Zinc is a trace element in the mammalian body, and increasing evidence is suggesting that it plays a critical role in bone development and in the differentiation of bone cells such as osteoblasts, osteoclasts, and chondrocytes. In vivo and in vitro studies have shown that zinc affects osteoclast differentiation. Zinc-sensitive ion channels have been reported. Zinc-related ion channel is ORAI1, which is a store-operated Ca2+ entry channel subunit, and zinc inhibits the activity of this channel. ORAI1 channels play a significant role in regulating osteoclastic Ca2+ oscillations during osteoclast differentiation. Knockdown of ORAI1 inhibited osteoclast differentiation. Zinc also inhibited osteoclastogenesis however, its inhibition was reduced by ORAI1 knockdown. Interestingly, zinc can change the osteoclastic membrane potential. Based on this, hyperpolarization-activated cyclic nucleotide-modulated (HCN) channels were investigated and were found to be highly expressed in osteoclasts. High concentrations of zinc chloride increase Ih current which is generated by HCN channels, suggesting a complicated relationship between HCN channels and zinc. Zinc plays various roles in bone physiology through zinc-modulated ion channels. Signaling of these ion channels would be promising targets for treating skeletal diseases.
Bone is the main storage medium for calcium (Ca2+) and zinc [1]. Although Ca2+-related signaling in bone biology is well studied [2-4], the biology of intracellular zinc signaling and related molecules remain unclear. Zinc is an essential trace element in physiological metabolic processes, including bone remodeling [5,6]. Numerous studies have shown that zinc deficiency leads to bone loss and increased risk of fractures [7-11]. Recently, some reports have demonstrated that zinc transporters plays critical roles in bone and cartilage development [5] and the pathology of osteoarthritis [12].
The mechanism of osteoclast differentiation and the related intracellular signaling has been abundantly studied [3,4,13,14]. The receptor activator of nuclear factor-κB (RANK)/RANK ligand (RANKL) signaling pathway induces changes in intracellular Ca2+ levels ([Ca2+]i); these changes lead to Ca2+-calcineurin-dependent dephosphorylation and activation of nuclear factor of activated T-cells cytoplasmic 1 (NFATc1), which translocates to the nucleus from the cytosol [4]. Addition of zinc to the culture medium inhibits osteoclast differentiation by decreasing [Ca2+]i after NFATc1 translocation [15]. Furthermore, zinc-related molecules such as ion channels and transporters also play important roles in osteoclast differentiation [14-17]. A recent study suggested that the endoplasmic reticulum-localized L-type voltage-gated Ca2+ channel, which is activated by membrane depolarization, is involved in intracellular zinc signaling [18]. In addition, our previous study showed that zinc influences osteoclastic membrane potential and hyperpolarization-activated cyclic nucleotide-modulated channels (HCN) [14].
This study focuses on the roles of zinc on osteoclasts, as well as the roles of zinc-modulated ion channels in osteoclast differentiation. Recent advances in the understanding of intracellular zinc signaling, including zinc-related ion channels, ORAI1 and HCNs in osteoclasts, are presented. Because zinc exerts inhibitory effects on the activity of various ion channels, the relationships between zinc and ion channels in bone physiology are described.
Cell culture
RAW 264.7 mouse osteoclast precursor-like cells (American Type Culture Collection, Manassas, VA, USA) were cultured in DMEM (GE Healthcare Japan, Tokyo, Japan) supplemented with 10% FBS and 1% penicillin and streptomycin. All cells were maintained at 37ºC and 5% CO2 in a humidified atmosphere. All chemicals were purchased from Wako Pure Chemical Industries (Osaka, Japan) unless noted otherwise. For osteoclast differentiation, RAW 264.7 cells were seeded at 2.0×105 cells/cm2 with the addition of soluble RANKL (sRANKL, 50 ng/mL; Oriental Yeast, Tokyo, Japan). TRAP staining was performed as previously described [3,14]. Orai1-siRNA and control-siRNA were purchased from Thermo Fisher Scientific K.K. (Yokohama, Japan). The siRNA was transfected into RAW cells using RNAi-MAX (Thermo Fisher Scientific K.K.) according to manufacturer protocols. TRAP-positive cells with more than three nuclei were defined as multinucleated osteoclasts.
