genomic tests in prostate cancer: a critical analysis. Minerva Urol Nefrol 67: 211-231. [Crossref]
- Allan GM, Chetner MP, Donnelly BJ, Hagen NA, Ross D, et al. (2011) Furthering the prostate cancer screening debate (prostate cancer specific mortality and associated risks). Can Urol Assoc J 5: 416-421. [Crossref]
- Vickers AJ, Sjoberg DD, Ulmert D, Vertosick E, Roobol MJ, et al. (2014) Empirical estimates of prostate cancer overdiagnosis by age and prostate-specific antigen. BMC Med 12: 26. [Crossref]
- Schröder FH1, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, et al. (2009) Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 360: 1320-1328. [Crossref]
- Cooperberg MR, Carroll PR1 (2015) Trends in Management for Patients with Localized Prostate Cancer, 1990-2013. JAMA 314: 80-82. [Crossref]
- Hayes JH, Barry MJ (2014) Screening for prostate cancer with the prostate-specific antigen test: a review of current evidence. JAMA 311: 1143-1149. [Crossref]
- Carroll PR, Parsons JK, Andriole G, Bahnson RR, Barocas DA, et al. (2014) Prostate cancer early detection, version 1.2014. Featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 12: 1211-1219. [Crossref]
- Wolf AM, Wender RC, Etzioni RB, Thompson IM, D'Amico AV, et al. (2010) American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin 60: 70-98. [Crossref]
- Fowler FJ Jr, Barry MJ, Walker-Corkery B, et al. (2006) The impact of a suspicious prostate biopsy on patients’ psychological, socio-behavioral, and medical care outcomes. J Gen Intern Med 21: 715-721. [Crossref]
- McNaughton-Collins M, Fowler FJ Jr, Caubet JF, et al. (2004) Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med 117: 719-725. [Crossref]
- Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM (2011) Complications after prostate biopsy: data from SEER-Medicare. J Urol 186: 1830-1834. [Crossref]
- Rosario DJ, Lane JA, Metcalfe C, Donovan JL, Doble A, et al. (2012) Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within Protect study. BMJ 344: d7894. [Crossref]
- Financial Burden of Cancer Care. [National Cancer Institute Website]. 2015. http://progressreport.cancer.gov/after/economic_burden
- Davis K, Abrams M, Stremikis K (2011) How the Affordable Care Act will strengthen the nation's primary care foundation. J Gen Intern Med 26: 1201-1203. [Crossref]
- Song Z (2014) Accountable Care Organizations in the U.S. Health Care System. J Clin Outcomes Manag 21: 364-371. [Crossref]
- https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/prostate-cancer-screening1
- Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P, Clinical Guidelines Committee of the American College of Physicians (2013) Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 158: 761-769. [Crossref]
- Kim SP, Karnes RJ, Nguyen PL, Ziegenfuss JY, Thompson RH, et al. (2014) A national survey of radiation oncologists and urologists on recommendations of prostate-specific antigen screening for prostate cancer. BJU Int 113: E106-111. [Crossref]
- Perez TY, Danzig MR, Ghandour RA, Badani KK, Benson MC, et al. (2015) Impact of the 2012 United States Preventive Services Task Force statement on prostate-specific antigen screening: analysis of urologic and primary care practices. Urology 85: 85- 89. [Crossref]
- Ghandour RA, McKiernan JM. Reply: To PMID 25440819. Perez TY, et al. (2015) Urology. 85:85-89. Impact of the 2012 United States Preventive Services Task Force statement on prostate-specific antigen screening: analysis of urologic and primary care practices. Urology 85: 90-91.
- Sammon JD, Pucheril D, Diaz M, Kibel AS, Kantoff PW, et al. (2014) Contemporary nationwide patterns of self-reported prostate-specific antigen screening. JAMA Intern Med 174: 1839-1841. [Crossref]
- Morgan T, Palapattu G, Wei J (2015) Screening for Prostate Cancer-Beyond Total PSA, Utilization of Novel Biomarkers. Curr Urol Rep 16: 63. [Crossref]
- Cohn JA, Wang CE, Lakeman JC, Silverstein JC, Brendler CB, et al. (2014) Primary care physician PSA screening practices before and after the final U.S. Preventive Services Task Force recommendation. Urol Oncol 32: 41. [Crossref]
- McCarthy M (2015) US guideline may have led to drop in PSA testing among primary care physicians, studies find. BMJ 350: h2906. [Crossref]
- Djavan B, Ravery V, Zlotta A, Dobronski P, Dobrovits M, et al. (2001) Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop? J Urol 166: 1679-1683. [Crossref]
- Schmidt M (2012) Reportable Results and Laboratories. Clinical Lab Products 42: 8-10. http://www.unquestinfo.com/images/uploads/CMS/226/clp_reportable_results_and_laboratories.pdf
- Wang X, Yu J, Sreekumar A, Varambally S, Shen R, et al. (2005) Autoantibody signatures in prostate cancer. N Engl J Med 353: 1224-1235. [Crossref]
- Schipper M, Wang G, Giles N, Ohrnberger J (2015) Novel prostate cancer biomarkers derived from autoantibody signatures. Transl Oncol 8: 106-111. [Crossref]
- Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, et al. (2009) Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst 101: 374-383. [Crossref]
- Hafron J, Ohmberger J, Singh S, Howard LE, Freedland SJ, Allen GG. The use of a novel autoantibody test (Apifiny®) to determine the risk of prostate cancer by community physicians: allowing prostate cancer screening without PSA.
- Hafron J, Ohmberger J, Arthurs J, Kernen KM, Howard LE, Freedland SJ, Allen GG. Clinical use of Apifiny® a novel serum based prostate cancer risk assessment multiplexed autoantibody assay.



