Aims: Right ventricular (RV) apical pacing (RVAP) has been the preferred pacing site for decades but recent evidence suggests chronic RVAP can deteriorate cardiac function. RV septal pacing (RVSP) has emerged as an alternative site but its benefits over RVAP remain unclear. This meta-analysis aims to compare the effect of RVSP and RVAP on cardiac function.
Methods: PubMed, EMBASE, and Cochrane were systematically searched for studies examining RVSP and RVAP. The inclusion criteria was randomized clinical trials comparing the effect of RVAP and RVSP on cardiac function and structure at follow-up. Data was pooled using random effects model.
Results: Twenty-five studies (N=2,315 patients) randomized into RVAP (n=1,028) and RVSP (n=1,287) were included in this meta-analysis. Pooled data across the studies showed RVSP patients achieved significantly higher mean gain in LVEF at the end of follow-up (standardized mean difference: SMD, 0.394, 95% CI: 0.715-0.073), a narrower QRS duration (SMD, -1.172, 95% CI: 0.672-1.672) and lower levels of serum B-type natriuretic peptide (BNP) (SMD, 0.328, 95% CI: 0.039-0.617, p=0.02). Although RVSP showed a positive trend towards protecting against both LV dyssynchrony and remodelling as well as having better lead performance (R-wave, pacing threshold and impedance), the difference was not significant (p > 0.01).
Conclusion: In patients with significantly impaired LV systolic function who are eligible for RV pacing, RVSP may improve their LV function and lower serum BNP levels but may not protect them against ventricular dyssynchrony and remodeling.
right ventricular apical pacing (rvap), right ventricular septal pacing (rvsp), pacemaker implantation, left ventricular (lv) function
Pacemaker or artificial endocardial pacing is a well-established treatment for multiple symptomatic bradyarrhythmias arising from an impairment in the heart’s electrical conduction system usually secondary to chronic atrioventricular block or sinus node dysfunction [1]. For close to five decades, the right ventricular apical pacing (RVAP) has remained the mainstay of pacemaker implantation due to technical ease of transvenous lead placement, electrode stability and treatment efficacy [2-4]. Clinical data in the past five decades also suggest chronic RVAP improves quality of life and life expectancy [5,6]. However, in some patients, chronic RVAP has been associated with intra- and inter-ventricular dyssynchrony [6] resulting in negative hemodynamic changes such as decreased cardiac output, increased myocardial workload and oxygen consumption, and altered neuro-hormonal and electrophysiological activities [3,5]. These hemodynamic changes can lead to the development of LV dysfunction, atrial fibrillation and heart failure [6].
Several non-apical positions have already been investigated including RV outflow tract (RVOT) in the septal region, septum, HIS-bundle and pulmonary infundibulum [3,4]. Of these, RVOT and RV septum are the most attractive options because of the relative technical ease of transvenous lead placement and electrode stability [6]. Experimental data supports RVSP but individual clinical trials provide inconsistent findings, and consequently, RVSP superiority to RVAP with regard to clinical outcomes, cardiac function and hemodynamic stability remains unclear [6-9]. Findings from previous four meta-analyses have also not firmly established beneficial outcomes of RVSP over RVAP [10-13]. They suggest that RVSP has a favourable effect on hemodynamic and on preserving LV systolic function for acute period (short-term) [10-12] or up to two years [13] but some of the studies reported wide confidence interval (CI) levels because of a large heterogeneity in the criteria of patient selection in individual studies [10-12]. Since then, additional clinical trials [3,4,6,7,14,15] examining other clinically relevant end-points with longer follow-up have accrued. The present meta-analysis seeks to extend the four previous meta-analysis by comparing their effect of RVAP and RVSP on LV systolic function (LVEF) and remodelling (LV volumes), LV systolic synchrony (Ts-SD) and long-term lead performance (R-wave sensing, stimulation threshold, and impedance).
Search strategy
We systematically searched PubMed, EMBASE, and Cochrane Central Register of Controlled Trials) from inception to October 2018 for RCTs comparing the effect of RVSP and RVAP on cardiac function and on lead performance after pacing. The search strategy included the following search terms: “cardiac pacing” OR “endocardial pacing” OR “pacing site” OR ‘pacemaker implantation” AND “heart ventricles” OR “ventricular” AND “controlled trials” OR “clinical trials”. We identified additional studies through a manual search of references of the included studies and review of included articles.
Inclusion criteria
The criteria for inclusion was as follows: the study (a) randomized subjects to either RVSP or RVAP; (b) compared RVSP and RVAP at baseline and after pacing; (c) reported cardiac functional outcomes, dyssynchrony and lead performance for both RVSP and RVAP; (c) provided data in an extractable form; and (d) followed patients for a period at least two months. Studies were excluded if they examined animal models, were conference papers, and were available only in abstract form. Finally, there was no restriction on publication language or publication period.
Data extraction
Two reviewers sequentially and independently screened each study against the inclusion criteria and subsequently collated data from all the included studies. Results from the two independent reviewers were then compared and any discrepancy resolved through discussion and consensus. Extracted data from each study was then summarized in a Microsoft Excel spreadsheet. The extracted data included first author, publication year, number of patients in RVSP and RVAP, follow-up period, common clinical or functional outcomes assessed and remarks on the optimal pacing site between RVSP or RVAP. If a study assessed more than one outcomes, each of the outcome was analysed independently.
