Cell survival and cell death should be well regulated in an orchestrated way for cell proliferation, differentiation, and development in multicellular organism. A variety of physiological outcomes in vertebrate is dependent on cell death modality in response to infectious pathogens and functionally damaged cells. Physiological and pathological roles of cell death are often addressed to describe normal and disease state. Particularly, immune responses can be affected from cell death modes of host cells and various immune-associated cells. Immunogenic cell death occurs in either an accidental or a regulated way could elicit the immune responses.
Representatively, apoptosis and necroptosis are involved in homeostasis of immune reaction. So far, apoptosis in immune system has been well defined, acting as deletion of self-recognizing immune cells and cytotoxic killing. However, it has been a growing body of evidence that alternative programmed cell death necroptosis could be involved in immune surveillance, although its underlying mechanism remains elusive. Therefore, necroptosis or programmed necrosis will be emphasized as a regulator of immunity. With immunological role of necroptosis, unmasking of necroptosis and its associated signaling pathway are also dealt in this review.
necroptosis, innate immunity, adaptive immunity, programmed cell death
Programmed cell death, apoptosis, occurs in an orchestrated fashion when cells are subjected to death stimuli. A series of caspases are required to drive apoptosis irrespective of context that intrinsic or extrinsic factor can trigger it. Under the condition that caspase is defective, various death inducer can actively convert apoptosis to necroptosis, showing atypical physiological outcomes via a different molecular route from apoptosis. Unlike apoptosis, rupture of cell membrane in necroptotic cells can release intracellular materials, which can give rise to immune responses. Originally, biological process of necroptosis was found in caspase8-compromised T cells and programmed cell death including apoptosis plays a key role in homeostasis of immune cells activated with specific antigens. Later, necroptosis-associated proteins and a signaling pathway were uncovered to define necroptosis a specialized programmed cell death. Primarily, this review will emphasize the underlying mechanism by which necroptosis can activate immune system and its physiological significances.
With growing knowledge on necroptosis, recently, it has been documented that induction of necroptosis plays a critical role in innate and adaptive immunity [1]. In general, cells undergoing apoptosis can be engulfed by macrophage or neighboring cells to be remove. Immunologically, apoptotic cells were completely degraded into debris via phagocytosis that intracellular molecules are not released to invoke immune reaction.
Necroptosis results in innate immunity by actively inducing cell death of infected cells or by provoking the immune system via the release of danger signals to clear pathogens. However, unmasking of necroptosis out of control may cause pathogenesis of diverse inflammatory diseases. For instance, induction of necroptosis by viral infection contributes to be beneficial or harmful to host.
Apoptosis vs. necroptosis
Programmed cell death is largely divided into apoptosis and necroptosis, which are clearly different in criteria of morphological and biochemical features. Originally, necroptosis is considered alternative cell death to apoptosis, but currently is recognized as a specialized form of programmed cell death. Specifically, necroptosis is executed by a cascade of cell signaling proteins in an orchestrated way in response to death stimuli such as TNFα and chemicals under a caspase-8 compromised condition. When it comes to immunology, cell death modes determine the physiological outcomes of immune responses in multicellular organism.
Signaling pathway for necroptosis and chemical inhibition of necroptosis-related proteins (Figure 1)
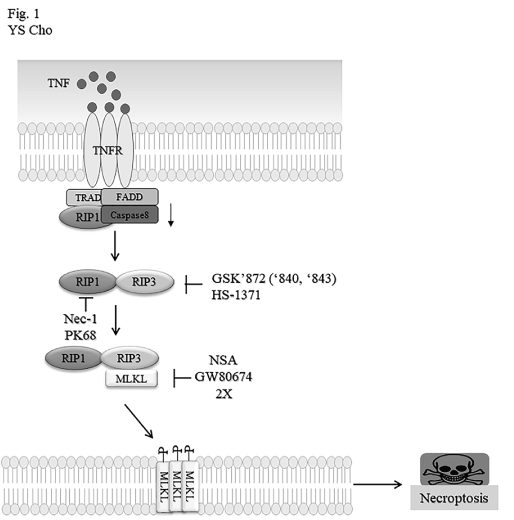
Figure 1. A signaling pathway leading to the necroptotic cell death and chemical regulation of necroptosis-related proteins. The signaling pathway for necroptosis is triggered upon ligation of TNFα family such as TNFα, FasL/CD95L and TRAIL (TNFα-related apoptosis-inducing ligand) to its cognate receptors. After internalization of TNFR signal complex 1, it is converted to the cytoplasmic complex II consisting of RIP1, RIP3, FADD, pro-caspase-8. Then, activated RIP3 recruits MLKL to induce the necrotic process. Based on necroptotic signaling pathway, some chemicals have been identified as pharmacological inhibitors of the necroptotic proteins. RIP1, RIP3 or MLKL can be specifically modulated by chemical inhibitors indicated.
