Objectives: CIMAvax-EGF is a therapeutic vaccine registered as switch maintenance therapy. This vaccine induces antibodies against self EGF that affect EGF-EGFR interaction. The aim of this study was to evaluate safety and efficacy of CIMAvax-EGF in the context of primary care.
Methods: A phase IV clinical trial was conducted in 65 Policlinic areas and 16 hospitals in Cuba during 3 years. A total of 1081 advanced NSCLC patients were included without other treatment options due to progressive disease or comorbidities. CIMAvax-EGF was administered by intramuscular injection in four sites of administration (4 subdoses of 0.25 ml), every 2 weeks the first 4 doses and after this induction phase monthly reinmunizations were given.
Results: A total of 927 patients (85.7 %) received at least one dose of CIMAvaxEGF. Most frequently adverse events related to vaccine were: injection-site reaction (14.5%), fever (7.0%), headache (5.8%), tremors (4.3%), and nausea (4.3%). Most of them were grade1 -2 according CTCAE v3. There were no deaths related to CIMAvaxEGF. The median overall survival (mOS) time for all vaccinated patients was 7.0 months, and in a subgroup of patient who received at least 4 doses of CIMAvaxEGF mOS was 9.98 months (n=715). Patients treated as switch maintenance therapy (n=97) reached a mOS of 12.1 months. In a subgroup of unfit patients (n=213) mOS was 3.97 months, but in those who completed the induction phase mOS was 7.36 months (n=124). Emotional function was improved at months 6 and 12 compared to baseline.
Conclusions: CIMAvaxEGF vaccine is a safe treatment option for advanced NSCLC that can be safely administered at primary level of health care. The median OS of treated patients (unselected population) compares with the results reported for second–line treatments and switch maintenance therapies. Patients who completed the induction phase of the treatment reached a better OS.
Lung cancer is the leading cause of cancer related-death worldwide. NSCLC represents about 85 % of all cases of lung cancer [1]. Despite of introduction of novel drugs, advanced NSCLC patients still have a poor outcome. Immunotherapy approaches in the last years have increased median overall survival mainly in selected populations. These efficacy data came from randomized controlled clinical trials most of the time, but real-world results sometimes are different. In unselected populations the presence of patients with poor PS, comorbidities, elderly patients, no systemic therapy, is more common than in clinical trial populations.
CIMAvax-EGF is a therapeutic cancer vaccine approved as switch maintenance therapy in Cuba, Peru, Paraguay, Colombia and Bosnia. In a cuban phase III trial was reported a clinical benefit in patients with high EGF serum concentration treated with the vaccine [2]. Also, this vaccine is under clinical evaluation for advanced NSCLC patients in Europe, United States and China. Herein we report our results from generalization of CIMAvax-EGF treatment in an unselected cohort of 1081 NSCLC patients treated at primary level of health care assistance. The objective of this study was to evaluate safety and efficacy of CIMAvax-EGF treatment in real-world conditions.
Study design
This was a phase IV prospective, multicenter study in advanced NSCLC patients without other treatment option due to progressive disease or comorbidities. A group of patients (n=97) was included to receive the vaccine as switch maintenance after first-line therapy, due to the nature of the study. Eligible patients were assigned to receive CIMAvax-EGF by intramuscular way with a total of 2.4 mg per doses, divided in 4 sites of injection. The induction phase included 4 doses every 2 weeks, and every 4 weeks during the maintenance phase. Criteria for stopping vaccination included voluntary withdrawal, unacceptable toxicity or worsening of the patient’s performance status. Patients who discontinued study treatment were followed until death, or study termination. The primary objective of the study was to describe the safety profile of CIMAvax-EGF administered at primary level of health care. Secondary objectives were to evaluate the overall survival (OS) and quality of life (QL) of the patients treated with CIAMvax-EGF.
The trial was approved by the local ethics review boards and notified to the cuban State Center for the Quality Control of Medicines and Medical Devices. All patients provided written informed consent before study participation. The trial was conducted in accordance to the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. It is registered at the Cuban Registry of Clinical Trials (www.registroclinico.sld.cu; ID: RPCEC00000181).
Patient's selection
Eligible patients were 18 years or older, with histo- or cytologically confirmed advanced NSCLC (6th edition AJCC staging system), without specific treatment option (ChT, RT) due to comorbidities or progressive disease. All patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 3, and adequate renal, hepatic and haematological functions.
