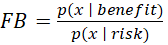Introduction: Maintenance is a therapeutic strategy that improves PFS and OS in advanced NSCLC. Racotumomab-alum is an anti-idiotypic vaccine that induces immunological response against N-glycolilated gangliosides, expressed in tumor cells. Nimotuzumab is a humanized anti-EGFR monoclonal antibody that has shown efficacy in cancer patients. The aim of this study was to evaluate safety and efficacy of racotumomab-alum or nimotuzumab versus docetaxel as switch maintenance therapy for advanced NSCLC.
Methods: Phase III, multicentre, open label, randomized trial was designed to enrol 743 stage IIIB-IV NSCLC patients, after first line therapy. Primary endpoint was OS. Patients were randomized (2:2:1) to receive racotumomab-alum, nimotuzumab or docetaxel. Racotumomab-alum treatment were five bi-weekly intradermal doses and re-immunizations every 4 weeks. Nimotuzumab arm received six weekly infusions, and bi-weekly maintenance doses. Docetaxel was used at 75 mg/m2 for 6 cycles if no evidence of progressive disease confirmed after 3 cycles. As switch maintenance therapy, both experimental drugs were classified as non-inferior to docetaxel, if 1- year OS rate was 36% using a 15% NI margin. Here we report the final analysis in non-progressor patients (n=238).
Results: 93 patients in each experimental arm and 52 in docetaxel arm with at least 1 year follow up were analyzed (ITT). The median OS and 1-year survival rate were 9.8 months (CI 90%: 8.8; 13.7) and 43.5 % with racotumomab, 11.2 months (CI 90%: 8.6 ;14.1) and 47.8 % with nimotuzumab and 8.6 months (CI: 5.9;11.3) and 31.0 % with docetaxel, respectively. Progression-free survival was similar in all groups: 4,4 months (CI 90% 4.1;5.6) in racotumomab, 4.6 months (CI 90%: 4.3; 6.5) with nimotuzumab and 4.0 months (CI 90%: 3.5; 5.9) with racotumomab.
Conclusions: Racotumomab-alum and Nimotuzumab were non-inferior to docetaxel as switch maintenance therapy. Both experimental treatments were safely administered at primary level of health assistance.
Maintenance strategies in advanced NSCLC are treatment options that prolong progression-free survival and overall survival. The main objective of this approach is to control the disease after the response achieved by first-line therapy, to keep the patient with good performance status and decrease symptoms. The election of a maintenance strategy depends on tumor histology, residual toxicity after first-line, response to platinum-based chemotherapy, performance status and patient choice [1,2].
Racotumomab-alum is therapeutic cancer vaccine composed by a murine monoclonal antibody and alum hydroxide as adjuvant. This vaccine can induce a specific antibody response against NeuGcGM3, in different type of cancer patients [3-8]. In NSCLC patients particularly, serum antibodies from vaccinated patients recognized and killed NeuGcGM3 expressing cell lines [9]. The expression of NglicolilGM3 has been reported in over 90 % of NSCLC [10,11]. The efficacy of racotumomab-alum versus placebo as switch maintenance therapy was reported in a phase II/III randomized controlled trial, with a significant benefit in terms of median OS and PFS for advanced NSCLC patients [12].
Nimotuzumab is humanized monoclonal antibody that recognizes the human epidermal growth factor receptor, blocking the binding of its ligands and inducing inhibition of cell proliferation, pro-apoptotic signals, ADCC, CDC, and decreasing VEGF production [13]. Clinical efficacy of antibody has been evaluated in brain tumours, head and neck, pancreatic and oesophageal cancer patients with favourable results [14-20]. In NSCLC patients nimotuzumab has been studied in Phase I clinical trials combined with first-line therapy, showing a favourable safety profile [21-23].
Docetaxel is a recommended switch maintenance therapy in the squamous NSCLC subtype. We selected this drug as a comparator in our trial using the reference from Fidias trial in which the median overall survival of the group treated immediately with docetaxel was 12.3 months compared with 9.7 months in the control group [24]. Our choice of non-inferiority design was based on the expectation that the median overall survival of experimental groups would be like docetaxel group and with a fewer number of adverse events. As switch maintenance therapy racotumomab-alum or nimotuzumab would be non-inferior to docetaxel if 1- year OS rate was 36% versus 51% using a 15% non- inferiority margin. Confirmation of non-inferiority in our study involved the pre-specification of a hazard ratio T/C below 1.5. The value of non-inferiority limit was stablished using the fixed value approach and is a fraction of a magnitude of the proven effect of the difference between docetaxel and placebo.