Signaling molecules of necroptotic machinery for PRRs considerably shares downstream of death receptors (DRs) including RIP3 and mixed lineage kinase domain-like pseudokinase (MLKL) [2,3]. As for adaptor proteins, RIP1 is essential downstream proteins for DR-mediated signaling pathway, while TRIF is prerequisite for TLR3/4-mediated signaling activation. Structurally, both RIP1 and TRIF contain a RHIM domain. In addition, Z-DNA sensor DAI bears two RHIM-like domains. Individually different signaling routes can converge to RIP3 activation through RHIM-RHIM interaction. Active RIP3 can recruit kinase-like domain of MLKL, leading to phosphorylation of MLKL in the activation loop. MLKL either acts as a scaffolding protein for recruitment of Na+ or Ca2+ channels or promotes the pore formation in plasma membrane. As a result, resulting phosphorylated MLKL leads to disintegration of plasma membrane and finally membrane rupture.
Necrostatin-1 (Nec-1) was firstly discovered to be a specific inhibitor of RIP1 kinase, and then later, PK68 has been optimized to be a selective RIP1 kinase with an EC50 of about 14-22 nM in human and mouse cells, not only protecting TNF-α-induced systemic inflammatory responses [4], but also suppressing metastasis of both melanoma cells and lung carcinoma cells in mice [5]. Another RIP family member RIP3 was also targeted by small molecules GSK’872, GSK’840 and GSK’843 [6]. These compounds are bound to RIP3 kinase domain with high affinity to inhibit kinase activity. HS-1371 was also identified as a novel specific RIPK3 inhibitor, which can bind to ATP-binding pocket of RIPK3 and inhibits kinase activity [7]. As described above, MLKL plays an executor of necroptosis through forming its oligomerization and membrane translocation to induce membrane-disrupting pores. Some inhibitors targeting MLKL have been developed. Necrosulfonamide (NSA) was for the first time discovered as a MLKL inhibitor, which covalently binds to Cys86 of human MLKL to block MLKL oligomerization [8]. Later, GW806742X was found to bind to the KLD of MLKL, finally abrogating plasma membrane translocation of MLKL [9].
Physiological and pathophysiological significances of cell death
Dysfunctional or infected host cells should be removed by apoptotic cell death with subsequent phagocytosis. Therefore, apoptosis is crucial in tissue homeostasis, development and various disease. Immunologically, it essential for the negative selection of lymphocytes reactive to self-antigens and a non-inflammatory process [10,11]. However, under caspase-compromised conditions that is subjected to tissue damage or infection, cells actively switch cell death from apoptosis to necroptosis. Unlike apoptosis, cells undergoing necroptosis are swollen to release intracellular debris to neighboring media and some endogenous molecules not only elicit inflammatory responses, but also recruit immune cells. In fact, viruses that express a caspase inhibitor can evade primary host defense mechanism apoptosis but could fail to disseminate by activating necroptosis of host, alternative programmed cell death. Therefore, necroptosis is of significance as alternative cell death of apoptosis to be masked in normal physiological condition. However, unregulated activation of necroptosis may lead to the pathogenesis of a broad range of inflammatory diseases [12]. Many researches have documented that necroptosis is implicated in multiple tissues subjected to ischemia-reperfusion condition. More intriguingly, a growing body of evidences has proposed that neurodegenerative diseases like Parkinson’s disease can be caused from necroptosis [13]. In vitro experiment, application of Nec-1 protects dopaminergic neurons from treatment of PC12 cells with 6-OHDA. This seems to be associated with the notion that RIP1 expression is high in the Parkinson disease model.
Targeted control of necropotosis-associated proteins like RIP1, RIP3 and MLKL protein may take advantage of the necroptosis for boosting innate immunity or treating inflammatory diseases.