Quality assessment
A modified version of the Oxford quality scoring system (Jadad scale) [16] was used to assess the quality of the included studies. The scoring system controls bias in three main research aspects: study design, subject recruitment or withdrawal and statistical analysis. Scoring involved responding 11 questions. The scoring system assigns two points first two questions and one point each for the remaining eight questions for a total score of 13 points. The 11 questions are as follows. (i) Was the study described as randomized? (ii) Was there concealment of randomisation? (iii) Was there a description of withdrawals and dropouts? (iv) Were study objective defined? (v) Were outcomes measured and define clearly? (vi) Was there a clear description of inclusion and exclusion criteria? (vii) Was the patient sample justified? (viii) Was there a clear description of interventions used? (ix) Was there a control group? (x) Were methods assessing adverse effects clearly described? (xi) Were statistical methods clearly described and justified?
Statistical analysis
Continuous data was expressed as mean and standard deviation (SD) while categorical data was expressed as frequencies and percentages. Comprehensive meta-analysis software was used to pool dichotomous data across studies and outcomes were treated as standardized mean difference (SMD) with corresponding 95% confidence interval (CI). The degree of heterogeneity across studies was calculated using the I2 statistic and a random model effect was used when I2 > 50% and a fixed model when I2 < 50%. P-value < 0.01 was considered statistically significant.
Search results
The search strategy yielded 749 unique citations. Of these, title and abstract screening excluded 709 articles while 40 were included for full text screening. A further 15 studies were excluded based on non-extractable data (6), no comparison between RVSP and RVAP (3), and having outcomes unrelated to LV function or structure (6). The remaining 29 studies met the eligibility criteria and were included for analysis [1,2,4,5,7,8,15,17-38]. Figure 1 provides a summary of the search process. However, additional four studies [20-23] were excluded from the final dataset due to difficulty in extracting data or lacked numerical data of interest this study.
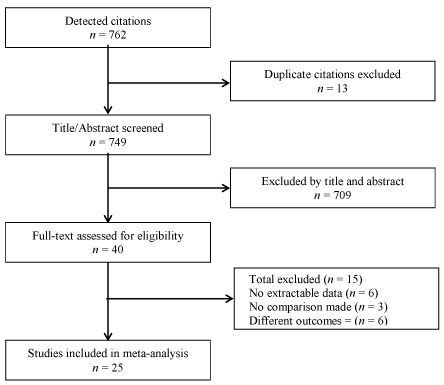
Figure 1. Flow chart of the search process
Study characteristics
Summaries of study and patient characteristics are provided in Table 1. Three studies [17,34,36] adopted a crossover design while the remaining adopted a parallel design [1,2,4,5,7,8,15,18,19,24-33,36]. Twenty-one studies [1,2,4,5,8,15,17-19,26-34,36-38] provided LVEF values at baseline and after pacing, eight [2,4,8,15,31,32,37,38] of them reported echocardiographic measures of changes in LV volumes (LVEDV and LVESV) (Table 2). Seventeen studies examined the effect of RVSP and RVAP on QRS [2,4,8,15,19,24-27,30-36,38]. Seven [4,8,15,24,35,38] of the 17 QRS studies also evaluated the effect on R-wave stimulation, pacing threshold and/or impedance (Table 3). Four studies examined tissue Doppler imaging (TDI)-defined LV synchrony [15,31,32,37] (Table 4). Three studies [7,17,25] assessed neuro-hormonal changes (B-type Natriuretic Peptide [BNP]) (Table 5). The 25 studies making the final dataset had a total of 2,315 patients randomized into RVAP (n = 1,028) and RVSP (n = 1,287) with a mean enrolled follow-up period of 14.04 months.