Patient eligibility
Patients with cytological or histological confirmation of Stage IIIB-IV NSCLC (7th edition TNM classification), with objective response or stable disease after first-line chemotherapy were evaluated to enter the trial. The inclusion criteria were: ECOG performance status 0 to 2, age over 18 years old, written informed consent, life expectancy of 6 months or more, adequate bone marrow, renal, and hepatic function, a time from end of first-line therapy to randomization up to 2 months. Patients with brain metastasis, acute infectious diseases, chronic or inflammatory uncontrolled diseases, severe allergic reactions history, pregnant or breastfeeding were excluded. Unfit patients for chemotherapy or previously treated with investigational drugs were also ineligible.
Study design
A Phase III, open label, randomized controlled trial with parallel groups was conducted in 24 hospital and 57 policlinic areas across 14 provinces in Cuba. Eligible patients were randomized 2:2:1 to receive racotumomab-alum, nimotuzumab or docetaxel. The randomization was performed using dynamic allocation of minimization system and balanced according to age, gender, ECOG performance status, response to prior therapy, disease stage, histology, and previous treatment. Patients allocated in racotumomab-alum group received five bi-weekly intradermal immunizations (1mg) and re-immunizations every 4 weeks. Nimotuzumab arm received 200 mg as a 1-hour intravenous infusion weekly for 6 weeks, followed by bi-weekly maintenance doses. Both drugs were administered until severe worsening of PS, unacceptable toxicity or patient requested discontinuation. Treatment was not discontinued at disease progression, even when other therapy line was administered concomitantly. Docetaxel was used at 75 mg/m2 of body surface for six cycles if no progressive disease was documented after 3rd cycle.
Patients were evaluated with complete medical history, physical examination, laboratory tests, radiologic imaging, and Quality of life assessments at baseline. These evaluations were performed every 3 months during the study period.
The primary objective of our study was to demonstrate the non-inferiority in terms of overall survival of racotomomab-alum or nimotuzumab versus docetaxel in an intent-to–treat analysis. As secondary objectives, we compared safety, quality of life, objective response rate and progression-free survival between treatment groups.
Statistical considerations
Sample size was estimated using East version 4.0 software considering a 10% alpha error and 77% of power. Based on results expected for Docetaxel treatment (51% of 1-year OS rate) and a non-inferiority margin of 15%, the trial hypothesized an expected 1-year OS around 36% for racotumomab-alum and nimotuzumab arms. Under 1:2:2 randomization and adding a 10% of dropout rate, a sample size of 233 subjects was estimated, 47 for Docetaxel and 93 for each racotumomab-alum and nimotuzumab, respectively. A one-sided confidence interval approach was used.
Kaplan-Meier estimates were used to assess the median time-to-event parameters: overall survival and progression-free survival. Results for each treatment arm were compared respect Docetaxel treatment, using the log-rank test. For the primary endpoint, non-inferiority of racotumomab-alum or nimotuzumab to docetaxel would be confirmed if the upper limit of CI of median overall survival HR was lower than 1.5. All tests of hypotheses were conducted using α= 0.1 level, with a 90% CI.
Overall survival was defined as the time from randomization until death from any cause and is measured in the intent-to-treat population. Objective response was evaluated using Response Evaluation Criteria in Solid Tumours version 1.1 [25] at baseline and every 3 months. Progression-free survival was calculated from randomization date to documented progressive disease or death. Quality of life was evaluated using QLQ-LC 30 and QLQ-LC 13 EORTC questionnaires (version 3). Adverse events were classified using the National Cancer Institute Common Terminology Criteria, version 3.
The global characterization adverse events (by patient) among groups were assessed by the chi-squared test.
As a measure for Benefit-Risk ratio, a Factor Bayes was estimated for each treatment arm. Given p(x | benefit) by the probability distribution function for benefit (Overall Survival rate at 1 year) and p(x | risk) by the probability distribution function for risk (any Grade 3-4 treatment-related adverse event) then the
Bayes Factor (FB) is: 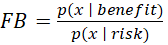
representing a summary of the evidence provided by the data in favor of benefit (red), as opposed to risk (blue). A value larger than 1 means a favorable benefit-risk ratio.
Patients
Between February 11, 2013, and January 21, 2016, 656 patients were screened for enter the trial. Two hundred and thirty-two advanced NSCLC patients were randomized, 93 to racotumomab-alum group, 93 to nimotuzumab group and 52 to docetaxel group. All of them were included in the intent-to-treat analysis. Eighteen patients (7.6%) did not receive any dose of assigned treatment, mainly due to worsening in PS, death, or patient request. At the data cut-off date December 2nd, 2016, 192 patients (80.7%) of 238 had died. Main cause of treatment discontinuation in all study groups was worsening of performance status (Figure 1).