Induction of necroptosis by pathogens and nonpathogens
Apoptosis, default programmed cell death, has been generally considered essential in biological processes such as development, differentiation and immune homeostasis of multicellular organism. Infection of host cells with pathogens actively induces apoptosis not so as to reproduce its progeny. In fact, apoptosis plays a key role as the primary defense machinery to remove pathogen-infected host cells. Meanwhile, some infection can cause necroptosis but not apoptosis.
For example, in the pathogenesis of pneumonia, pore-forming toxins (PFT) kill the respiratory epithelial cells (REC) in a necroptosis mode with simultaneous caspase activation [14,15]. During this process, caspase activity enhances the release of alarmins to the extracellular media, which lead to interleukin-6 production. Streptococcus pneumoniae and Staphylococcus aureus can mediate cell death of macrophage through RIP1/RIP3/MLKL signaling axis. Its necroptosis contributes to failure in host defense machinery due to the loss of immune cells [16]. Additionally, intracellular bacterial pathogens can induce various necroptotic pathological conditions. Salmonella typhimurium can cause macrophage loss via type I IFN-dependent necroptosis [17]. Infection of macrophage with Mycobacterium tuberculosis results in activation of TNF signaling with recruitment of RIP1 and RIP3 to execute necroptosis [18]. Additionally, necroptosis can be initiated by activation of death receptors for viral nucleic acids, and viral proteins [19]. Various TLRs including TLR3, TLR7 and TLR9 can sense viral nucleic acids and transduce different signals via the adaptor MyD88 to finally execute necroptosis under a caspase-compromised condition [20]. However, various viruses have evolved to suppress its host’s programmed cell death like apoptosis and necroptosis for propagation. In fact, viruses have a variety of evasion mechanisms to premature cell death of host cells subjected to infection. Virus perpetuation is depending on the ability to suppress or bypass host’s default cell death, whereas apoptosis-defective host copes with induction of alternative cell death to apoptosis. In a sense, therefore, necroptosis plays a key role in protecting host from spreading of virions that can inhibit apoptosis through suppression of caspase (Table 1).
Though the evidence that virus mediates necroptosis via TLR remains clarified, antiviral defense machinery of host via TNF-mediated necroptosis has long been suggested by infection of vaccinia virus that can encode an inhibitor of apoptosis. Necroptosis can be unmasked under the condition that vaccinia virus (VV) infected host [21]. VV expresses the B13R protein that suppresses caspase 8 activity, and its infection renders host sensitive to TNF-induced necroptosis. Therefore, it is suggested that necroptosis is essential for the antiviral strategy to VV infection. Another protein MC159, viral FLICE-like inhibitory protein (vFLIP) that encoded in human poxvirus Molluscum contagiosum virus (MCV), inhibits not only necroptosis, but also caspase-8-induced apoptosis.
Viral nucleic acids can initiate cytosolic receptor-mediated necroptosis during DNA and RNA infection. DNA-dependent activator of IRFs (DAI), encoded by the Zbp1 gene acts as a sensor of vDNA to promote necroptosis in MEFs infected with mouse cytomegalovirus (MCMV) [22]. Its DAI-driven necroptosis requires RIP3, but not RIP1. Therefore, antiviral mechanism of DAI and RIP3 was proved with a virus expressing a mutant viral inhibitor of RIP activation (vIRA) protein.
HSV-1 and HSV-2 promote necroptosis in murine cells, and viral RHIM domain-containing proteins ICP6 and ICP10 are responsible for this cell death event. Homodimers of ICP bind to RIP1/RIP3 via RHIM;RHIM domain to initiate MLKL-dependent necroptosis [23]. Recently, H7N9 influenza A virus has been known to accelerate necroptosis in human monocytes when combined with the pan-caspase inhibitor, IDN6556 [24]. Like MCMV, moreover, influenza A virus activates both apoptotic and necroptotic cell death in MEFs and human lung epithelial cells through DAI/RIP3-dependent route. Particularly, DAI-dependent apoptosis differs from TNFR-mediated cell death in a sense that RIP1 expression, but not its kinase activity is required for cell death [19].