Patients who had received immunotherapy for NSCLC or other investigational drug, patients with known hypersensitivity to any component of the formulation, patients that were pregnant or in lactation, patients with autoimmune, uncontrolled chronic diseases or history of inflammatory demyelinating diseases of the peripheral or CNS, acute allergic conditions or history of severe allergic reactions, patients with brain metastases or other primary neoplastic lesion were excluded from the study. Furthermore, patients with uncontrolled intercurrent illness including active infection, symptomatic congestive heart failure, unstable angina, cardiac arrhythmia and psychiatric disorders, patients with a malignant disease in the previous 5 years except skin cancer (not melanoma), patients receiving systemic corticosteroids at the time of inclusion and patients with positive serology for Hepatitis B, C or HIV were also excluded from this study.
Study assessments
The primary endpoint was safety. Adverse events were registered after every vaccine administration by medical examination and clinical laboratory tests performed to the patients during treatment and follow up, until patient’s death or lost to follow up. All these events were graded according to Common Terminology Criteria for Adverse Events (NCI-CTCAE version 3.0). Overall survival was the secondary endpoint, defined as the time from randomization until the date of death from any cause. Patients who were still alive at the clinical cutoff date were censored at the date at which they were last confirmed to be alive. Quality of life was measured applying EORTC QLQ-30 and QLQ-13 questionnaires at the beginning of the study and every 3 months.
Statistical analysis
Descriptive methods were used for patient’s characteristics, treatment exposure and safety analysis. For the analysis of overall survival, median values and 95% CI were estimated by Kaplan-Meir methodology. For quality-of-life analysis values of scores of the scales was described and compared to baseline using the paired rank nonparametric test with Wilcoxon signs. Statistical analysis was performed with SPSS program (version 21.0).
Patient characteristics
Between july 13, 2009 and january 10, 2013, 1081 NSCLC patients were included in this phase IV, multicenter clinical trial. Demographic and baseline characteristics are shown in Table 1. The mean age of population in study was 65.5, and there was a prevalence of male sex (65.1%), smokers (56.9%), and ECOG PS 1 (40.2%). The most frequent histological subtype was adenocarcinoma (37.4%) and most common clinical stage were IIIB (44.4%) and IV (44.2%).A small number of patients with early stage was included with recurrent disease (7.9%). In 6 patients there was not available clinical stage. The EGFR mutation status was unknown in all included patients (1041). Data from first-line treatment was available in 1035 patients, 79.7 % of them received chemotherapy and 45.6% radiotherapy.
Table 1. Patient demography and baseline characteristics SD standard deviation, ECOG Eastern Cooperative Oncology Group, PS performance status, EGFR epidermal growth factor receptor, NSCLC nos no otherwise specified non-small cell lung cancer, NA not available.
ITT Population (n= 1041) |
Age (years) |
Mean (Range) |
65.5 (28-94) |
Gender |
Female |
363 (34.9%) |
Male |
677 (65.1%) |
NA |
1 (0.1%) |
ECOG PS |
0 |
272 (26.1%) |
1 |
419 (40.2%) |
2 |
230 (22.1%) |
3 |
119 (11.4%) |
NA |
1 (0.1%) |
Smoking status |
Ex-smoker |
589 (56.9%) |
Smoker |
331 (31.8%) |
Non-smoker |
115 (11.0%) |
NA |
6 (0.6%) |
Histological subtype |
Adenocarcinoma |
389 (37.4%) |
Squamous cell carcinoma |
328 (31.5%) |
Large cell carcinoma |
197 (18.9%) |
NSCLC, nos |
121 (11.6%) |
NA |
6 (0.6%) |
Clinical Stage |
IIIA |
31 (2.9%) |
IIIB |
462 (44.4%) |
IV |
460 (44.2%) |
Other (recurrent) |
82 (7.9%) |
NA |
6 (0.6%) |
EGFR mutation status |
Unknow |
1041 (100%) |
First-line oncoespecific therapy (n=1035) |
Chemotherapy |
825 (79.7%) |
Radiotherapy |
472 (45.6%) |
Safety
Safety evaluable population was composed by 932 patients (89.5 %) who received at least one dose of CIMAvax-EGF. Ten percentages of the included patients didn’t receive any dose due to several reasons (worsening of PS, lost to follow up, death). Seventy hundred and nine patients (68.1 %) completed the induction phase of the treatment (four doses or more) and 195 (18.7%) were immunized for one year or more (more than 15 doses).