Gene expression assay
Gene expression assay was performed as previously described [3,14]. Total RNA was extracted using a RNeasy kit (Qiagen K. K., Tokyo, Japan) according to the manufacturer’s protocol. First-strand cDNA was synthesized from total RNA using a high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific). The amount of total RNA used in each reaction of synthesis was 1 μg. Quantitative real-time PCR (qRT-PCR) was performed using a StepOne real-time PCR system (Thermo Fisher Scientific), SYBR Green (Fast SYBR Green master mix, Thermo Fisher Scientific), and specific forward and reverse primers. Transcript levels were normalized relative to those of glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Electrophysiology
Electrophysiological recordings were made from RAW-derived osteoclasts cultured for 3–5 days after addition of RANKL as previously described [14,19]. For whole cell recordings, the standard external solution contained 145 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM HEPES (pH 7.3), or 10 mM 2-morpholinoethanesulfonic acid (MES) (pH 5.5), and 0.1% bovine serum albumin. To investigate the effects of high concentration of zinc, 1mM ZnCl2 was added to the bath. The pipette solutions contained 130 mM K-gluconate, 20 mM KCl, 3 mM MgCl2, 1 mM EGTA, 1 mM ATP, 0.5 mM GTP, and 10 mM HEPES (pH 7.3). The pH of the bath and pipette solutions was adjusted by adding NaOH and KOH. Osmolality was maintained between 280 and 300 mOsm.
The borosilicate glass pipettes had a resistance of 5–8 MΩ. The reference electrode was an Ag-AgCl wire connected to the bath solution through a Ringer agar bridge. The zero current potential before formation of the gigaseal was taken as 0 mV. Current signals were recorded with an Axopatch 200A amplifier (Axon Instruments, Foster City, CA, USA), digitized at 1–2 kHz with a Digidata 1200 analog-digital converter (Axon Instruments), and analyzed using pCLAMP software (Axon Instruments). Voltage steps (from -30 to −150 mV) were applied at holding potential of -30 mV every 10-30 s.
Zinc and ORAI1 in osteoclasts
Ion channels that are selective for zinc have not been identified yet. However, zinc modulates the activities of various ion channels [20-22]. One of these channels, the voltage-gated H+ channel (H+ channel), is highly expressed in osteoclasts and contribute to regulation of the intracellular pH [19,23]. Zinc inhibits proton currents generated from H+ channels suggesting that zinc affects osteoclastic intracellular pH. Recently, H+ channels in RAW264.7-derived osteoclasts are reported to be activated by extracellular phosphates, and are suggested to support production of reactive oxygen species (ROS), an essential mediator in RANK-RANKL signaling cascade [24]. ROS products are decreased by inhibition of H+ channel, which might down-regulate osteoclastogenesis.
The other zinc-related ion channel is the ORAI1 Ca2+ channel, which is a store-operated Ca2+ entry (SOCE) channel subunit (25). ORAI1 Ca2+ channels play an important role in the maintenance of osteoclastic Ca2+ oscillations during osteoclast differentiation [16]. The knockdown of ORAI1 inhibits osteoclastogenesis and decreases nuclear translocation of NFATc1. It should be noted that zinc inhibits the activity of the SOCE channel and decreases Ca2+ entry into cells [26-28]. Based on these reports, we have investigated the relationships between zinc and ORAI1 during osteoclast differentiation in RAW 264.7 cells. ORAI1-siRNA transfection significantly reduced mRNA expression of ORAI1 compared with control-siRNA transfection (Figure 1a). Zinc inhibited tartrate-resistant acid phosphatase (TRAP) activity in controls; however, less reduction in TRAP activity was observed in ORAI1-knockdown cells (Figure 1b). This result indicates that zinc-induced inhibitory effects on osteoclastogenesis include, in part, the inhibition of ORAI1 activity.