Viral infection, on the contrary, can interfere with the cell death-related signaling pathways of the host to potentiate virus propagation by encoding anti-apoptotic proteins which increase its ability to replicate inside the host cell. Upton et al. have shown that viral proteins of mouse cytomegalovirus (M45-encoded viral inhibitor of RIP activation) containing the RHIM domain interact with RIP1 and RIP3 to inhibit virus-induced cell death [21]. Specifically, viral inhibitor of RIPK activation (vRIA) disrupts the binding of RIPK3 with DNA-dependent activator of IRFs (DAI) which results in suppression of cytomegalovirus-mediated necroptosis. Similar to MCMV, human cytomegalovirus encodes immediate early gene 1(IE1), which acts as a blockade of necroptosis by interfering with signaling pathway downstream of MLKL [25]. Besides microbial infections, necroptotic cell death has been found in pathological conditions like ischemia-reperfusion injury and atherosclerosis [26]. It has been demonstrated that other sterile inflammation like alcoholic and drug-induced liver damage is associated with necroptosis. Under these contexts, use of necrostatin-1 or knockout of RIP3 significantly protects liver cells from induction of necroptosis [27,28]. Interestingly, type 2 diabetes mellitus (T2DM) promotes TNFR-mediated necroptosis of alveolar macrophage upon Mycobacterium tuberculosis [29].
Recognition of PAMP and DAMP
Inflammation can be provoked by pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) resulting from necroptotic cell death. Especially, PAMPS are detected by pathogen recognition receptors like TLRs, RLRs and NLRs. DAMP includes lipids, sugars and nucleic acids and can be also recognized by PAMP receptors. Both PAMP and DAMP augments the inflammatory response against pathogens, even though excessive dysregulated inflammation can lead to chronic inflammatory diseases or autoimmune diseases [30].
Role of necroptosis in innate immunity
Innate immunity can be provided by immune cells to detect the molecules derived from infectious agents including bacteria, viruses, yeast and fungi. Pathogens or endogenous molecules are recognized as foreign pathogens-associated molecular patterns (PAMPs) or danger associated molecular patterns (DAMPs) by pathogen recognition receptors like toll like receptors (TLRs) and Z-DNA sensor DAN [21,31]. Representatively, TLR3 and TLR4 binds double-stranded RNA and lipopolysaccharide (LPS), respectively [32]. Accordingly, once binding to those receptors, it plays a key role in cytokine production, dendritic cell maturation, induction of programmed cell death and presentation of antigen [33]. A series of signaling activation provokes proinflammatory cytokines via mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-kB pathway. The programed cell death apoptosis leads to removal of infected cells by macrophage. When caspase-8 is compromised, default apoptosis TLR4 transduce signals through TRIF, which subsequently interacts with RIP3 through RHIM motif of them [34,35] and then mediates necroptosis in bone-marrow derived macrophages (BMDMs) [34]. Activation of TLR3 or TLR4 signaling pathway produces proinflammatory cytokine TNFα, which can partly drive necroptosis. Plasma membrane of necroptotic cells is ruptured to release DAMPs like high mobility group box 1 protein (HMGB1) into the surrounding environment, which induce inflammation that can cause to recruit various immune cells to the inflamed sites [36]. In some way, initiation of necroptosis and subsequent activation of innate immunity could protect organisms from microbial infection as a first line of defense. Moreover, parasitic diseases such as leishmaniasis and malaria result in hemolysis, leading to anemia. The resulting release of hemoglobin (Hb) and its degradation product heme activate TLR4, leading to RIP1/3-driven necroptosis [37].
Escherichia coli expressing NleB1 protein inhibits apoptosis as well as necroptosis via modifying arginine residue in proteins containing death domains such as FADD and RIPK1 [38]. In contrast, NleB1-deficient E. coli can no longer colonize in the intestine, suggesting that bacteria are induced to cell death in the host.
In addition to this, RIP3 deficiency in combination with caspase-8 or FADD is vulnerable to Yersinia infection [39]. Therefore, caspase-8 and RIP3 are crucial players to regulate innate immune responses. Moreover, Mycobacterium tuberculosis induces RIP3-dependent necroptosis with accompanying TNFα expression, which provides host defense against tuberculosis. During tuberculosis infection, RIP1/RIP3 are involved in producing TNF-induced reactive oxygen species in infected macrophage (Table 2) [29,40].
Intriguingly, mice lacking RIP3 does not exhibit virus-induced necroptosis but are susceptible to viral infection, demonstrating that failure of necroptosis can be detrimental to the host under a specific condition like viral infection with compromised apoptosis [41].
Necroptosis in adaptive immune responses
T cell population is largely maintained by apoptosis via intrinsic or extrinsic pathway. Whereas T cells with defective FADD or caspase 8 can actively die in a necroptotic route upon ligation of T cell receptor with antigen.