Safety information is summarized in Table 2. A total of 7884 adverse events were recorded during the study, regardless causality. Only 4.1% of them were classified as grade 3-4 and 3.7 as serious AE. Around 60 % of all AE registered were related to the vaccine administration, and 6 of them was classified as serious AE. The frequency of treatment-related adverse events is shown in Table 3. The most common treatment-related adverse events (all grades) were injection-site pain (23.9%), fever (10.32%), headache (8.55 %), tremor (10.3%) and nausea (6.1%). The majority of all described treatment-related adverse events were classified as mild or moderate. The serious AE related to CIMAvax-EGF were bronchospasm, cerebral ischemia, arrhythmia, dyspnea, sweating and arterial hypotension. These SAE were present in 3 patients and causes treatment discontinuation. There was no death related to CIMAvax-EGF administration.
Table 2. Adverse events summary.
Safety population (n=923) |
|
|
Adverse event |
Number of EA |
Percentage |
Any AE |
7884 |
100 |
AE grade 3-4 |
322 |
4.08 |
SAE |
282 |
3.65 |
AE grade 5 |
72 |
0.91 |
AE related to vaccine |
4746 |
60.2 |
AE grade 3-4 related to vaccine |
54 |
0.68 |
SAE related to vaccine |
6 |
0.076 |
AE grade 5 related to vaccine |
0 |
0 |
Table 3. Most common vaccine-related adverse events.
Adverse event |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Total |
N |
% |
N |
% |
N |
% |
N |
% |
N |
% |
Injection-site pain |
872 |
22.66 |
263 |
31.16 |
2 |
4.17 |
0 |
0.00 |
1137 |
23.96 |
Fever |
392 |
10.20 |
97 |
11.50 |
1 |
2.10 |
0 |
0.00 |
490 |
10.32 |
Headache |
349 |
9.10 |
52 |
6.20 |
5 |
10.40 |
0 |
0.00 |
406 |
8.55 |
Tremor |
277 |
7.20 |
53 |
6.30 |
5 |
10.40 |
0 |
0.00 |
335 |
7.06 |
Nausea |
270 |
7.00 |
17 |
2.00 |
1 |
2.10 |
0 |
0.00 |
288 |
6.07 |
Chills |
215 |
5.60 |
33 |
3.90 |
0 |
0.00 |
0 |
0.00 |
248 |
5.23 |
Vomiting |
183 |
4.80 |
21 |
2.50 |
0 |
0.00 |
0 |
0.00 |
204 |
4.30 |
Malaise |
159 |
4.10 |
35 |
4.10 |
0 |
0.00 |
0 |
0.00 |
194 |
4.09 |
Arthralgia |
98 |
2.50 |
32 |
3.80 |
2 |
4.20 |
0 |
0.00 |
132 |
2.78 |
Dizziness |
113 |
2.90 |
13 |
1.50 |
1 |
2.10 |
0 |
0.00 |
127 |
2.68 |
Dyspnea |
73 |
1.90 |
29 |
3.40 |
7 |
14.60 |
1 |
0.00 |
110 |
2.32 |
Hypotension |
86 |
2.20 |
19 |
2.30 |
4 |
8.30 |
1 |
0.00 |
110 |
2.32 |
Dry mouth |
80 |
2.10 |
8 |
0.90 |
1 |
2.10 |
0 |
0.00 |
89 |
1.88 |
Injection-site induration |
64 |
1.70 |
18 |
2.10 |
0 |
0.00 |
0 |
0.00 |
82 |
1.73 |
Myalgia |
62 |
1.60 |
13 |
1.50 |
0 |
0.00 |
0 |
0.00 |
75 |
1.58 |
Fatigue |
60 |
1.60 |
7 |
0.80 |
0 |
0.00 |
0 |
0.00 |
67 |
1.41 |
Asthenia |
43 |
1.10 |
8 |
0.90 |
0 |
0.00 |
0 |
0.00 |
51 |
1.07 |
Overall survival
One thousand and sixteen patients were included in OS analysis. Due to the nature of the study, 65 patients were excluded because missing information or lost to follow up. Median overall survival in all included patients (n= 1016) was 6.0 months (95% CI: 5.4; 6.5) (Figure 1A). In those patients who received at least one dose of CIMAvax-EGF (safety evaluable population) the median OS was 7.0 months (95% CI: 6.2; 7.8) (Figure 1B). The 1- and 2- year OS rates were 34.7 % and 17.9%, respectively. In those patients who completed the induction phase of the treatment (n=709) the mOS was 9.98 months (95% CI: 8.8;10.98) and 44.1 % and 23.3% of the patients were alive at 1 and 2 years, respectively (Supplementary Figure 1). The subset of patients treated as switch maintenance with CIMAvax-EGF (n=91) achieved a median OS of 12.1 months (95% CI: 9.1; 15.1) and OS rates at 1 and 2 years were 50.0 % and 24.9%, respectively (Figure 2). In patients unfit for chemotherapy (n=213) median OS was 3.97 months irrespective of the treatment adherence, but in those who completed the induction phase of the treatment mOS was 7.36 months (n=124) (Supplementary Figure 2).