Table 1. Summary of study characteristics
First Author
[Reference No.] |
Year |
No. of Patients |
Follow-up (Months) |
Is Septal better |
Outcomes/Remarks |
Apical |
Septal |
Alhous, MH. et al. [15] |
2015 |
8 |
14 |
2 |
± |
RVSP improves LVEF and LV synchrony CRT candidates |
Arnold, CT. et al. [18] |
2009 |
17 |
17 |
36 |
- |
RVSP in inferior to RVAP. Has poorer LEVF and dyssynchrony |
Atteia, I. et al. [38] |
2012 |
20 |
20 |
6 |
+ |
RVSP has less adverse effects on LV function/dyssynchrony than RVAP |
Bai, M. et al. [28] |
2016 |
46 |
50 |
12 |
+ |
RVSP reduces deleterious effects of RVAP in selected patients |
Cano, O. et al. [29] |
2010 |
46 |
47 |
12 |
+ |
RVSP reduces RVAP-induces LV dyssynchrony |
Chen, K. et al. [30] |
2014 |
47 |
45 |
18 |
+ |
RVSP has betted clinical utility in AV-block and LVEF:35-40% |
Cho, GY et al. [31] |
2011 |
45 |
34 |
1 |
± |
RVSP has a smaller increase in QRS than RVAP. Additional studies required |
Domenichini, G. et al. [27] |
2012 |
28 |
31 |
48 |
- |
RVSP confers no clinical benefits over RVAP. |
Fat-Hung, TSE. et al. [26] |
2009 |
12 |
12 |
18 |
+ |
Upgraded RVSP reverses deleterious effects of RVAP in chronic RV pacing |
Gong, X. et al. [32] |
2009 |
48 |
48 |
12 |
± |
RVSP has no benefits over RVAP in preventing cardiac remodeling |
Kikuchi, M. et al. [24] |
2012 |
70 |
79 |
24 |
+ |
RVSP is safer and has favorable clinical benefits than RVAP |
Leclercq C. et al. [8] |
2015 |
132 |
131 |
12 |
± |
RVSP is non-inferior to RVAP for LV reverse modelling at 6 months. |
Lewicka-Nowak et al. [33] |
2006 |
14 |
13 |
9 |
+ |
In normal LV function RVSP reduces unfavorable effects of chronic RVAP |
Mera, F. et al. [34] |
1999 |
-- |
12 |
2 |
+ |
RVSP has better LV function in LV dysfunction and chronic AF patients |
Mizukami, A. et al. [5] |
2016 |
223 |
223 |
25 |
± |
RVSP did not show superior medium-term advantages over RVAP |
Molina, L. et al. [2] |
2014 |
34 |
37 |
12 |
+ |
RVSP has better clinical and LV function at 12 months |
Nikoo, MH et al. [7] |
2011 |
39 |
35 |
2 |
± |
No significant differences between RVSP and RVAP |
Occhetta, et al. [4] |
2015 |
33 |
244 |
21 |
+ |
RVSP is safe and effective and reverses deleterious effect of chronic RVAP |
Ren, X. et al. [35] |
2009 |
39 |
36 |
12 |
+ |
RVOT has stable lead performance and no serious complication. |
Victor, F. et al. [19] |
1999 |
10 |
6 |
3 |
± |
At 3 months RVOT has no symptomatic/hemodynamic benefit than RVAP |
Victor, F. et al. [36] |
2006 |
-- |
28 |
3 |
+ |
RVSP preserves LV function in LVEF≤45% than RVAP. |
Wada, T. et al. [25] |
2011 |
46 |
44 |
36 |
± |
RVSP has no clear clinical benefits compared to RVAP. |
Wang, F. et al. [37] |
2011 |
29 |
31 |
12 |
± |
RVSP is non-inferior to RVAP in intraventricular dyssynchrony/LV volumes |
Zanon, F. et al. [17] |
2008 |
-- |
12 |
3 |
+ |
RVSP is superior to RVAP in LV dyssynchrony and mitral regurgitation |
Zou, C. et al. [1] |
2015 |
42 |
38 |
24 |
+ |
RVSP has fewer adverse effects on patients with normal cardiac function. |
Table 2. Summary of studies examining left ventricular function
First Author
[Reference No.] |
LVEF |
LVEDV |
LVESV |
Apical |
Septal |
Apical |
Septal |
Apical |
Septal |
Baseline |
Pacing |
Baseline |
Pacing |
Baseline |
Pacing |
Baseline |
Pacing |
Baseline |
Pacing |
Baseline |
Pacing |
Alhous, MH. et al. [15] |
29±7 |
28±7 |
29±7 |
32±6 |
205±52 |
207±47 |
205±52 |
207±68 |
146±41 |
150±40 |
146±41 |
132±53 |
Arnold, CT. et al. [18] |
52±1 |
47±2 |
54±8 |
53±1 |
|
|
|
|
|
|
|
|
Atteia, I. et al. [38] |
67±7 |
61±7 |
68±7 |
68±8 |
|
49±6 |
|
47±6 |
|
62±7 |
|
69±8 |
Bai, M. et al. [28] |
59±6 |
54±8 |
57±6 |
57±5 |
|
|
|
|
|
|
|
|
Cano, O. et al. [29] |
62±6 |
63±8 |
64±8 |
67±7 |
|
|
|
|
|
|
|
|
Chen, K. et al. [30] |
|
38±7 |
|
42±2 |
|
|
|
|
|
|
|
|
Cho, GY et al. [31] |
60±8 |
56±9 |
62±8 |
63±8 |
85±28 |
89±33 |
107±37 |
100±28 |
38±16 |
38±21 |
44±21 |
40±16 |
Domenichini, G. et al. [27] |
54±8 |
53±1 |
52±1 |
47±2 |
|
|
|
|
|
|
|
|
Fat-Hung, TSE. et al. [26] |
58±4 |
59±6 |
55±3 |
60±30 |
|
|
|
|
|
|
|
|
Gong, X. et al. [32] |
68±6 |
66±7 |
68±6 |
68±5 |
84±32 |
78±18 |
83±25 |
79±16 |
27±10 |
27±10 |
27±11 |
26±7 |
Leclercq C. et al. [8] |
30±8 |
38±11 |
29±8 |
36±10 |
215±84 |
178±81 |
221±94 |
188±99 |
154±72 |
115±68 |
158±83 |
126±87 |