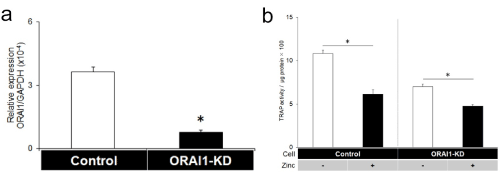
Figure 1. ORAI1 knockdown reduced the zinc-induced inhibitory effects on osteoclastogenesis in RAW264.7 cells. (a: OARI1 knockdown by siRNA transfection, the mRNA expression of ORAI1 was reduced at 24 h after transfection of control-siRNA or ORAI1-siRNA (Mean ± SD, n=5, *p<0.01). b: TRAP activities in the presence or absence of zinc chloride (100 μM) in control and ORAI1-knockdown (ORAI1-KD) cells 3 days after the addition of RANKL (50 ng/ml) (n=8). *P<0.01. Zinc inhibited osteoclastogenesis (1st vs. 2nd column from the left); however, its inhibition was reduced by ORAI1 knockdown (3rd vs. 4th column)
Recently we found that zinc transiently hyperpolarizes osteoclastic cell membrane potential (14), although the mechanism of such changes to the membrane potential remains unclear. This finding implies that zinc regulates cell function via voltage-gated ion channels. Based on our findings, we focused on HCNs, which have important functions in excitable cells [29-32]. HCNs are non-selective cation channels that control the rhythmic activity of cardiac myocytes and the timing of neuron firing, and are known as “pacemaker channels” [32,33]. The physiological roles of HCNs have been investigated in the heart and brain because all four subunits (HCN1-4) have been cloned. One of these subunits, HCN1, localizes abundantly in the cerebellum, hippocampus, and cortex, and is considered to be involved in learning and memory [32,34]. HCNs exert their effects by generating specific hyperpolarization-activated currents (known as Ih or If), which were recognized in the 1970s [35]. Ih plays a significant role in determining the resting membrane potential in these excitable cells [33,36].
Zinc-induced hyperpolarization of osteoclastic cell membrane potential is reduced by ZD7288, which is an HCN inhibitor [14]. Both HCN1 and HCN4 are expressed in osteoclasts: the main subtype is HCN4, based on qRT-PCR analysis and Ih current recording from whole-cell patch clamps. To confirm the relationships between HCN4 and zinc, HCN4 was knocked down using siRNA. Zinc-induced inhibition of osteoclastogenesis was diminished. The investigation of the effects of membrane potentials on osteoclast differentiation was challenging because the non-invasive methods were required for regulating the membrane potential. To resolve this problem, we have generated the RAW cell line that expresses a light-driven pump (Arch). The Arch is activated by yellow-green light and hyperpolarizes via proton transport [37]. Using this light-controlled system, the hyperpolarization was shown to promote osteoclastogenesis. Therefore, zinc inhibits osteoclast differentiation through intracellular signaling cascade, while osteoclast differentiation is promoted via zinc-induced hyperpolarization. Under physiological conditions, zinc-induced promotion of osteoclastogenesis (via changes to the membrane potential) is suppressed by HCN function (Figure 2). These findings suggest that zinc affects the activity of voltage-gated ion channels via changes to the membrane potential.
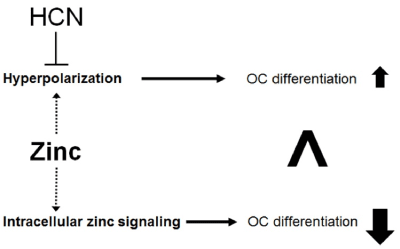
Figure 2. The proposed relationships between HCN and zinc in osteoclastogenesis
In addition to these results, we have found that high concentrations of zinc chloride increase Ih current (Figure 3a), suggesting a complicated relationship between HCN channels and zinc. This increase in Ih current implies the acceleration of HCN channel activity; thus, high concentrations of zinc itself would reduce hyperpolarization-induced osteoclastogenesis. Although the mechanism of the effects of high zinc concentrations on bone physiology is unclear, the resorption lacuna might be exposed to high concentrations of zinc (Figure 3b) during bone resorption. Effects of high concentrations of zinc on osteoclasts will be investigated in our subsequent experiments involving HCN channels.