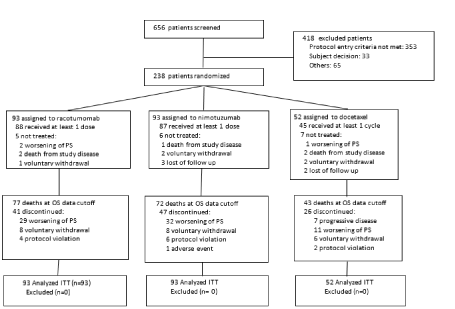
Figure 1: CONSORT diagram.
Baseline characteristics and demography are shown in Table 1. The three study arms were well balance for demographic and disease characteristics. Two patients in docetaxel group did not receive first-line chemotherapy. Regarding response to first-line therapy, 12 patients were included by mistake in these setting of non-progressing patients. All of them were included in the ITT analysis.
Table 1. Patient demography and baseline characteristics.
ITT population (n=238) |
Docetaxel
(n=52) |
Nimotuzumab
(n=93) |
Racotumomab
(n=93) |
Age |
≤ 60 years |
19 (36.5) |
35 (37.6) |
31 (33.3) |
> 60 years |
33 (63.5) |
58 (62.4) |
62 (66.7) |
Mean ± SD |
62.13 ± 8.06 |
62.61 ± 9.16 |
63.98 ± 9.11 |
Median ± IR |
62.00 ± 11 |
64.00 ± 15 |
65.00 ± 12 |
Gender |
Male |
37 (71.2) |
55 (59.1) |
51 (54.8) |
Female |
15 (28.8) |
38 (40.9) |
42 (45.2) |
Ethnic origin |
|
|
|
White |
32 (61.5) |
62 (66.7) |
66 (71.0) |
Afro-caribbean |
12 (23.1) |
13 (14.0) |
9 (9.7) |
Other |
8 (15.4) |
18 (19.4) |
18 (19.4) |
Disease stage |
IIIB |
14 (28.6) |
38 (41.8) |
32 (35.2) |
IV |
35 (71.4) |
53 (58.2) |
59 (64.8) |
NA |
1(1.9) |
2(2.2) |
2(2.2) |
ECOG PS |
0 |
26 (53.1) |
43 (47.8) |
45 (49.5) |
1 |
22 (44.9) |
45 (50.0) |
45 (49.5) |
2 |
1 (2.0) |
2 (2.2) |
1 (1.1) |
NA |
3 (5.8) |
3 (3.2) |
3(3.2) |
Smoking status |
Current smoker |
17 (33.3) |
24 (26.1) |
36 (39.6) |
Former smoker |
28 (54.9) |
58 (63.0) |
46 (50.5) |
Never smoker |
6 (11.8) |
10 (10.9) |
9 (9.9) |
NA |
1(2.0) |
1(1.1) |
2(2.2) |
Histology |
Squamous cell carcinoma |
19 (36.5) |
27 (29.3) |
28 (30.1) |
Adenocarcinoma |
18 (34.6) |
33 (35.9) |
33 (35.5) |
Large cell carcinoma |
10 (19.2) |
19 (20.7) |
15 (16.1) |
NSCLC NOS |
2 (3.8) |
11 (12.0) |
16 (17.2) |
Other |
3 (5.8) |
2 (2.2) |
1 (1.1) |
NA |
0(0) |
1(1.1) |
0(0) |
First-line treatment |
Chemotherapy |
50 (96.2) |
93 (100.0) |
93 (100,0) |
Radiotherapy |
16 (30.8) |
35 (37.6) |
33 (35.5) |
Response to first-line |
Complete response |
3 (5.8) |
6 (6.5) |
6 (6.5) |
Partial response |
16 (30.8) |
34 (36.6) |
34 (36.6) |
Stable disease |
30 (57.7) |
48 (51.6) |
47 (50.5) |
Progression disease |
2 (3.8) |
5 (5.4) |
5 (5.4) |
NA |
1 (1.9) |
0 (0.0) |
1 (1.1) |
Abbreviation: NOS, not otherwise specified. NA, not available
The median number of doses received in racotumomab arm was 11 with a range of one to 31. In Nimotuzumab group the median was 16 with a range of one to 68. In docetaxel arm the median number was 4 with a range of one to six (data not shown).
There was a small number of patients in experimental arms that received a second–line therapy at documented progressive disease: 15 patients in nimotuzumab group (16.1%) and 13 patients in racotumomab arm (14%). There were no patients treated with second-line therapy in docetaxel arm. The most common regimen used was carboplatino/paclitaxel since there was no availability of registered drugs as pemetrexed, erlotinib, or checkpoint inhibitors in our country (data not shown).