Lewicka-Nowak et al. [33] |
56±11 |
47±8 |
54±7 |
53±9 |
|
|
|
|
|
|
|
|
Mera, F. et al. [34] |
|
55±16 |
43±10 |
51±14 |
|
|
|
|
|
|
|
|
Mizukami, A. et al. [5] |
69±13 |
70±8 |
70±13 |
71±8 |
|
|
|
|
|
|
|
|
Molina, L. et al. [2] |
52±10 |
54±10 |
57±10 |
61±10 |
71±34 |
62±22 |
66±32 |
68±30 |
36±27 |
32±21 |
34±26 |
33±25 |
Occhetta, et al. [4] |
|
43±9 |
49±11 |
53±11 |
98±22 |
139±31 |
100±37 |
104±40 |
47±14 |
79±22 |
49±27 |
55±31 |
Victor, F. et al. [19] |
51±9 |
48±1 |
49±6 |
45±9 |
|
|
|
|
|
|
|
|
Victor, F. et al. [36] |
38±5 |
37±4 |
38±5 |
42±5 |
|
|
|
|
|
|
|
|
Wang, F. et al. [37] |
64±7 |
63±5 |
65±5 |
63±4 |
95±36 |
83±19 |
96±21 |
81±18 |
36±22 |
31±10 |
34±9.8 |
30±8 |
Zanon, F. et al. [17] |
59±7 |
61±10 |
59±7 |
63±12 |
|
|
|
|
|
|
|
|
Zou, C. et al. [1] |
66±12 |
51±10 |
65±14 |
62±14 |
|
|
|
|
|
|
|
|
*LVEF: Left ventricular ejection fraction; +=Yes; - = No; ± = No difference; Missing apical patients = cross-over study
Table 3. Summary of studies examining lead performance (QRS, R-wave, Threshold and Impedance)
First Author [Reference No.] |
QRS |
R-wave |
Pacing Threshold |
Impedance |
Apical |
Septal |
Apical |
Septal |
Apical |
Septal |
Apical |
Septal |
Alhous, MH. et al. [15] |
196±26 |
179±20 |
|
|
0.9±0.3 |
1.0±0.5 |
497±105 |
539±64 |
Atteia, I. et al. [38] |
162±5.9 |
148±6.9 |
11.9±1.7 |
11.7±1.6 |
0.53±0.17 |
0.52±0.19 |
625±89 |
633±94 |
Chen, K. et al. [30] |
175±20 |
153±18 |
|
|
|
|
|
|
Cho, GY et al. [31] |
163±18 |
152±26 |
|
|
|
|
|
|
Domenichini, G. et al. [27] |
158±17 |
150±15 |
|
|
|
|
|
|
Fat-Hung, TSE. et al. [26] |
171±4 |
160±4 |
|
|
|
|
|
|
Gong, X. et al. [32] |
177±23 |
161±22 |
|
|
|
|
|
|
Kikuchi, M. et al. [24] |
176±25 |
149±24 |
15.3±9.1 |
12.4±6.6 |
0.62±0.3 |
0.92±0.3 |
800±397 |
581±334 |
Leclercq C. et al. [8] |
140±26 |
136±26 |
14.2±6.9 |
13.8±6.8 |
0.8±0.3 |
0.7±0.3 |
676±146 |
762±172 |
Lewicka-Nowak et al. [33] |
178±19 |
177±21 |
|
|
|
|
|
|
Mera, F. et al. [34] |
170±11 |
158±10 |
|
|
|
|
|
|
Molina, L. et al. [2] |
158±30 |
146±46 |
11.3±3.7 |
12.3±5.4 |
0.7±0.4 |
0.7±0.2 |
711±175 |
610±120 |
Occhetta, et al. [4] |
165±10 |
122±9 |
|
|
|
0.8±0.5 |
|
540±116 |
Ren, X. et al. [35] |
177±21 |
138±23 |
10.7±4.4 |
11.4±5.1 |
0.91±0.2 |
0.92±0.2 |
568±198 |
592±201 |
Victor et al. [19] |
163±22 |
164±19 |
|
|
|
|
|
|
Victor, F. et al. [36] |
170±40 |
145±40 |
|
|
|
|
|
|
Wada, T. et al. [25] |
162±14 |
147±17 |
|
|
|
|
|
|
*HF: Heart Failure; AF: Atrial fibrillation
Table 4. Summary of studies examining LV systolic dysynchrony
First Author
[Reference No.] |
Ts-SD (ms) |
Apical |
Septal |
Baseline |
Pacing |
Baseline |
Pacing |
Alhous, MH. et al. [15] |
50±19* |
43±14 |
50±19 |
37±17 |
Cho, GY et al. [31] |
33.3±11.9** |
36.5±16.1 |
33.3±11.9** |
38.6±14.6 |
Gong, X. et al. [32] |
26.4±14.4 |
35.3±15.3 |
24.8±15.5 |
28.3±15.1 |
Wang, F. et al. [37] |
33.0±18.4 |
32.5±21.0 |
34.5±29.3 |
33.4±25.4 |
*Mean of all patients; **No baseline values, Ts-SD obtained from controls; Ts: time to the peak systolic velocity with reference to the QRS complex; SD: standard deviation of the time difference in 12 basal and mid segments
Table 5. Summary of studies examining changes in B-type natriuretic peptide
First Author [Reference No.] |
Year |
No. of Patients |
Follow-up (Months) |
BPN |
Apical |
Septal |
|
Apical |
Septal |
Nikoo, MH et al. [7] |
2011 |
39 |
35 |
2 |
494±292 |
310±292 |
Wada, T. et al. [25] |
2011 |
46 |
44 |
36 |
141±141 |
119±139 |
Zanon, F. et al. [17] |
2008 |
NR |
12 |
3 |
74.1±62 |
69±57 |
*BNP: B-type natriuretic peptide; NR: Not Reported
Results of analyses
LV function: Left ventricular systolic function (LVEF values) were reported in 21 RCTs at follow-up for both RVAP and RVSP groups. Pooled data at the end of follow-up showed RVSP patients achieved a significant increase in mean gain in LVEF compared to RVAP (SMD, 0.394, 95% CI: 0.715-0.073, p = 0.01). Heterogeneity between the studies was also high (I2 = 89.7%, p < 0.01) (Figure 2). To ensure the changes in LVEF were not attributed to differences in baseline LVEF values, we pooled LVEF baseline data across studies. There was no significant differences between RVSP and RVAP (SMD, 0.15, 95% CI: 0.05-0.25) and there was no evidence of heterogeneity across the 21 studies (I2 = 0.00%, p < 0.974).