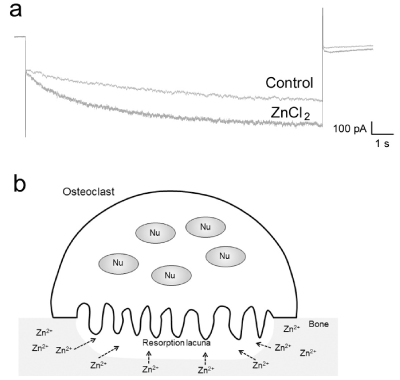
Figure 3. Effects of high concentrations of zinc on Ih currents (a: Representative whole-cell currents evoked by hyperpolarization pulses (-150 mV) applied at a holding potential of -30 mV, before (Control) and after addition of ZnCl2 (100 μM). ZnCl2 increased hyperpolarization-activated inward current in RAW-derived OC. b: The proposed physiological condition for high concentration of zinc. Nu: nucleus)
Zinc is abundant in bone; however, its physiological role in bone biology is unclear. Zinc directly affects osteoclastic cell functions via zinc transporters. Because zinc functions as an inhibitor of certain types of ion channels and causes changes to the membrane potential, understanding zinc-modulated ion channel functions would lead to novel findings in bone biology.
The authors declare no conflict of interest.
- Gomez S, Rizzo R, Pozzi-Mucelli M, Bonucci E, Vittur F, et al. (1999) Zinc mapping in bone tissues by histochemistry and synchrotron radiation-induced X-ray emission: Correlation with the distribution of alkaline phosphatase. Bone 25: 33-38.
- Kuroda Y, Hisatsune C, Nakamura T, Matsuo K, Mikoshiba K (2008) Osteoblasts induce Ca2+ oscillation-independent NFATc1 activation during osteoclastogenesis. Proc Natl Acad Sci USA 105: 8643-8648.
- Notomi T, Ezura Y, Noda M (2012) Identification of two-pore channel 2 as a novel regulator of osteoclastogenesis. J Biol Chem 287: 35057-35064. [Crossref]
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, et al. (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3: 889-901.
- Fukada T, Hojyo S, Furuichi T (2013) Zinc signal: A new player in osteobiology. J Bone Miner Metab 31: 129-135. [Crossref]
- Fukada T, Civic N, Furuichi T, Shimoda S, Mishima K, et al. (2008) The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways. PLoS One 3: e3642.
- Eberle J, Schmidmayer S, Erben RG, Stangassinger M, Roth HP (1999) Skeletal effects of zinc deficiency in growing rats. J Trace Elem Med Biol 13: 21-26. [Crossref]
- Elmstahl S, Gullberg B, Janzon L, Johnell O, Elmståhl B (1998) Increased incidence of fractures in middle-aged and elderly men with low intakes of phosphorus and zinc. Osteoporos Int 8: 333-340. [Crossref]
- Hie M, Iitsuka N, Otsuka T, Nakanishi A, Tsukamoto I, et al. (2011) Zinc deficiency decreases osteoblasts and osteoclasts associated with the reduced expression of Runx2 and RANK. Bone 49: 1152-9.
- Ovesen J, Møller-Madsen B, Nielsen PT, Christensen PH, Simonsen O, et al. (2009) Differences in zinc status between patients with osteoarthritis and osteoporosis. J Trace Elem Med Biol 23: 1-8.
- Rico H, Villa LF (2000) Zinc, a new coherent therapy for osteoporosis? Calcif Tissue Int 67: 422-423. [Crossref]
- Kim JH, Jeon J, Shin M, Won Y, Lee M, et al. (2014) Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell 156: 730-743. [Crossref]
- Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423: 337-342.
- Notomi T, Kuno M, Hiyama A, Ohura K, Noda M, et al. (2015) Zinc-induced effects on osteoclastogenesis involves activation of hyperpolarization-activated cyclic nucleotide modulated channels via changes in membrane potential. J Bone Miner Res 30: 1618-1626.
- Park KH, Park B, Yoon D S, Kwon SH, Shin DM, et al. (2013) Zinc inhibits osteoclast differentiation by suppression of Ca2+-Calcineurin-NFATc1 signaling pathway. Cell Commun Signal 11: 74.
- Hwang SY, Putney JW (2012) Orai1-mediated calcium entry plays a critical role in osteoclast differentiation and function by regulating activation of the transcription factor NFATc1. FASEB J 26: 1484-1492.