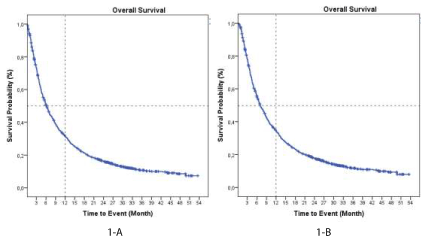
Figure 1. Kaplan Meier plot of overall survival .1-A, all included patients (n=1016), 1-B Safety evaluable population (n=927).
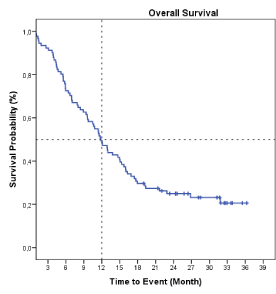
Figure 2. Kaplan Meier plot of overall survival in non-progressor patients (n=91).
Quality of life
Quality of life data of 234 patients with at least one evaluation after baseline was analyzed. Mean of absolute values of scores at baseline and month 6 and 12 was compared. Comparison of functional scales is show in Figure 3. The mean values are higher at month 6 and 12 compared to baseline, but there is only a statistical difference between them in emotional function scores compared to baseline (p=0.001 baseline vs M6; p=0.024 baseline vs M 12). In terms of symptoms there was a significant improvement in fatigue score between baseline and month 6 (p=0.013) (Supplementary Figure 3), as well as most of the symptoms evaluated in QLQ-LC13 questionnaire at month 6 (Supplementary Figure 4).
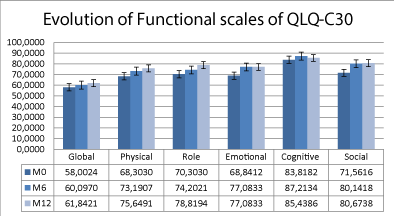
Figure 3. Evolution of functional scores of quality-of-life questionnaire QLQ-C30.
Results from this study confirm the safety profile of CIMAvax-EGF in the context of primary care assistance. The frequency of AE described here is consistent with previous clinical studies with the vaccine at secondary level of health care. Most part of vaccine-related adverse events was mild or moderate. A very low percentage of patients in these setting of population presented a serious AE related to CIMAvax-EGF and there was no death related.
In terms of efficacy the median overall survival in vaccinated patients compares with reported data from second-line drug studies. The median overall survival in our study (6.0 m) is similar to docetaxel (7.5 m), erlotinib (6.7 m) and pemetrexed (8.3 m), in unselected population [3-5]. Also, it is inferior to other results in this scenario with checkpoint inhibitors (nivolumab: 12.2 m non-squamous NSCLC, 9.2 m squamous NSCLC; atezolizumab: 12.6 m; pembrolizumab: 14.9 m) [6-9]. The lack of other lines of therapy in our population and the presence of 213 patients unfit for chemotherapy could be the reasons for this minor median OS.
