Research on heart failure (HF) has traditionally focused on the left-ventricular heart failure (LHF) with little consideration for the right-ventricular heart failure (RHF). Thus, precise knowledge of the role of the right ventricle (RV) in health and disease has lagged far behind that of the left ventricle (LV). Recently, increased recognition of the importance of RV failure in the development of HF has motivated increased research interest to improve clinical management of HF. However, research on RHF has remained fragmented and precise diagnostic and therapeutic methods are not well established. The present paper seeks to conduct a review of published research evidence including two meta-analyses of diagnosis and clinical management methods to advance knowledge on clinical status, diagnosis and management of RHF.
right heart failure, right ventricular failure, right ventricular dysfunction
The right ventricle (RV) has been traditionally considered a moderately passive conduit between systemic and pulmonary circulations with infrequent involvement in cardiac diseases. Very little research has been devoted to understand the precise pathophysiological mechanisms leading to RV dysfunction and specific interventions required to preserve the RV structure and function. Moreover, specific professional practice guidelines on the management of right ventricular heart failure (RHF) are lacking. However, due to increasing volumes of clinical data demonstrating the involvement of RV dysfunction in various cardiac diseases, RV failure may play a specific and important role in the development of HF. This review seeks to combine available research evidence on RHF to advance the clinical knowledge of its etiopathophysiology, diagnosis and clinical management.
The initial description of the role of the right ventricle (RV) in human circulation appearing in a 1616 treatise “De Moru Cordis” established the key function of the RV is pulmonary perfusion rather than nourishment as originally believed [1]. However, over many subsequent decades, research focus shifted to the LV physiology. Underlying the shift were reports that the RV was a passive connection for systemic and pulmonary circulations [2] supported by demonstrations that complete destruction of the RV free wall in an open pericardial dog model [3] or the substitution of the RV free wall by a synthetic patch [4] did not impair the overall cardiac performance. These findings skewed the few studies on RV in the first half of the 20th Century to investigate the hypothesis that the human circulation could function adequately in the absence of the RV contractile function [5]. In the early 1970s, researchers, cardiologists and cardiac surgeons began to recognize the importance of the RV when evaluating procedures to palliate the right-heart hypoplasia [6,7]. In a study of six patients with RV myocardial infarction (MI), RV dysfunction was reported to have an overall deleterious effect on cardiac hemodynamics and performance. The six patients exhibited severe hypotension, reduced peripheral perfusion and severely impaired pressure in the RV [8]. Subsequent studies established that the key function of the RV is maintaining acceptable low pressure in the systemic circulation more than pulmonary perfusion [9]. Since then, the role of RV in various diseased conditions including heart failure (HF), RV myocardial infarction [MI], congenital heart disease (CHD) and pulmonary hypertension (PH) has been well-documented [2].
Despite the long-established research into HF, cardiovascular diseases specialists have struggled with its precise definition. The American Heart Association/American College of Cardiologists (AHA/ACC) provide a broad definition: HF is a complex clinical syndrome in the setting of structural or functional heart disorders impairing ventricular ability to fill or eject blood [10]. Characterized by dyspnea, fatigue and clinical signs of congestion, HF is an important cause of frequent hospitalization, poor quality of life and shortened life expectancy, and considered the final common pathway to various heart diseases [10]. The definition takes into account that LV or RV dysfunction in isolation may not be sufficient to cause symptoms of heart failure, or clinical manifestations of HF may occur even without any demonstrable LV systolic dysfunction or RV contractile dysfunction. The definition also recognized that HF might develop due to the left or right heart failure or both.
On the other hand, the definition of RHF is a little more specific, usually targeting deleterious effects on the right ventricular function. The Heart Failure Association (HFA) and the Working Group on Pulmonary Circulation and RV Function of the European Society of Cardiologists (ESC) guidelines define acute RHF as a rapidly progressive syndrome with systemic congestion in the setting of impaired RV filling and/or decreased RV outflow output [11]. Mostly, RV failure increases RV afterload or preload followed by RV chamber dilatation and tricuspid regurgitation [11]. Greyson [12] defines RHF as the inability of the RV to eject sufficient blood for pulmonary circulation at a normal central venous pressure. Despite RHF definitional variance, the basis of the definitions is altered cardiac hemodynamics and the inadequacy of pulmonary circulation.
RV failure, as the primary presentation of decompensated HF, accounts for 2.2% of HF admissions and in more than 20% of the cases occurs secondary to acute LV failure [13]. The prevalence of RHF has significant geographical variation. In ESC Egyptian HF registry, RHF is present in 4.5% of patients with acute HF compared to 3% in other ESC regions [14]. RV failure accounts for nearly 20% of all deaths secondary to congestive heart failure (CHF) [15]. It is also a major determinant of clinical outcomes in patients with various forms of CHDs, which subjects the RV to abnormal loading conditions [16]. RV failure affects patients with acquired heart diseases because it imposes an independent effect on the prognosis of obstructive pulmonary diseases [17] and RV hemodynamic function closely correlates with mortality rates in patients with primary pulmonary hypertension (PH) [18]. The involvement of the RV function has also been reported in pulmonary thromboembolic diseases and acute respiratory syndrome [19]. The RV appears to be relatively resistance to infarction and recovers even after chronic occlusion [20] but in patients with inferior MI with RV involvement have an elevated risk of death and arrhythmias [21].