Efficacy
The ITT median overall survival time in the group of patients treated with racotumomab was 9.8 months (CI 90%: 8.8; 13.7) compared to 8,6 months (CI 90%: 5.9;11.3) in those included in docetaxel arm. The one-year OS rate was 43.5 % for racotumomab and 31 % for docetaxel group. In the group treated with nimotuzumab the median OS was 11.2 months (CI 90%: 8.6 ;14.1) and the one-year OS rate was 47.8 %. Both experimental treatments were non-inferior to docetaxel in the ITT analysis for the OS endpoint. The non-inferiority condition was confirmed because the upper limit of the CI of the hazard ratio of nimotuzumab arm [90% CI NI HRT/C (0; 1.06)] and racotumomab arm [90% CI NI HRT/C (0; 1.08)] was under de non-inferiority margin (HR T/C =1.5) (Figure 2).
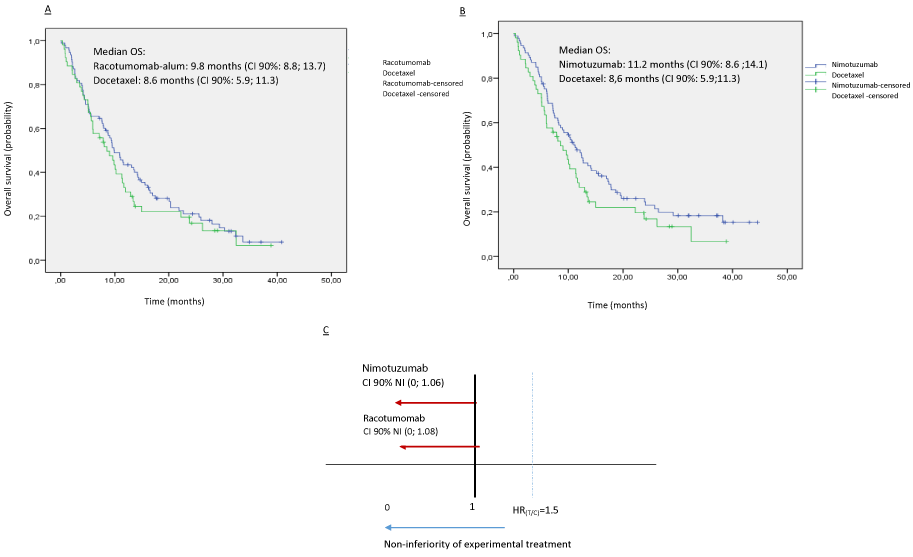
Figure 2: Kaplan-Meier curves of Overall survival on ITT population. A: Racotumomab versus docetaxel. B: Nimotuzumab versus Docetaxel. C: Confidence intervals of hazard ratios and non-inferiority margin.
Progression-free survival analysis is shown in Figure 3. Median PFS in racotumomab-alum arm was 4,4 months (CI 90% 4.1;5.6), compared with 4.0 months (CI 90%: 3.5; 5.9) in docetaxel arm. Patients treated in nimotuzumab group obtained a median PFS of 4.6 months (CI 90%: 4.3; 6.5). There were no differences between experimental groups versus docetaxel regarding this endpoint. Log-rank test P values were: 0.578 for racotumomab and 0.203 for nimotuzumab comparison versus docetaxel, respectively.
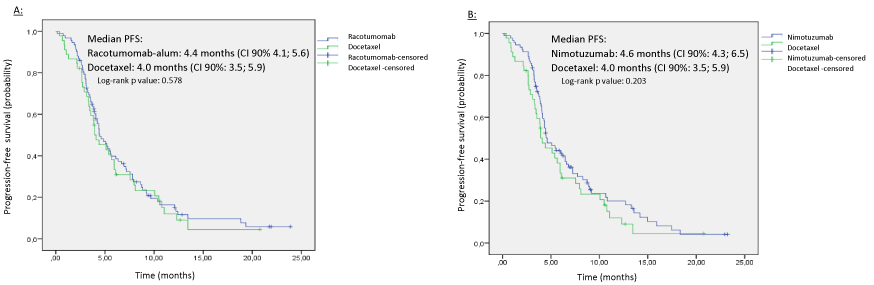
Figure 3: Kaplan-Meier curves of Progression-free survival on ITT population. A: Racotumomab versus docetaxel. B: Nimotuzumab versus Docetaxel.
There were no differences observed in terms of objective response between treatment arms in the evaluated population. Disease control rate at month 3 was: 50 % in nimotuzumab arm, 55.8% in racotumomab arm, and 56.4% in docetaxel arm. Similar behaviour was observed in the following evaluations. Analysing best overall response across the study, similar proportions of patients achieved a disease control rate between treatment arms (data not shown).