The group of patients treated as switch maintenance therapy reached a median OS of 12.1 months (ITT population). In this scenario the efficacy of CIMAvax-EGF compares with other drugs registered: docetaxel (12.3 months), pemetrexed (13.4 m) and erlotinib (12.0 m) [10-12]. This result is also consistent with median overall survival reported in the phase III trial of CIMAvax-EGF (10.83 m).
As in previous trial with CIMAvax-EGF vaccine those patients who completed the induction phase of the treatment obtained a benefit in terms of overall survival. It has been observed in our study in different settings: second-line, switch maintenance and unfit patients. It should be noted the existence of a tail in the OS curves of those populations that reflects the minor probability of patient’s death in that period.
Quality of life data was evaluable only in 25.1 % (n=234) of patients treated with CIMAvax-EGF. There was a significant difference between baseline and post-treatment evaluations at month 6 and 12 in emotional function and fatigue symptoms. Also, most of the QLQ-LC 13 symptoms were significant different at month 6 vs baseline. The type of the study and the small number of patients at each evaluation could affect the interpretation of these data.
In conclusion, CIMAvax-EGF is an effective and safe treatment option for advanced NSCLC patients treated at primary level of health care. Also, this vaccine can be administered for a long-term period without cumulative toxicity due to its favorable safety profile. The completion of the induction phase is a critical point for developing a protective response ensuring a clinical stabilization of the disease.
The trial was funded by Center of Molecular Immunology and Cuban Ministry of Public Health.
We thank all the participating patients and their families, staffs of all the primary care units and hospitals involved, and National Coordinating Center for Clinical Trials as the CRO of the study.
- 1. Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, et al. (2014) SEER Cancer Statistics Review, 1975- 2014, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017.
- 2. Rodriguez PC, Popa X, Martinez O (2016) A Phase III Clinical Trial of the Epidermal Growth Factor Vaccine CIMAvaxEGF as Switch Maintenance Therapy in Advanced Non-Small Cell Lung Cancer Patients. Clin Cancer Res 22: 3782-3790. [Crossref]
- 3. Shepherd FA, Dancey J, Ramlau R, Gralla R, O'Rourke M, et al. (2000) Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18: 2095-03. [Crossref]
- 4. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, et al. (2005) Erlotinib in Previously Treated Non–Small Cell Lung Cancer. N Eng J Med 353: 123-132. [Crossref]
- 5. Hanna N, Shepherd FA, Fossella FV, Pereira JR, Marinis FD, et al. (2004) Randomized Phase III Trial of Pemetrexed versus Docetaxel in Patients with Non–Small-Cell Lung Cancer Previously Treated with Chemotherapy. J Clin Oncol 22: 1589-97. [Crossref]
- 6. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, et al. (2015) Nivolumab versus Docetaxel in Advanced Non-Squamous Non-Small Cell Lung Cancer. N Eng J Med 373: 1627-1639. [Crossref]
- 7. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, et al. (2015) Nivolumab versus Docetaxel in Advanced Squamous Cell Non-Small Cell Lung Cancer. N Eng J Med 373: 123-135. [Crossref]
- 8. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, et al. (2016) Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387: 1837-1846. [Crossref]
- 9. Herbst RS, Baas P, Kim D, Felip E, Pérez-Gracia JL, et al. (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1- positive, advanced non-small-cell lung cancer (KEYNOTE-010): A Randomised controlled trial. Lancet 387: 1540-1550. [Crossref]
- 10. Fidias PM, Dakhil SR, Lyss AP, Loesch DM, Waterhouse DM, et al. (2008) Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 27: 591-598. [Crossref]
- 11. Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, et al. (2009) Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double blind, phase 3 study. Lancet 374: 1432-1440. [Crossref]
- 12. Capuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczésna A, et al. (2010) Erlotinib as maintenance treatment in advanced non small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 11: 521-529. [Crossref]
Editorial Information
Editor-in-Chief
Sam Cheol Kim
Chosun University
South Korea
Article type
Research Article
Publication History
Received: Sep 05, 2021
Accepted: Oct 01, 2021
Published: Oct 06, 2021
Copyright
©2021 Hernandez M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Hernandez M, Ortiz RA, Salomon E, Acosta S, Santiesteban E, et al. (2021) Safety and efficacy of CIMAvax-EGF vaccine for the treatment of real-world non-small cell lung cancer patients. Int Clin Med 5: DOI: 10.15761/ICM.1000195.