In the normal human heart, the RV is the most anteriorly located cardiac chamber immediately behind the sternum and delimited by the tricuspid and pulmonary valves. It is described based on three components: (a) inlet consisting of tricuspid valve, chordae tendinease and papillary muscles; (b) trabeculated apical myocardium; and (c) infundibulum corresponding to the smooth myocardial outflow region [22]. The normal RV wall thickness is 2-3mm at end-diastole, which is less than 8-11 mm of LV thickness. Its contraction is peristaltic-like, beginning at the apex and moves in a wave towards the outflow tract [23]. The RV has a higher proportion of myosin heavy chain isoform than the LV resulting in more rapid but less efficient contraction [24]. The primary role of the RV is the maintenance of low atrial pressure, which optimizes venous return and provides sustained low-perfusion through the lungs. Tissue pressure in the RV usually does not exceed aortic root systolic pressure to permit continued coronary flow throughout cardiac cycle from the right atrial to the lungs [12]. The continuous ejection of blood from the atria is possible because the pulmonary vascular bed has low pressure and resistance, and high compliance circuit [6]. In a conscious dog with normal and elevated RV pressures, coronary perfusion of the RV under normal hemodynamic conditions is balanced between systolic and diastolic intervals [25].
The continuous coronary perfusion pattern of the RV differs from that of the LV, in which tissue pressure rises during systole to systemic levels meaning coronary perfusion of the LV is confined to diastolic interval [25]. The result is considerably different hemodynamics (atrial mean, ventricular systolic and diastolic, and mean pressure, and vascular resistance) between the RV and LV. The RV also uses less than a sixth of the effort of the LV to move the same volume of blood (Table 1).
Table 1. Normal hemodynamics for RV and LV
Pressure |
Pulmonary RV/RA |
Systemic LV/LA |
Pressure mm Hg, Average, Range (±) |
Atrial Mean |
2-7 |
2-12 |
Ventricular Systolic |
15-28 |
90-140 |
Ventricular Diastolic |
0-8 |
4-12 |
Ventricular Mean |
10-16 |
65-105 |
Resistance: dyne/sec/cm-5 Avg. |
Vascular |
123±54 |
2130±450 |
Source: Greyson, 2008, p. S58 [12]
Despite the hemodynamic differences, there is an important ventricular interdependence in RV failure. Systolic or diastolic interdependence involves the concept that the size, the shape and compliance of the RV affects the size, shape and pressure-volume relationship of the LV and vice-versa through direct mechanical interactions [2]. Ventricular interdependence is always present but becomes more pronounced with changes in loading conditions such as those associated with RV failure. The main anatomical determinant of the RV-LV interdependence is the shared interventricular septum (IVS) while others include the insertion of anterior and posterior ends of the RV free wall into the IVS, the encircling fibers, and the pericardium [13]. Acute dilatation of the RV shifts the IVS leftward both in systole and in diastole as both RV and LV compete for space within the pericardium, in the process changing the LV geometry. Acute RV distension may cause an increase in pericardial constraint, contributing to low cardiac output due to decreased LV distensibility, preload and ventricular elastic resistance [11].
The main causes of RHF are structural and functional impairment that decrease the ability of the RV to eject blood into the pulmonary circulation. The causes include pressure overload, volume overload, ischemia and infarction, intrinsic MI, inflow limitation, complex congenital malformation and pericardial diseases [26-30] (Table 2).
Table 2. Causes of right heart failure
Mechanism
|
Specific Etiology
|
Pressure overload (afterload) |
Left-sided heart failure, acute pulmonary embolism, pulmonary hypertension and RVOT obstruction |
Volume Overload |
Tricuspid regurgitation, pulmonary regurgitation, atrial septal defect, anomalous pulmonary venous return or carcinoid syndrome. |
Ischemia and infarction |
RV myocardial ischemia or infarction |
Intrinsic myocardial infarction |
Cardiomyopathy or infiltrative diseases, or arrhythmogenic right ventricular dysplasia |
Inflow limitation |
Tricuspid stenosis, superior vena cava stenosis |
Complex congenital malformation |
Tetralogy of Fallot, double outlet RV with mitral atresia, or hypoplastic RV |
Pericardial disease |
Constrictive pericarditis |
Pulmonary hypertension is emerging as the most common cause of RHF. In most cases, pulmonary hypertension occurs in the setting of LV failure due to LV systolic dysfunction, LV diastolic dysfunction or left-sided valvular heart disease [13]. A variety of congenital heart defects may also lead to RV failure due to increased afterload (pressure overload), volume overload or both. Septal defects are common causes of RV failure since they subject the RV to volume overload because of shunting blood from the left side of the heart. Fallot's tetralogy may also lead to RV hypertrophy and subsequently RV failure resulting from RV outlet obstruction. In adult patients with treated Fallot's tetralogy using transanular patch to repair the RV outflow tract, RV failure may still occur due to pulmonary regurgitation [27].