In fact, it has been documented that necroptosis is involved in homeostasis of T cell in response to activation stimuli. Mice defective in caspase-8 fails to maintain T cell homeostasis, reducing T cell numbers [42]. Impaired proliferation of T cells upon TCR activation is demonstrated in T cells deficient in caspase-8 or FADD. In the absence of caspase-8 activity, failure in expansion of T cells is caused by the activation of necroptosis. This notion was strongly corroborated by the experiment that necrostatin-1 (Nec-1) reversed the expansion defect of T cells with loss of caspase-8 or FADD. Furthermore, genetic knockdown of RIP1 or deletion of RIP3 can rescue the defective T cell proliferation in caspasw-8 -/- mice [43,44]. Under physiological conditions, caspase 8 regulates the survival of activated T cells by masking necroptotic pathways.
HIV infection highly induces necroptosis of T-cells that bear low caspase-8 activity and potentiates the TNFα-mediated cell death [45]. Recently, it has been demonstrated that colonizing Pneumococci promotes adaptive immunity via induction of necroptosis [15]. In this article, asymptomatic colonization of Pneumoniae induces necroptosis of nasopharyngeal epithelial cells and enhances accompanying CD11c+ antigen presenting cells in submucosa.
Besides infectious agents, injection of necroptotic cancer cells for vaccination elicits efficient anti-tumor immunity [46] (Table 2). Membrane rupture of necroptotic cells release DAMPs (ATP and HMGB1), which promote maturation of dendritic cell, cross priming of cytotoxic T cells and production of IFN-γ in response to antigen. It is of note that immunogenicity of necroptosis is not related to NF-κB activation. Intramural administration of mRNA encoding necroptosis executioner MLKL blunt growth of human lymphoma by inducing antitumor immunity directed against neo-epitopes [47].
As described above, necroptosis exerts a beneficial effect on innate and adaptive immunity to microbial infection. However, tissue injury-derived necroptosis could lead to pathogenesis of various retinal diseases retinal diseases including age-related macular degeneration (AMD), diabetic retinopathy, and retinal detachment, all of which can result in irreversible blindness [48-50]. Moreover, RIPK/MLKL-mediated necroptosis plays a key role in destructive inflammatory responses to cause other various diseases like cancer, neuronal dysfunction and inflammatory diseases [51-54]. Induction of necroptosis could exert not only beneficial effects, but also harmful damage on cells, resulting in host’s immune defensive processes and pathological disorders, respectively. Accordingly, required is more extensively study on the signaling crosstalk between apoptosis and necroptosis and pharmacological regulation of necroptosis regulators for the treatment of necroptosis-associated diseases. Finally, this review will be helpful to comprehend how necroptosis could elicit immunological consequences depending on infectious contexts.
There is no conflict of interest in the publication of this article.
- Lu JV, Chen HC, Walsh CM (2014) Necroptotic signaling in adaptive and innate immunity. Semin Cell Dev Biol 35: 33-39. [Crossref]
- Su Z, Yang Z, Xie L, DeWitt JP, Chen Y (2016) Cancer therapy in the necroptosis era. Cell Death Differ 23: 748-756. [Crossref]
- Dhuriya YK, Sharma D (2018) Necroptosis: A regulated inflammatory mode of cell death. J Neuroinflammation 15: 199. [Crossref]
- Wang Y, Wang H, Tao Y, Zhang S, Wang J, et al. (2014) Necroptosis inhibitor necrostatin-1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience 266: 91-101.
- Hou J, Ju J, Zhang Z, Zhao C, Li Z, et al. (2019) Discovery of potent necroptosis inhibitors targeting ripk1 kinase activity for the treatment of inflammatory disorder and cancer metastasis. Cell Death Dis 10: 493. [Crossref]
- Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, et al. (2014) Rip3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell 56: 481-495. [Crossref]
- Park HH, Park SY, Mah S, Park JH, Hong SS, et al. (2018) Hs-1371, a novel kinase inhibitor of rip3-mediated necroptosis. Exp Mol Med 50: 125.