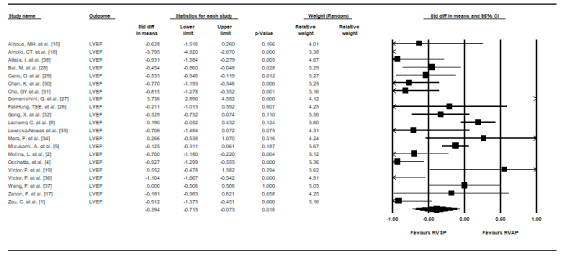
Figure 2. Standard mean differences in LVEF between RVSP and RVAP
Cardiac remodelling: In eight studies [2,4,8,15,31,32,37,38], cardiac remodelling was assessed by changes in LV diastolic and systolic volumes measured using LVEDV and LVESV. When the data was pooled across the eight studies, there was a trend towards reduced LV volumes in the RVSP group but the difference was not statistically supported: LVEDV: (SMD, 0.14, 95% CI: -0.16-0.44, p = 0.03, I2 = 77.8%) and LVESV: (SMD, 0.02, 95% CI: 0.33-0.38, p = 0.90, I2 = 78.6%). Three studies [8,33,34] examined the effect of RVSP and RVAP on LV end-diastolic (LVEDD) and end-systolic diameter (LVESD) and all reported no significant differences at the end-of follow-up.
LV Systolic synchrony: Four studies [15,31,32,37] examined the effect of RVAP and RVSP on LV systolic synchrony. While different parameters were used, the common measure was LV systolic dyssynchrony index, Ts-SD, defined as the standard deviation of the time to peak myocardial systolic velocity of all 12 left ventricular segments. Pooled data at follow-up showed no statistically significant differences in mean Ts-SD between RVAP and RVSP but there was a trend towards increased LV systolic dyssynchrony in RVAP (SMD, 0.151, 95% CI: -0.097-0.398, p = 0.233) (Figure 3). There was also mild heterogeneity between studies in this sub-analysis (I2 = 35.02%, p = 0.20).
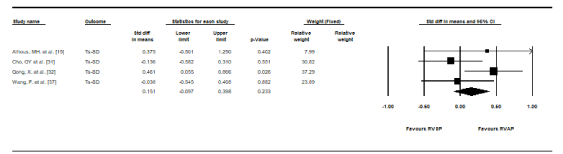
Figure 3. Standard mean differences in Ts-SD between RVSP and RVAP
Lead performance: Lead performance was assessed using QRS duration, R-wave, pacing threshold and impedance. Pooled data from 17 RCTs [2,4,8,15,19,24-27,30-36,38] that provided data on baseline and paced QRS duration revealed at the end of follow-up, the RVAP group had significantly prolonged mean QRS duration compared to the RVSP group (SMD, 1.172, 95% CI: 0.672-1.672, p = 0.00) with a high heterogeneity (I2 = 94.34%, p = 0.00) (Figure 4). Pooled data from seven studies [4,8,15,24,35,38] reveal the RVSP group had slightly higher mean R-wave (SMD, 0.029, 95% CI: 0.274-0.216), pacing threshold (SMD, 0.149, 95% CI: 0.645-0.348) and impedance (SMD, 0.106, 95% CI: 0.592-0.380) but the difference was not statistically significant (p > 0.01).
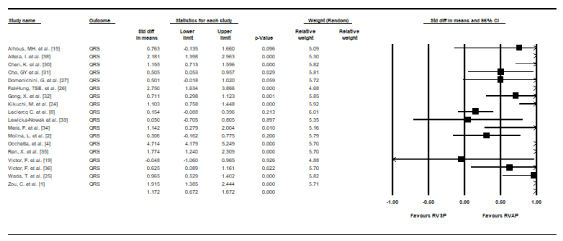
Figure 4. Standard mean differences in QRS Duration between RVSP and RVAP
Natriuretic peptides: B-type natriuretic peptides (BNP) is an important biomarker of the severity of heart failure and its levels are useful in reflecting hemodynamic changes resulting from different pacing modes. Three studies [7,17,25] assessed BNP levels after RVAP and RVSP. When pacing BNP levels were pooled, RVAP had higher mean values but the difference with RVSP lacked statistically significant support (SMD, 0.328, 95% CI: 0.039-0.617, p = 0.026) (Figure 5). There was low heterogeneity between the studies (I2 = 23.55%, p = 0.27).