Quality of life assessment had similar results in the three arms across the study. A reduced number of patients had available information of these endpoint at baseline and one moment after: 30 patients in docetaxel arm, 55 in nimotuzumab arm and 56 in racotumomab group. In transversal analysis at month 3 was detected a significant difference in the mean values of global health status, full function, cognitive function, and symptoms like pain, dysphonia, chest pain and constipation, in nimotuzumab treated patients (data not shown).
Safety
A summary of safety information is shown in Table 2. Sixty-one percentage of patients in docetaxel group, 78.5 % in nimotuzumab group and 81.7 % in racotumomab group have presented at least one adverse event, regardless causality with study treatment. Grade 3-4 treatment-related adverse events were registered in 11.5 % of docetaxel group, 1.1 % of nimotuzumab group and 1.1 % of racotumomab group, detecting a statistically significative relationship between Docetaxel treatment respect to Nimotuzumab and Racotumomab, respectively (p=0.008). SAE related to the study treatment were reported in 4.4 of docetaxel arm and 1.15 % of nimotuzumab arm. In racotumomab arm there was no report of treatment-related SAE.
Table 2: Adverse Event Summary.
|
Docetaxel
(n=52) |
Nimotuzumab
(n=93) |
Racotumomab
(n=93) |
Patients with any adverse event |
32 (61,5) |
73 (78,5) |
76 (81,7) |
Patients with any treatment-related adverse event |
23 (44,2) |
57 (61,3) |
54 (58,1) |
Patients with any Grade 3-4 adverse event |
11 (21,2) |
21 (22,6) |
16 (17,2) |
Patients with any Grade 3-4 treatment-related adverse event |
6 (11,5) |
1 (1,1) |
1 (1,1) |
Patients with any serious adverse event |
6 (11,5) |
24 (25,8) |
18 (19,4) |
Patients with any serious treatment-related adverse event |
2 (4,4) |
1 (1,15) |
0 (0) |
Adverse Event
|
183 (100) |
1166 (100) |
806 (100) |
Treatment-related adverse event |
122 (66,7) |
402 (34,5) |
343 (42,6) |
Grade 3-4 adverse event |
12 (6,5) |
44 (3,8) |
14 (1,7) |
Grade 3-4 treatment-related adverse event |
8 (4,4) |
3 (0,26) |
1 (0,12) |
Serious adverse event |
10 (5,5) |
33 (2,8) |
20 (2,5) |
Treatment-related serious adverse event |
2 (1,1) |
1 (0,1) |
0 (0) |
Most common racotumomab-related adverse events were: injection-site reaction (29. 8%), myalgia (19.0 %), fever (5.3 %) and arthralgia (5.0 %). In patients treated with nimotuzumab most frequent adverse events were: myalgia (21.9 %), fever (6.0 %), headache (5.7 %) and nausea (5.2 %). In docetaxel arm most frequent registered adverse events were: nausea (14.8 %), diarrhoea (14.8 %), anaemia (9.8 %) and asthenia (5.7 %). Most of the treatment-related adverse events were classified as mild or moderate (Table 3). Only 3 SAE were related to the study treatment: one patient treated with nimotuzumab with hypertension, and in docetaxel group were reported one pain in limbs and one anaemia grade 3, classified as treatment-related SAE.
Table 3: Most common treatment-related adverse events.
|
Racotumomab-alum |
Nimotuzumab |
Docetaxel |
Adverse event
|
All grades |
Grade 3-4 |
All grades |
Grade 3-4 |
All grades |
Grade 3-4 |
Arthralgia |
17(5.0) |
0(0) |
17(4.2) |
0(0) |
1(0.8) |
0(0) |
Fever |
18(5.3) |
0(0) |
24(6.0) |
0(0) |
4(3.3) |
0(0) |
Myalgia |
65(19.0) |
0(0) |
88(21.9) |
3(0.7) |
3(2.5) |
0(0) |
Injection-site reaction |
102(29.8) |
0(0) |
0(0) |
0(0) |
0(0) |
0(0) |
Malaise |
10(2.9) |
0(0) |
9(2.2) |
0(0) |
3(2.5) |
0(0) |
Mareos |
11(3.2) |
0(0) |
4(1.0) |
0(0) |
0(0) |
0(0) |
Nausea |
5(1.5) |
0(0) |
21(5.2) |
0(0) |
18(14.8) |
0(0) |
Vomiting |
7(2.0) |
0(0) |
11(2.7) |
0(0) |
4(3.3) |
1(0.8) |
Headache |
1(0.3) |
0(0) |
23(5.7) |
0(0) |
0(0) |
0(0) |
Weakness in limbs |
0(o) |
0(0) |
14(3.5) |
0(0) |
0(0) |
0(0) |
Cramps in limbs |
3(0.9) |
0(0) |
11(2.7) |
0(0) |
1(0.8) |
0(0) |
Asthenia |
7(2.0) |
0(0) |
4(1.0) |
0(0) |
7(5.7) |
0(0) |
Anorexia |
9(2.6) |
0(0) |
8(2.0) |
0(0) |
5(4.1) |
0(0) |
Anemia |
2(0.6) |
0(0) |
8(2.0) |
0(0) |
12(9.8) |
1(0.8) |
Diarrhea |
0(0) |
0(0) |
1(0.25) |
0(0) |
18(14.8) |
1(0.8) |
NOTE: Data are number of adverse events (%)
The benefit/risk ratio between treatment groups was assessed (see Figure 4, Supplemental Data 1). The odds for benefit were larger than for risk (Bayes Factor) for the three treatments groups, indicating a favorable benefit-risk balance. However, for Docetaxel treatment, FB=2.6 (<3) suggesting weak evidence for differentiating between benefit and risk. In Nimotuzumab and Racotumomab groups FB is greater than 15, allow us to think in stronger evidence in the favour of benefit. Redefining risk as any serious adverse event, FB=7.75 for Docetaxel arm, that is, moderate evidence in favour of benefit. In the other arms, FB is greater than 15 too, maintaining the idea of strong benefit-risk balance.