- Sun L, Wang H, Wang Z, He S, Chen S, et al. (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of rip3 kinase. Cell 148: 213-227. [Crossref]
- Yan B, Liu L, Huang S, Ren Y, Wang H, et al. (2017) Discovery of a new class of highly potent necroptosis inhibitors targeting the mixed lineage kinase domain-like protein. Chem Commun (Camb) 53: 3637-3640. [Crossref]
- Opferman JT (2008) Apoptosis in the development of the immune system. Cell Death Differ 15: 234-242. [Crossref]
- Nagata S (2018) Apoptosis and clearance of apoptotic cells. Annu Rev Immunol 36: 489-517. [Crossref]
- Pasparakis M, Vandenabeele P (2015) Necroptosis and its role in inflammation. Nature 517: 311-320. [Crossref]
- Wu JR, Wang J, Zhou SK, Yang L, Yin JL, et al. (2015) Necrostatin-1 protection of dopaminergic neurons. Neural Regen Res 10: 1120-1124. [Crossref]
- Gonzalez-Juarbe N, Bradley KM, Riegler AN, Reyes LF, Brissac T, et al. (2018) Bacterial pore-forming toxins promote the activation of caspases in parallel to necroptosis to enhance alarmin release and inflammation during pneumonia. Sci Rep 8: 5846. [Crossref]
- Riegler AN, Brissac T, Gonzalez-Juarbe N, Orihuela CJ (2019) Necroptotic cell death promotes adaptive immunity against colonizing pneumococci. Front Immunol 10: 615. [Crossref]
- Ahn D, Prince A (2017) Participation of necroptosis in the host response to acute bacterial pneumonia. J Innate Immun 9: 262-270. [Crossref]
- Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, et al. (2012) Type i interferon induces necroptosis in macrophages during infection with salmonella enterica serovar typhimurium. Nat Immunol 13: 954-962. [Crossref]
- Sridharan H, Upton JW (2014) Programmed necrosis in microbial pathogenesis. Trends Microbiol 22: 199-207. [Crossref]
- Orzalli MH, Kagan JC (2017) Apoptosis and necroptosis as host defense strategies to prevent viral infection. Trends Cell Biol 27: 800-809. [Crossref]
- Seya T, Shime H, Takaki H, Azuma M, Oshiumi H, et al. (2012) Tlr3/ticam-1 signaling in tumor cell rip3-dependent necroptosis. Oncoimmunology 1: 917-923. [Crossref]
- Upton JW, Kaiser WJ, Mocarski ES (2010) Virus inhibition of rip3-dependent necrosis. Cell Host Microbe 7: 302-313. [Crossref]
- Maelfait J, Liverpool L, Bridgeman A, Ragan KB, Upton JW, et al. (2017) Sensing of viral and endogenous rna by zbp1/dai induces necroptosis. EMBO J 36: 2529-2543. [Crossref]
- Huang Z, Wu SQ, Liang Y, Zhou X, Chen W, Li L, et al. (2015) Rip1/rip3 binding to hsv-1 icp6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe 17: 229-242. [Crossref]
- Lee ACY, Zhang AJX, Chu H, Li C, Zhu H, et al. (2019) H7n9 influenza a virus activation of necroptosis in human monocytes links innate and adaptive immune responses. Cell Death Dis 10: 442. [Crossref]
- Omoto S, Guo H, Talekar GR, Roback L, Kaiser WJ, et al. (2015) Suppression of rip3-dependent necroptosis by human cytomegalovirus. J Biol Chem 290: 11635-11648. [Crossref]
- Zhe-Wei S, Li-Sha G, Yue-Chun L (2018) The role of necroptosis in cardiovascular disease. Front Pharmacol 9: 721. [Crossref]
- Wang S, Ni HM, Dorko K, Kumer SC, Schmitt TM, et al. (2016) Increased hepatic receptor interacting protein kinase 3 expression due to impaired proteasomal functions contributes to alcohol-induced steatosis and liver injury. Oncotarget 7: 17681-17698. [Crossref]
- Takemoto K, Hatano E, Iwaisako K, Takeiri M, Noma N, et al. (2014) Necrostatin-1 protects against reactive oxygen species (ros)-induced hepatotoxicity in acetaminophen-induced acute liver failure. FEBS Open Bio 4: 777-787. [Crossref]
- Stutz MD, Ojaimi S, Allison C, Preston S, Arandjelovic P, et al. (2018) Necroptotic signaling is primed in mycobacterium tuberculosis-infected macrophages, but its pathophysiological consequence in disease is restricted. Cell Death Differ 25: 951-965. [Crossref]
- Jounai N, Kobiyama K, Takeshita F, Ishii KJ (2012) Recognition of damage-associated molecular patterns related to nucleic acids during inflammation and vaccination. Front Cell Infect Microbiol 2: 168. [Crossref]
- Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, et al. (2013) Toll-like receptor 3-mediated necrosis via trif, rip3, and mlkl. J Biol Chem 288: 31268-31279. [Crossref]
- Lien E, Means TK, Heine H, Yoshimura A, Kusumoto S, et al. (2000) Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest 105:497-504. [Crossref]
- Iwasaki A, Medzhitov R (2004) Toll-like receptor control of the adaptive immune responses. Nat Immunol 5: 987-995. [Crossref]
- He S, Liang Y, Shao F, Wang X (2011) Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A 108: 20054-20059. [Crossref]
- Kaiser WJ, Offermann MK (2005) Apoptosis induced by the toll-like receptor adaptor trif is dependent on its receptor interacting protein homotypic interaction motif. J Immunol 174: 4942-4952. [Crossref]
- Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, et al. (2015) Ripk1 and nf-kappab signaling in dying cells determines cross-priming of cd8(+) t cells. Science 350: 328-334. [Crossref]
- Barbosa LA, Fiuza PP, Borges LJ, Rolim FA, Andrade MB, et al. (2018) Ripk1-ripk3-mlkl-associated necroptosis drives leishmania infantum killing in neutrophils. Front Immunol 9: 1818. [Crossref]
- Lung TWF, Pearson JS, Schuelein R, Hartland EL (2014) The cell death response to enteropathogenic escherichia coli infection. Cell Microbiol 16: 1736-1745. [Crossref]
- Weng D, Marty-Roix R, Ganesan S, Proulx MK, Vladimer GI, et al. (2014) Caspase-8 and rip kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci U S A 111: 7391-7396. [Crossref]
- Roca FJ, Ramakrishnan L (2013) Tnf dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 153: 521-534. [Crossref]
- Orozco S, Oberst A (2017) Ripk3 in cell death and inflammation: The good, the bad, and the ugly. Immunol Rev 277: 102-112. [Crossref]
- Bell BD, Leverrier S, Weist BM, Newton RH, Arechiga AF, et al. (2008) Fadd and caspase-8 control the outcome of autophagic signaling in proliferating t cells. Proc Natl Acad Sci U S A 105: 16677-16682. [Crossref]
- Zhang H, Zhou X, McQuade T, Li J, Chan FK, et al. (2011) Functional complementation between fadd and rip1 in embryos and lymphocytes. Nature 471: 373-376. [Crossref]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, et al. (2011) Rip3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471: 368-372. [Crossref]
- Pan T, Wu S, He X, Luo H, Zhang Y, et al. (2014) Necroptosis takes place in human immunodeficiency virus type-1 (hiv-1)-infected cd4+ t lymphocytes. PLoS One 9: e93944. [Crossref]
- Aaes TL, Kaczmarek A, Delvaeye T, De Craene B, De Koker S, et al. (2016) Vaccination with necroptotic cancer cells induces efficient anti-tumor immunity. Cell Rep 15: 274-287. [Crossref]
- Van Hoecke L, Van Lint S, Roose K, Van Parys A, Vandenabeele P, et al. (2018) Treatment with mrna coding for the necroptosis mediator mlkl induces antitumor immunity directed against neo-epitopes. Nat Commun 9: 3417. [Crossref]
- Hanus J, Anderson C, Wang S (2015) RPE necroptosis in response to oxidative stress and in AMD. Ageing Res Rev 24: 286-298. [Crossref]
- Feenstra DJ, Yego EC, Mohr S (2013) Modes of retinal cell death in diabetic retinopathy. J Clin Exp Ophthalmol 4: 298. [Crossref]
- Trichonas G, Murakami Y, Thanos A, Morizane Y, Kayama M, et al. (2010) Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc Natl Acad Sci U S A 107: 21695-21700. [Crossref]
- Gong Y, Fan Z, Luo G, Yang C, Huang Q, et al. (2019) The role of necroptosis in cancer biology and therapy. Mol Cancer 18: 100. [Crossref]
- Zhang S, Tang MB, Luo HY, Shi CH, Xu YM (2017) Necroptosis in neurodegenerative diseases: A potential therapeutic target. Cell Death Dis 8: e2905. [Crossref]
- Li S, Ning LG, Lou XH, Xu GQ (2018) Necroptosis in inflammatory bowel disease and other intestinal diseases. World J Clin Cases 6: 745-752. [Crossref]
- Weinlich R, Oberst A, Beere HM, Green DR (2017) Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol 18: 127-136. [Crossref]