- Khadeer MA, Sahu SN, Bai G, Abdulla S, Gupta A (2005) Expression of the zinc transporter ZIP1 in osteoclasts. Bone 37: 296-304. [Crossref]
- Yamasaki S, Hasegawa A, Hojyo S, Ohashi W, Fukada T, et al. (2012) A novel role of the L-type calcium channel α1D subunit as a gatekeeper for intracellular zinc signaling: Zinc wave. PLoS One 7: e39654. [Crossref]
- Sakai H, Li G, Hino Y, Moriura Y, Kawawaki J, et al. (2013) Increases in intracellular pH facilitate endocytosis and decrease availability of voltage-gated proton channels in osteoclasts and microglia. J. Physiol 591: 5851-5866.
- Banke TG, Wickenden AD (2009) Intracellular zinc irritates TRPA1. Nat Chem Biol 5: 141-142. [Crossref]
- Takeshita K, Sakata S, Yamashita E, Fujiwara Y, Kawanabe A, et al. (2014) X-ray crystal structure of voltage-gated proton channel. Nat Struct Mol Biol 21: 352-357.
- Schwiebert EM, Liang L, Cheng NL, Williams CR, Olteanu D, et al. (2005) Extracellular zinc and ATP-gated P2X receptor calcium entry channels: New zinc receptors as physiological sensors and therapeutic targets. Purinergic Signal 1: 299-310.
- Mori H, Sakai H, Morihata H, Kawawaki J, Amano H, Yamano T, et al. (2003) Regulatory mechanisms and physiological relevance of a voltage-gated H+ channel in murine osteoclasts: Phorbol myristate acetate induces cell acidosis and the channel activation. J Bone Miner Res 18: 2069-2076.
- Li G, Miura K, Kuno M (2017) Extracellular phosphates enhance activities of voltage-gated proton channels and production of reactive oxygen species in murine osteoclast-like cells. Pflügers Arch. - Eur. J Physiol 469: 279-292.
- Kar P, Nelson C, Parekh AB (2011) Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca2+ release-activated Ca2+ (CRAC) channels. J Biol Chem 286: 14795-14803.
- Huang J, van Breemen C, Kuo KH, Hove-Madsen L, Tibbits GF (2006) Store-operated Ca2+ entry modulates sarcoplasmic reticulum Ca2+ loading in neonatal rabbit cardiac ventricular myocytes. Am J Physiol Cell Physiol 290: C1572- C1582. [Crossref]
- Itagaki K, Kannan KB, Livingston DH, Deitch EA, Fekete Z, et al. (2002) Store-operated calcium entry in human neutrophils reflects multiple contributions from independently regulated pathways. J Immunol 168: 4063-4069.
- Gore A, Moran A, Hershfinkel M, and Sekler I (2004) Inhibitory mechanism of store-operated Ca2+ channels by zinc. J Biol Chem 279: 11106-11111.
- Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z (2002) Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci 5: 1185-1193.
- Endo, T, Tarusawa, E, Notomi, T, Kaneda, K, Hirabayashi, M, et al. (2008) Dendritic Ih ensures high-fidelity dendritic spike responses of motion-sensitive neurons in rat superior colliculus. J Neurophysiol 99: 2066-2076.
- Kang Y, Notomi T, Saito M, Zhang W, Shigemoto R (2004) Bidirectional interactions between h-channels and Na+-K+ pumps in mesencephalic trigeminal neurons. J Neurosci 24: 3694-3702.
- Notomi T, Shigemoto R (2004) Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol 471: 241-276.
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M (1998) A family of hyperpolarization-activated mammalian cation channels. Nature 393: 587-91.
- Robinson RB Siegelbaum SA (2003) Hyperpolarization-activated cation currents: From molecules to physiological function. Annu Rev Physiol 65: 453-480.
- Noma A, Irisawa H (1976) A time- and voltage-dependent potassium current in the rabbit sinoatrial node cell. Pflugers Arch Eur J Physiol 366: 251-258.
- Pape HC (1996) Queer current and pacemaker: The hyperpolarization-activated cation current in neurons. Annu Rev Physiol 58: 299-327.
- Chow BY, Han X, Dobry AS, Qian X, Chuong AS, et al. (2010) High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463: 98-102. [Crossref]