In our randomized controlled trial advanced NSCLC patients treated as switch maintenance with nimotuzumab or racotumomab-alum achieved a median overall survival like docetaxel group. The non-inferiority hypothesis proposed in the protocol was confirmed. However, the median survival in our trial is slightly inferior to the OS obtained in the reference trial reported by Fidias et al, and in other maintenance study with pemetrexed (m OS: 13.4 months) or erlotinib (mOS: 12.0 months). In the study reported by Perol, et al of pemetrexed versus placebo a 51 % of pemetrexed arm and 67 % of placebo arm received anticancer systemic therapy after discontinued study drug [26]. In the erlotinib maintenance trial published by Capuzzo, et al 71 % in erlotinib arm and 72 % in placebo arm received any post-study drug at progression [27]. In our trial only 16.1 % and 14 % of patients in experimental arms received second-line therapy with no preferred drugs for this setting. It could be the main reason for our inferior result.
Progression-free survival was similar between treatment arms: 4.4 months in racotumomab arm, 4.6 months in nimotuzumab arm and 4.0 months in docetaxel arm. This median PFS is consistent with the reported by Ciuleanu, et al [28] in maintence trial of pemetrexed vs best supportive care (mPFS 4.3 m vs 2.6 m), and with 12.3 weeks of median PFS in erlotinib maintenance arm reported by Capuzzo. As we expected similar efficacy in our experimental arms, a difference in mPFS would not be a rational result.
In terms of quality of life our results showed no difference between treatment arms. This result is consistent with the Fidias trial, and with many other unable to demonstrate a difference in quality of life. Our main objective in this trial was to demonstrate a non-inferior efficacy of racotumomab and nimotuzumab, but we couldn’t obtain an achievement in quality of life of included patients. The loss of follow up in some cases, the early death in others, and the missing information in patients with a good treatment adherence could affect the analysis of this endpoint in our study.
In terms of safety data, we confirmed the favorable toxicity profile of racotumomab and nimotuzumab in advanced NSCLC patients. Most treatment-related adverse events in experimental arms were mild or moderate. There was no death related to racotumomab or nimotuzumab. We expected to observe a fewer incidence of treatment related adverse event, but we couldn’t confirm that. In our opinion in docetaxel arm there was a low detection and registration rate of treatment-related adverse event. The frequency of administration of patients included in docetaxel arm was minor that the experimental groups, so, the probability to detect adverse event in 6 administrations was fewer than in 11 doses and 16 that was the median number of doses in racotumomab and nimotuzumab arm, respectively.
In conclusion, our study confirms the efficacy of racotumomab and nimotuzumab as switch maintenance therapy for advanced NSCLC in a non-inferiority approach comparison with docetaxel. Both drugs were well tolerated and would be administered in long-term use as a maintenance treatment option, with the aim to increase the overall survival of treated patients.
