Abstract
Objective: This work characterizes the electrocorticograms in a small case series retrospectively in which Responsive Neurostimulation (RNS -Neuropace™) recorded subclinical seizure activity during Covid-19 infection in patients who tested positive for SARS-CoV-2.
Methods: After gaining institutional IRB approval, we performed a retrospective review of our RNS database and identified patients who received the initial series (without boosters) of SARS-CoV-2 vaccines after the World Health Organization declared the pandemic’s start date of March 11, 2020. We quantified the inventories of ictal and interictal discharges including short and long episode counts, channel saturations, magnet-swipes, and histogram data counts as arbitrary potentially surrogate correlates of seizure activity as other articles denote. Statistical analyses were conducted using R version 4.1.1 and tables were produced using the package gtsummary. Wilcoxon rank sum test was used for comparison of groups. A p-value of < 0.05 was considered significant.
Results: Increases in Long Episodes (LEs indicating >30 seconds of activity) exhibited a statistically significant increase in the four weeks of these post infection with a p value of <0.05 in 50 % of patients. There was an overall modest increase in the total number of total daily episode counts after initial infection with SARS-CoV-2 during this 4-week post exposure period, although this observation was not statistically significant.
Significance: Despite the small number of patients identified and although other confounders may not be excluded, RNS is a valuable method of studying the electrocorticograms of the ambulatory SARS-CoV-2 population.
Key words
intractable seizures, SARS-CoV-2, Responsive neurostimulation
Key Points
- RNS is a valuable method of studying the electrocorticograms of patients with SARS-CoV-2
- RNS identifies that subclinical seizure activity is increased in the SARS-CoV-2 population
- Further studies of the SARS-CoV-2 population using quantification that RNS allows may yield future valuable observations
Introduction
This work identifies ambulatory patients with intractable epilepsy who were infected with SARS-CoV-2 who had subclinical seizures in the non-acute ambulatory setting and neither the patients themselves nor did any caretakers report any seizures in this group who had been previously implanted with an RNS-Responsive NeuroStimulation device (Neuropace™) at our institution. The study concept originated from retrospective electrocorticogram reviews at office visits during which increases in seizure counts were noted by visual inspection of the histogram dataset tallied by the RNS device leading to querying if there had been any prior SARS-CoV-2 infection among these patients.
Methods
After obtaining institutional IRB approval, we performed a retrospective review of our RNS database and identified patients within the patient data management system (PDMS) who had tested positive for acute SARS-CoV-2 after March 11, 2020 which was designated the start of the Covid-19 pandemic by the World Health Organization (WHO) [1]. We quantified the daily inventories of ictal and interictal discharges, short and long episode counts, channel saturations, magnet-swipes, and thus the daily histogram data counts as other articles utilizing RNS as a method of monitoring and quantifying such information identify that such inventory may represent surrogate markers of seizure activity [2-4]. Statistical analyses were conducted using R version 4.1.1 and tables were produced using the package gtsummary [5]. Wilcoxon rank sum test was used for comparison of groups. A p-value of <0.05 was considered significant.
Of note, three out of the four patients (A, B and D) each completed their vaccination series prior to being infected with SARS-CoV-2 as noted in the tables. Patient C was noted to be unvaccinated against SARS-CoV-2 at the time of study.
Results
We identified four patients from our RNS database who tested positive for SARS-CoV-2 infection, three males and one female, and 3 of these patients had bitemporal epilepsy and 1 had occipital localization. The patients that reported SARS-CoV-2 infection, did not report any clinical seizure activity and such SARS-CoV-2 infection status was queried retrospectively at office visits for these cases that identified an increase in seizure activity upon visual inspection of the histograms. These patients were queried about SARS-CoV-2 infection since clinically it seemed no other readily identifiable factors such as anti-seizure medication noncompliance or changes, device changes, or other variables which might have potentially contributed to such increases as literature might suggest [3,4,6]. Potential confounders of histogram data which included RNS adjustments, dates of anti-seizure medication changes, and other notable clinical circumstances (one patient reported urosepsis, another started Vaping THC, CBD products, and nicotine in the months acutely prior to SARS-CoV-2 infection) were tabulated below in the figures. The number of daily seizure episodes as well as the previously noted histogram data identifying the number of long episodes were tabulated and analyzed as noted in the figures and tables below. A modest increase in the overall number of daily seizure activity was noted within 4 weeks of the infection (See Table and Figures 1 & 2). Increases in Long Episodes (LEs) were defined as seizure activity lasting more than 30 seconds and there was a statistically significant increase in the four weeks of these post infection with a p value of <0.05 in 2 patients- 50 % of our series, see table and figures. There was an overall modest increase in the total number of daily episode counts after initial infection with SARS-CoV-2 within 4 weeks post exposure, although this was not a statistically significant finding.