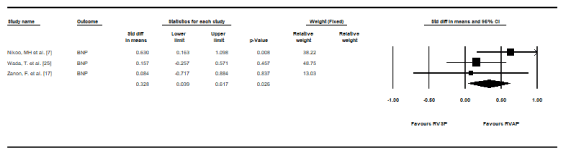
Figure 5. Standard mean differences in plasma BNP Levels between RVSP and RVAP
The present meta-analysis compared the effects of RVAP and RVSP on ventricular function, remodelling and dyssynchrony on patients who are eligible for cardiac pacing. The effect of RVAP and RVSP on lead performance and plasma BNP levels after pacing was also compared. The results indicate RVSP is superior in preserving LV function (LVEF) and on narrowing QRS duration compared to RVAP. The findings also suggest a positive trend of RVSP providing better protection against cardiac remodelling (reducing LVESV and LVEDV) and LV systolic dyssynchrony but the difference is not significant. There was also a non-significant difference between RVAP and RVSP on R-wave, pacing threshold and impedance. Finally, while RVSP showed a positive trend in preserving serum BNP levels, when compared to RVAP, but the difference was not significant. In overall, the findings suggest modest benefits of RVSP over RVAP but not significant to conclude which of the two pacing sites produce superior clinical outcomes.
The present findings are consistent with those of two previous meta-analyses, which reported RVSP achieved significantly higher mean LVEF values compared to RVAP as well as offered better protection for interventricular synchrony and cardiac function [11-12]. While the present analysis did not examine the relationship between baseline LVEF and RVSP-associated LVEF improvement, previous meta-analyses suggest that patients with significantly impaired baseline LV function (LVEF < 35%), elderly or with longer follow-up period (> 12 months) can achieve the greatest benefits when using RVSP [10,12]. On the other, hand, for patients eligible for pacing with preserved baseline LVEF, there is no evidence of either RVSP or RVAP producing significant and clinically important difference in preserving LVEF [12].
The effect of RVAP-associated deleterious effect on LV function has been attributed to ventricular dyssynchrony and remodelling [2,4,20]. However, in the present findings, although RVAP induces greater LV systolic dyssynchrony and ventricular remodelling (changes in LV systolic volumes – LVESV and changes in LV diastolic volumes (LVEDV), the difference with RVSP are minimal. These findings support three previous RCTs that reported in patients with normal LV function, there is insignificant differences in the effect of RVAP and RVSP on LV structure (LVEDD and LVESD) [8,32-34]. These findings suggest that RVSP does not provide superior protection against LV systolic dyssynchrony and cardiac remodelling after 12 months of pacing in patients with normal cardiac function, although it causes more synchronous LV contraction. Individual studies also show pacing may have different outcomes in different patient groups. RVSP reduces atrial fibrillation in patients with sick sinus syndrome [21] while RVAP may have detrimental ventricular remodelling in patients with congenital heart block [21-23].
The present findings also find RVSP achieves a greater narrowing of QRS duration after pacing compared to RVAP. The difference may be explained by reports that RVSP initiates ventricular depolarization in the septal wall across the mitral septal papillary muscle, where pacing activation starts. As a result, RVSP has a narrower QRS duration than RVAP leading into the LV contractions that are more efficient. On the other hand, longer QRS duration in RVAP means a more de-synchronization effect on LV than on RVSP [2]. In addition, while RSVP shows a trend on higher pacing threshold, R-wave sensitivity and lead impedance, the difference was not significant. Ren [35] reported stable lead performance and no serious complications for RVSP relative to RVAP. These findings suggest RVSP may be considered as a first choice pacing-site because of long-term stable lead performance and reduced complications but additional RCTs are warranted to confirm these benefits.
Finally, serum BNP levels is an important biomarker for cardiac dysfunction [7]. In the present study, we used BNP levels to determine hemodynamic changes associated with different pacing modes. We associated RVSP with significantly lower levels of BNP compared to RVAP. In addition, lower BNP levels in RVSP patients has been shown to be independent of sex, age and LVEF [7]. Since BNP are neuro-hormones secreted by the heart in relation to changes in pressure [7,25,26], higher levels may suggest the degree of cardiac dysfunction. Further, two of the included studies [24,25] suggested non-significant lower rate of hospitalization and death between RVSP and RVAP patients (event free RVSP: 2 years, 98%; RVAP: 81%, p < 0.05) [24] and hospitalization (RVSP: 4.4% vs. RVAP 6.8%, p>0.01) [25]. The findings suggest RVSP may provide better protection against cardiac dysfunction compared to RVAP but long-term studies are warranted to confirm this benefit.