RANIDO Trial Research Group: Liudmila Herrera, Martha Gómez, Humberto Gómez, Odette Martínez, Ania E. Fernández, Vivian Matos, Conrado E. Fernández, Marileidi C. Valdivia, Katerin Gómez, Cintia M. Rodriguez, Maribel Alonso, Maria C. García, Menelio N. Noriega, Maritza Artiles, Irma García, Gustavo Pichs, Zenaida Carbonell, gala N. Romero, Nieves O. Gonzalez, Gisela Tamayo, Deward Torrecilla, Odelaine Guzmán, Guillermo O. Guirola, Zulema Andrade, Maritza Monzón, Yaima García, Niurba Hernandez, Jorge Jiménez,Yunia Hernandez, Maria N. Florido, José Soler, Noralis Lara, Idalia Hernández, Eva Salomón, Madelaine Serra, Ivon García, Joaquín Hernández, Carlos Perez, Lucia V. Núñez, Rachidys B. Reyes, Miosotis Yero, Rolando Valdes, Aivin Cruz, Zurama Griffit, Yaima Piloña, Yanet Menéndez, Aymara Calderin, Pedro Pablo Guerra, Evelyn Galano, Alicia Vargas, Alina Perez, Elena Rodríguez, Daimy Bajo, Clara Ballagas, Maritza Ramos, Rolando Uranga, Yaima Muñoz, Mayra Ramos, Zaima Mazorra, Zuyeng Gonzalez, Yoisbel Moreno, Daymys Estevez, Lazara García , Aliuska Frias, Mabel Alvarez, Yanela Santiesteban, Yuliannis Santiesteban, Meylan Cepeda, Geidy Lorenzo, Milagros Domeneq, Yaimarelis Saumel, Leticia Cabrera, Ana R. Vals, Ania Gorte, Jose J. Hernández, Martha Lugiollo, Delmis Batista.
View Supplementary Data
- National Comprehensive Cancer Network (2019) Clinical Practice Guidelines V4, 2019, Non-Small Cell Lung cancer.
- Planchard D, Popat S, Kerr K, Novello S, Smit EF, et al. (2018) Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29(Suppl 4): iv192–iv237. [Crossref]
- Neninger E, Diaz RM, de la Torre A, Rives R, Díaz A, et al. (2007) Active immunotherapy with 1E10 anti-Idiotype vaccine in patients with small cell lung cancer. Report of a Phase I Trial. Cancer Biol Ther 6: 145-150. [Crossref]
- Guthmann MD, Castro MA, Cinat G, Venier C, Koliren L, et al. (2006) Cellular and humoral immune response to N-Glycolyl-GM3 elicited by prolonged immunotherapy with an anti-idiotypic vaccine in high-risk and metastatic breast cancer patients. J Immunother 29: 215–223. [Crossref]
- Alfonso M, Diaz A, Hernandez AM, Pérez A, Rodríguez E, et al. (2002) An Anti-Idiotype Vaccine Elicits a Specific Response to N-Glycolyl Sialic Acid Residues of Glycoconjugates in Melanoma Patients. J Immunol 168: 2523–2529. [Crossref]
- Cacciavillano W, Sampor C, Venier C, Gabri MR, de Dávila MTG, et al. (2015) A Phase I Study of the Anti-Idiotype Vaccine Racotumomab in Neuroblastoma and Other Pediatric Refractory Malignancies. Pediatr Blood Cancer 62: 2120-2124. [Crossref]
- Alfonso S, Diaz RM, de la Torre A, Santiesteban E, Aguirre F, et al. (2007) 1E10 Anti-Idiotype Vaccine in Non-Small Cell Lung Cancer. Experience in Stage IIIb/IV Patients. Cancer Biol Ther 6: 1847-1852. [Crossref]
- Hernández AM, Toledo D, Martinez D, Griñán T, Brito V, et al. (2008) Characterization of the antibody response against NeuGcGM3 ganglioside elicited in non-small cell lung cancer patients immunized with an anti-idiotype antibody. J Immunol 181: 6625-6634. [Crossref]
- Hernández AM, Rodríguez N, González JE, Reyes E, Rondón T, et al. (2011) Anti-NeuGcGM3 antibodies, actively elicited by idiotypic vaccination in non-small cell lung cancer patients, induce tumor cell death by an oncosis-like mechanism. J Immunol 186: 3735-3744. [Crossref]
- Blanco R, Rengifo CE, Cedeno M, Frómeta M, Rengifo E, et al. (2012) Immunoreactivity of the 14F7Mab (Raised against N-Glycolyl GM3 Ganglioside) as a Positive Prognostic Factor in Non-Small-Cell Lung Cancer. Pathol Res Int 235418.