Table 1. TABLE 1: Note that N=number of days with datapoints/RNS upload data for analysis
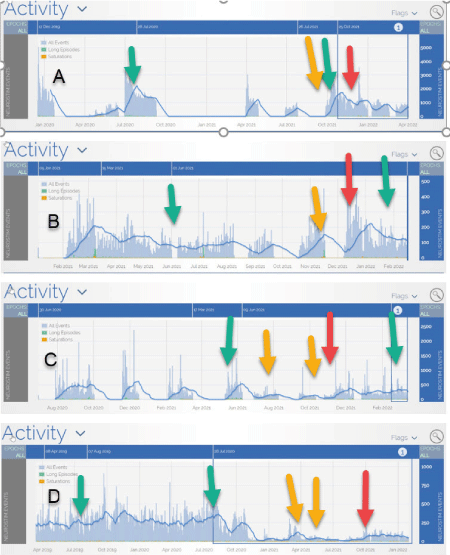
Figure 1: Histograms showing daily inventory of all day-to-day seizure activities (interictal and ictal activities). Covid-19 date of acute infection (when patient reported positive SARS-CoV-2 testing) is indicated by RED arrows, 2 most recent medication changes are indicated with YELLOW arrows, and the 2 most recent changes in RNS therapy are denoted by the Green Arrows

Figure 2: Histograms of Long episodes (green bars) and saturations of channels (Yellow bars) showing Covid-19 date of acute infection (RED arrows), 2 most recent medication changes (YELLOW arrows), 2 most recent changes in RNS therapy (Green Arrows), and in one patient the purple arrow coincides with initiation of vaping Nicotine and CBD containing products
Discussion
There is a literature regarding SARS-CoV-2 during the Covid-19 pandemic that delineates the association with seizures [7-13]. Both sporadic and more fulminant seizures that may progress to status epilepticus have been described along with severe and acute neurologic manifestations resulting from a SARS-CoV-2 associated neuroinflammatory syndrome and articles exist that contain descriptions of the notable EEG findings during the Covid-19 pandemic [7-13]. The biologically mediated mechanisms of the SARS-CoV-2 virus on seizure activity in epilepsy patients remains unknown after approximately 2 years of the pandemic. Confounding factors such as stress, social and or socioeconomic issues, or circumstantial factors such as missed medication or lack of sustained supply of medications and so forth may be responsible for the occurrence of seizure activity in this population in a literature referencing other world events and calamities, but those articles are limited by the subjective self-reporting of seizures [14-17]. Responsive neurostimulation (RNS) devices which provide ongoing electrocorticogram monitoring present an opportunity for objectively studying seizure activity in epilepsy patients in various circumstances- including a recent article noting the association of seizure activity with RNS devices in conjunction with verifying such patient reports [2-4]. In this study, we retrospectively analyze RNS data of epilepsy patients with prior documented SARS-CoV-2 infection comparing activity on the electrocorticograms 4 weeks before with activity 4 weeks post infection in patients who had no such reports whatsoever of any seizures and these were cases noted at office visits to have retrospectively appreciated increases in tallies on histogram dataset counts from the electrocorticograms that seemed unexplained otherwise at the time of the observations which led to inquiry about SARS-CoV-2 infection. Viral subtypes of the SARS-CoV-2 were not identified. To our knowledge, this is the first report to provide this unique clinically surprising perspective of noting such increased in activity on the electrocorticogram review at ambulatory office visits and subsequently enquiring about SARS-CoV-2 infection that led to this observation. Several case reports denote that there may be an association between SARS-CoV-2 and non-convulsive status epilepticus but such did not occur in our ambulatory patient population with RNS and the current literature suggests that some patients exhibited electrographic seizures during SARS-CoV-2 infection while undergoing continuous inpatient video EEG monitoring- but these patients seem distinct from our group as these previously reported cases exhibited underlying significant encephalopathy and abnormal neuroimaging and were managed in real time within an acute inpatient intensive care setting and not in the non-acute ambulatory outpatient office setting as occurred here [8-13].
Despite our findings, it is not obvious what accounted for the electrographic seizure activity exactly whether it was due to the SARS-CoV-2 virus itself or other confounding socioeconomic factors related to the pandemic or other factors such as sleep disturbances, depression, or the effects of a febrile or viral illness might have on increasing seizure activity already mentioned above [14-19]. As an attestation of the bias of subjective reporting that potentially limits these reports, our study also offers the unique perspective regarding such ambulatory and intractable epilepsy patients who have previously implanted RNS devices that can reliably document seizures in patients who also do not accurately report seizures if at all. Additionally, these 4 patients remained ambulatory and required no specific care, had no anti-seizure medication adjustments other than as noted, had no immediate device programming changes in relationship to the results we report, nor did they require hospitalization or any form of inpatient management post infection with SARS-CoV-2.