In conclusion, in selected patients, RVAP may deteriorate LV systolic function, and these patients may benefit from RVSP. The most important benefit after at least 12 months follow-up include improved LV systolic function (LVEF), narrow QRS duration and lower levels of serum BNP. RVSP also shows a positive trend towards protection against LV systolic dyssynchrony and ventricular remodeling, and stable lead performance but these benefits are not significant. In patients eligible for cardiac pacing with significantly depressed LV systolic function, RVSP may improve cardiac function. It is important to determine baseline LV systolic function to identify patients who may potentially benefit from RVSP. However, additional studies examining the contribution of pacing duration, sex, age and baseline LVEF are warranted to confirm the benefits of RVSP over RVAP in protecting against cardiac dysfunction.
- Zou C, Song J, Li H, Huang X, Liu Y et al. (2015) Right ventricular outflow tract septal pacing is superior to right ventricular apical pacing. J Am Heart Assoc 4: 1-7. [Crossref]
- Furman S, Schwedel JB (1959) An intracardiac pacemaker for Stokes-Adams seizures. New Engl J Med 261: 943-948. [Crossref]
- Molina L, Sutton R, Gandoy W, Reyes N, Lara S et al. (2014) Medium‐term effects of septal and apical pacing in pacemaker‐dependent patients: A double‐blind prospective randomized Study. Pacing Clin Electrophysiol 37: 207-214. [Crossref]
- Klein N, Pfeiffer D, Klein M (2016) Right apical, biventricular, and right high septal ventricular pacing: a comparison of procedural burden and long-term electrical performance. Archives of Medicine 8: 1-7.
- Occhetta E, Quirino G, Baduena L, Nappo R, Cavallino C et al. (2015) Right ventricular septal pacing: Safety and efficacy in a long term follow up. World J Cardiol 7: 490-498. [Crossref]
- Mizukami A, Matsue Y, Naruse Y, Kowase S, Kurosaki K et al. (2016) Implications of right ventricular septal pacing for medium-term prognosis: Propensity-matched analysis. Int J Cardiol 220: 214-218. [Crossref]
- Algazzar AS, Moharram MA, Katta AA, Soltan GM, ElAziz WF (2015) Comparison of early effects of right ventricular apical pacing on left ventricular functions in single and dual chamber pacemakers. Egyptian Heart Journal 67: 129-135.
- Nikoo MH, Ghaedian MM, Kafi M, Jorat MV, Fakhrpour A et al. (2011) Effects of right ventricular septal versus apical pacing on plasma natriuretic peptide levels. J Cardiovasc Dis Res 2: 104-109. [Crossref]
- Leclercq C, Sadoul N, Mont L, Defaye P, Osca J et al. (2016) Comparison of right ventricular septal pacing and right ventricular apical pacing in patients receiving cardiac resynchronization therapy defibrillators: the SEPTAL CRT Study. Eur Heart J 37: 473-483 [Crossref]
- De Cock CC, Giudici MC, Twisk JW (2003) Comparison of the haemodynamic effects of right ventricular outflow-tract pacing with right ventricular apex pacing. Europace 5: 275-278. [Crossref]
- Shimony A, Eisenberg MJ, Filion KB, Amit G (2012) Beneficial effects of right ventricular non-apical vs. apical pacing: a systematic review and meta-analysis of randomized-controlled trials. Europace 14: 81-91. [Crossref]
- Weizong W, Zhongsu W, Yujiao Z, Mei G, Jiangrong W et al. (2013) Effects of right ventricular nonapical pacing on cardiac function: a meta‐analysis of randomized controlled trials. Pacing Clin Electrophysiol 36: 1032-1051. [Crossref]
- Hussain MA, Furuya‐Kanamori LU, Kaye G, Clark J (2015) The Effect of right ventricular apical and nonapical pacing on the short‐and long‐term changes in left ventricular ejection fraction: A systematic review and meta‐analysis of randomized‐controlled trials. Pacing Clin Electrophysiol 38: 1121-1136. [Crossref]
- Muto C, Calvi V, Botto GL, Pecora D, Ciaramitaro G et al. (2014) Is there a right place to pace the right ventricle? Evaluation of apical and septal positions in a pacemaker population: Study protocol for a prospective intervention-control trial. Contemp Clin Trials 39: 320-326. [Crossref]
- Alhous MH, Small GR, Hannah A, Hillis GS, Frenneaux M et al. (2015) Right ventricular septal pacing as alternative for failed left ventricular lead implantation in cardiac resynchronization therapy candidates. Europace 17: 94-100. [Crossref]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ et al. (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17: 1-2. [Crossref]
- Zanon F, Bacchiega E, Rampin L, Aggio S, Baracca E et al. (2008) Direct his bundle pacing preserves coronary perfusion compared with right ventricular apical pacing: a prospective, cross-over mid-term study. Europace 10: 580-587. [Crossref]
- Arnold CT, Allman C, Vidaic J, Tie H, Hopkins AP et al. (2009) Long-term impact of right ventricular septal versus apical pacing on left ventricular synchrony and function in patients with second-or third-degree heart block. Am J Cardiol 103: 1096-1101. [Crossref]
- Victor F, Leclercq C, Mabo P, Pavin D, Deviller A et al. (1999) Optimal right ventricular pacing site in chronically implanted patients. J Am Coll Cardiol 33: 311-316. [Crossref]
- Barin ES, Jones SM, Ward DE, Camm A, Nathan AW (1991) The Right ventricular outflow tract as an alternative permanent pacing site: Long‐term follow‐up. Pacing Clin Electrophysiol 14: 3-6. [Crossref]
- Minamiguchi H, Nanto S, Onishi T, Watanabe T, Uematsu M et al. (2011) Low atrial septal pacing with dual-chamber pacemakers reduces atrial fibrillation in sick sinus syndrome. J Cardiol 57: 223-230. [Crossref]
- Liu L, Tang J, Peng H, Wu S, Lin C et al. (2015) A long-term, prospective, cohort study on the performance of right ventricular pacing leads: comparison of active-fixation with passive-fixation leads. Sci Rep 5: 7662. [Crossref]
- Thambo JB, Bordachar P, Garrigue S, Lafitte S, Sanders P et al. (2004) Detrimental ventricular remodelling in patients with congenital complete heart block and chronic right ventricular apical pacing. Circulation 110: 3766-3372. [Crossref]
- Kikuchi M, Tanno K, Miyoshi F, Munetsugu Y, Onuma Y et al. (2012) Long-term effectiveness of right septal pacing vs. right apical pacing in patients with atrioventricular block. Journal of Arrhythmia 28: 214-218.