- Hayashi N, Chiba H, Kuronuma K, Go S, Hasegawa Y, et al. (2013) Detection of N-Glycolyated gangliosides in non-small cell lung cancer using GMR8 monoclonal antibody. Cancer Sci 104: 43–47. [Crossref]
- Alfonso S, Valdés A, Santiesteban E, Flores YI, Areces F, et al. (2014) A randomized, multicenter, placebo-controlled clinical trial of racotumomab-alum vaccine as switch maintenance therapy in advanced non-small-cell-lung cancer patients. Clin Cancer Res 20: 3660-3671. [Crossref]
- Crombet-Ramos T, Rak J, Pérez R, Viloria-Petit A (2002) Antiproliferative, antiangiogenic and proapoptotic activity of h-R3: A humanized anti-EGFR antibody. Int J Cancer 101: 567-575. [Crossref]
- Massimino M, Biassoni V, Miceli R, Schiavello E, Warmuth-Metz M, et al. (2014) Results of nimotuzumab and vinorelbine, radiation and re-irradiation for diffuse pontine glioma in childhood. J Neurooncol 118: 305-312. [Crossref]
- Cabanas R, Saurez G, Alert J, Reyes A, Valdes J, et al. (2014) Prolonged use of nimotuzumab in children with central nervous system tumors: safety and feasibility. Cancer Biother Radiopharm 29: 173-178. [Crossref]
- Wang Y, Pan L, Sheng X, Chen S, Dai J (2014) Nimotuzumab, a humanized monoclonal antibody specific for the EGFR, in combination with temozolomide and radiation therapy for newly diagnosed glioblastoma multiforme: First results in Chinese patients. Asia Pac J Clin Oncol 12: e23-e29. [Crossref]
- Crombet T, Osorio M, Cruz T, Roca C, Castillo R, et al. (2004) Use of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients. J Clin Oncol 22: 1646-1654. [Crossref]
- Ramos TC, Figueredo J, Catala M, González S, Selva JC, et al. (2006) Treatment of High-Grade Glioma Patients with the Humanized Anti-Epidermal Growth Factor Receptor (EGFR) Antibody h-R3. Report from a Phase I/II Trial. Cancer Biol Ther 5: 375-379. [Crossref]
- Schulteis B, Reuter D, Ebert MP, Siveke J, Kerkhoff A, et al. (2017) Gemcitabine combined with the monoclonal antibody Nimotuzumab is an active first-line regimen in KRAS wildtype patients with locally advanced or metastatic pancreatic cancer: a multicenter, randomized, phase IIb study. Ann Oncol 28: 2429-2435. [Crossref]
- de Castro Junior G, Segalla JG, de Azevedo SJ, Andrade CJ, Grabarz D, et al. (2018) A randomised phase II study of chemoradiotherapy with or without nimotuzumab in locally advanced oesophageal cancer: NICE trial. Eur J Cancer 88: 21-30. [Crossref]
- Bebb G, Smith C, Rorke S, Boland W, Nicacio L, Sukhoo R, et al. (2010) Phase I clinical trial of the anti-EGFR monoclonal antibody nimotuzumab with concurrent external thoracic radiotherapy in Canadian patients diagnosed with stage IIb, III or IV non-small cell lung cancer unsuitable for radical therapy. Cancer Chemother Pharmacol 67: 837-45. [Crossref]
- Choi HJ, Sohn JH, Lee CG, Shim HS, Lee I, et al. (2011) A phase I study of nimotuzumab in combination with radiotherapy in stagesIIB-IV non-small cell lung cancer unsuitable for radical therapy: Korean results. Lung Cancer 71: 55-59. [Crossref]
- Govind Babu K, Prabhash K, Vaid AK, Sirohi B, Diwakar RB, et al. (2014) Nimotuzumab plus chemotherapy versus chemotherapy alone in advanced non-small-cell lung cancer: a multicenter, randomized, open-label Phase II study. Onco Targets Ther 7: 1051–1060. [Crossref]
- Fidias PM, Dakhil SR, Lyss AP, Loesch DM, Waterhouse DM, et al. (2009) Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small cell lung cancer. J Clin Oncol 27: 591-598. [Crossref]
- Eisenhauera EA, Therasseb P, Bogaerts J, Schwartz LH, Sargent D, et al. (2009) New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45: 228-247. [Crossref]
- Perol M, Chouaid C, Perol D, Barlési F, Gervais R, et al. (2012) Randomized, Phase III Study of Gemcitabine or Erlotinib Maintenance Therapy Versus Observation, with Predefined Second-Line Treatment, After Cisplatin-Gemcitabine Induction Chemotherapy in Advanced Non–Small-Cell Lung Cancer. J Clin Oncol 30: 3516-3524. [Crossref]
- Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczésna A, et al. (2010) Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 11: 521–529. [Crossref]
- Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, et al. (2009) Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 374: 1432–1440. [Crossref]
Editorial Information
Editor-in-Chief
Kleanthis Giannoulis
Thessaloniki School of Medicine, Greece
Article Type
Research article
Publication history
Received: September 07, 2021
Accepted: September 23, 2021
Published: September 26, 2021
Copyright
©2021 Hernandez M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Hernandez M, Neninger E, Ortiz RA, Santiesteban E, Camacho K, et al. (2021) Safety and Efficacy of Racotumomab-alum or Nimotuzumab versus Docetaxel as switch maintenance therapy for advanced non-small cell lung cancer patients: a Phase III open label randomized non-inferiority trial. Glob Surg 7: DOI: 10.15761/GOS.1000230.