Conclusions
In summary, our study indicated a subclinical increase in seizure activity following infection with SARS-CoV-2 with a statistically significant increase in Long Episodes (LEs) associated with a p<0.05 within four weeks post infection in 50% of our small sample as outlined. RNS technology therefore provides a unique opportunity to quantitatively study seizure activity in patients with SARS-CoV-2 infection. In future studies, matching of subjects based on age, gender, duration of RNS therapy, location or type of seizures, and the severity of infection or subtypes of SARS-CoV-2 infections along with other clinical factors may be considered which might allow a more definitive and scientifically valid prospective study design for full delineation of the effects of SARS-CoV-2 on seizure activity utilizing the objective data quantification RNS devices offer.
Conflict of Interests
All the authors declare that there are no conflicts of interests to be disclosed.
References
- https://www.who.int [Accessed 30 May 2022].
- Leguia MG, Andrzejak RG, Rummel C, Fan JM, Mirro EA, et al. (2021) Seizure Cycles in Focal Epilepsy. JAMA Neurol 78: 454-463. [Crossref]
- Oster JM, Tatum P, Monigan C, Kryzanski J (2022) Seizures Noted by Responsive Neurostimulation From e-Cigarette Use (Vaping). J Clin Neurophysiol 39: 1-3. [Crossref]
- Karakas C, Ward R, Hegazy M, Skrehot H, Haneef Z (2022) Seizure control during the COVID-19 pandemic: Correlating Responsive Neurostimulation System data with patient reports. Clin Neurophysiol 139: 106-113. [Crossref]
- Sjoberg DD, Whiting K, Curry M, Lavery JA, Larmarange J (2021) Reproducible Summary Tables with the gtsummary Package. The R Journal 13: 570-580.
- Quraishi IH, Mercier MR, Skarpaas TL, Hirsch LJ (2020) Early detection rate changes from a brain-responsive neurostimulation system predict efficacy of newly added antiseizure drugs. Epilepsia 61: 138-148. [Crossref]
- Mao L, Jin H, Wang M, Hu Y, Chen S, et al. (2020) Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol 77: 683-690. [Crossref]
- Galanopoulou AS, Ferastraoaru V, Correa DJ, Cherian K, Duberstein S, et al. (2020) EEG findings in acutely ill patients investigated for SARS-CoV-2/COVID-19: A small case series preliminary report. Epilepsia Open 5: 314-324. [Crossref]
- LaRovere KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, et al. (2021) Neurologic Involvement in Children and Adolescents Hospitalized in the United States for COVID-19 or Multisystem Inflammatory Syndrome. JAMA Neurol 78: 536-547. [Crossref]
- Hwang ST, Ballout AA, Mirza U, Sonti AN, Husain A, et al. (2020) Acute Seizures Occurring in Association With SARS-CoV-2. Front Neurol 11: 576329. [Crossref]
- El Aidaoui K, Ait Benhamou R, Hazim A, Haoudar A, El Kettani C (2021) COVID-19: A Potential Cause of Non-convulsive Status Epilepticus. Cureus 13: e15041. [Crossref]
- Parihar J, Tripathi M, Dhamija RK (2020) Seizures and Epilepsy in Times of Corona Virus Disease 2019 Pandemic. J Epilepsy Res 10: 3-7. [Crossref]
- Asadi-Pooya AA (2020) Seizures associated with coronavirus infections. Seizure 79: 49-52. [Crossref]
- Albert DVF, Das RR, Acharya JN, Lee JW, Pollard JR, et al. (2020) The Impact of COVID-19 on Epilepsy Care: A Survey of the American Epilepsy Society Membership. Epilepsy Curr 20: 316-324. [Crossref]
- Rosengard JL, Donato J, Ferastraoaru V, Zhao D, Molinero I, et al. (2021) Seizure control, stress, and access to care during the COVID-19 pandemic in New York City: The patient perspective. Epilepsia 62: 41-50. [Crossref]
- Hao X, Zhou D, Li Z, Zeng G, Hao N, et al. (2020) Severe psychological distress among patients with epilepsy during the COVID-19 outbreak in southwest China. Epilepsia 61: 1166-1173. [Crossref]
- Huang S, Wu C, Jia Y, Li G, Zhu Z, et al. (2020) COVID-19 outbreak: The impact of stress on seizures in patients with epilepsy. Epilepsia 61: 1884-1893. [Crossref]
- Malow BA (2004) Sleep deprivation and epilepsy. Epilepsy Curr 4: 193-195. [Crossref]
- Rosman NP, Colton T, Labazzo J, Gilbert PL, Gardella NB, et al. (1993) A controlled trial of diazepam administered during febrile illnesses to prevent recurrence of febrile seizures. N Engl J Med 329: 79-84. [Crossref]