- Wada T, Watanabe A, Kawada S, Koide Y, Naito Y et al. (2011) Effect of alternative pacing sites between the right ventricular apex and septum in heart failure with a preserved ejection fraction. Journal of Arrhythmia 27: PJ2-PJ032.
- Fat-Hung TSE, Wong KK, Siu CW, Zhang XH, Ho WY et al. (2009) Upgrading pacemaker patients with right ventricular apical pacing to right ventricular septal pacing improves left ventricular performance and functional capacity. J Cardiovasc Electrophysiol 20: 901-905. [Crossref]
- Domenichini G, Sunthorn H, Fleury E, Foulkes H, Stettler C et al. (2012) Pacing of the interventricular septum versus the right ventricular apex: a prospective, randomized study. Eur J Intern Med 23: 621-627. [Crossref]
- Bai M, Li Q, Jiang G, Zhang L, Wang T, Zhang Z (2016) Comparison of effectiveness of right ventricular mid-septal pacing vs. apical pacing: a randomized-controlled trial. Eur Heart J Suppl 18: F12-F18. [Crossref]
- Cano O, Osca J, Sancho-Tello MJ, Sánchez JM, Ortiz V et al. (2010) Comparison of effectiveness of right ventricular septal pacing versus right ventricular apical pacing. Am J Cardiol 105: 1426-1432. [Crossref]
- Chen K, Mao Y, Liu SH, Wu Q, Luo QZ et al. (2014) Is right ventricular mid-septal pacing superior to apical pacing in patients with high degree atrioventricular block and moderately depressed left ventricular function? J Zhejiang Univ Sci B 15: 507-514. [Crossref]
- Cho GY, Kim MJ, Park JH, Kim HS, Youn HJ et al. (2011) Comparison of ventricular dyssynchrony according to the position of right ventricular pacing electrode: a multi-centre prospective echocardiographic study. J Cardiovasc Ultrasound 19: 15-20. [Crossref]
- Gong X, Su Y, Pan W, Cui J, Liu S et al. (2009) Is right ventricular outflow tract pacing superior to right ventricular apex pacing in patients with normal cardiac function? Clin Cardiol 32: 695-699. [Crossref]
- Lewicka-Nowak E, Dabrowska-Kugacka A, Tybura S, Krzyminska-Stasiuk E, Wilczek R et al. (2006) Right ventricular apex versus right ventricular outflow tract pacing: prospective, randomised, long-term clinical and echocardiographic evaluation. Kardiologia Polska 64: 1082. [Crossref]
- Mera F, DeLurgio DB, Patterson RE, Merlino JD, Wade ME et al. (1999) A Comparison of ventricular function during high right ventricular septal and apical pacing after his‐bundle ablation for refractory atrial fibrillation. Pacing Clin Electrophysiol 22: 1234-1239. [Crossref]
- Ren X, Zhang S, Pu J, Wang F (2009) Long-term follow-up of right ventricular outflow tract septal pacing. J Geriatric Cardiology 6: 71-74. [Crossref]
- Victor F, Mabo P, Mansour H, Pavin D, Kabalu G et al. (2006) Randomized comparison of permanent septal versus apical right ventricular pacing: Short‐term results. J Cardiovasc Electrophysiol 17: 238-242. [Crossref]
- Wang F, Shi H, Sun Y, Wang J, Yan Q et al. (2012) Right ventricular outflow pacing induces less regional wall motion abnormalities in the left ventricle compared with apical pacing. Europace 14: 351-357. [Crossref]
- Atteia I, Alazab A, Hussein K, Abeed N, Nagi H (2012) Right ventricular apical versus septal pacing: impact on left ventricular synchrony and function. Crit Care 16: 1-89. [Crossref]
Editorial Information
Editor-in-Chief
Article Type
Review Article
Publication history
Received date: September 09, 2020
Accepted date: September 21, 2020
Published date: September 30, 2020
Copyright
©2020 Albakri A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Albakri A (2020) Right ventricular septal or apical? which is optimal positioning in pacemaker implantation: A systematic review and meta-analysis. Integr Mol Med 7 DOI: 10.15761/IMM.1000411