Pathophysiologic mechanisms of RHF may vary depending on the precipitating condition. In most cases, RV failure occurs in the setting of a combination of depressed cardiac contractility, RV volume overload, and RV afterload resulting from a combination of cardiac conditions [31-33]. (Figure 1).
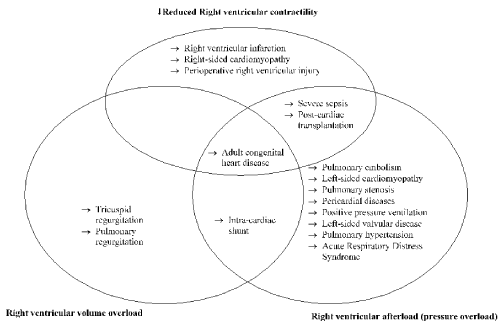
Figure 1. Cardiac conditions causing right ventricular failure [25]
The main pathophysiologic mechanisms of right heart failure (RHF) are reduced RV contractility, RV volume overload and RV pressure overload. RV infarction, RV cardiomyopathy and peri-operative injury to the RV during cardiac surgery leading to to impaired RV contractility. Severe sepsis may also cause RV failure through impairing bi-ventricular contractility and increasing pulmonary vascular resistance. Pulmonary embolism (PE), pulmonic stenosis, pulmonary arterial hypertension (PAH), and PH with left heart disease or with associated lung disease or chronic thromboembolic disease may result into increased RV pressure overload. Pulmonary vascular pressure and PH may impaired RV contractility as well as result in RV dysfunction in patients with cardiac transplantation. Valvular heart disease such as primary tricuspid regurgitation due to endocarditis may cause RHF through increased RV volume overload. Several other cardiac disorders such as adult congenital heart diseases and intra-cardiac shunts may also contribute to RV failure through a combination of pathophysiologic mechanisms
Cardiac conditions such as ischemia and infarction depress the RV contractile function leading to the inability of the RV to handle even normal loading conditions [27,28]. RV ischemia causes the RV chamber to dilate and impair diastolic function with a concomitant increase in RV end-diastolic pressure. The increased pressure causes a leftward shift of the inter-ventricular septum towards an under-filled LV. In turn, the resulting RV dilatation in the setting of limited pericardial compliance causes elevated intra-pericardial pressure, which adds constraints on both RV and LV filling. These changes cause a depressed right-sided output, reduced LV preload and a reduction in the overall cardiac output [27]. Since the thin-walled RV is less muscular than the LV, it is less suited to sustain compensation for acute increases in afterload such as in the setting of pulmonary embolism. In pulmonary embolism, the degree of the obstruction of the pulmonary artery is a critical factor in predicting the degree of RV dysfunction [34]. Of the three pathologic mechanisms, increased RV afterload (pressure overload) is emerging as a leading pathologic mechanism of RHF (Figure 2).
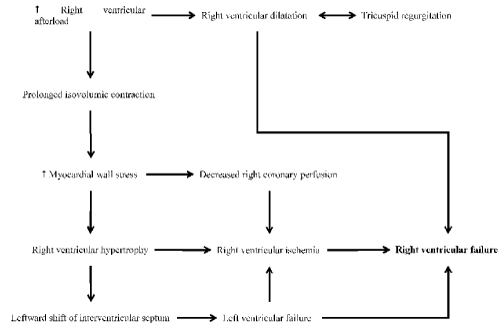
Figure 2.Pathophysiologic mechanisms of RHF due to increased RV afterload [37]
Right ventricular (RV) afterload may cause prolonged isovolumic contraction leading to an increase in myocardial wall stress. In turn, increased myocardial wall stress causes RV hypertrophy and the shift of the interventricular septum towards the LV causing LV failure and ultimately heart failure. RV ischemia may also result from RV hypertrophy and decreased coronary perfusion, contributing to RV failure. Increased RV afterload may also cause RV dilatation, which may lead to tricuspid regurgitation or RV heart failure
Acute increases in RV afterload increases wall tension resulting in dilated RV chamber and impaired diastolic and systolic function [35]. Increased RV afterload also causes a leftward shift of the inter-ventricular septum leading to impaired filling of the LV chamber in the setting of a non-complaint pericardium [35]. Acute tricuspid regurgitation secondary to RV dilatation and systolic dysfunction decreases RV cardiac output and reduces LV preload. Increased RV wall tension concomitant with decreased systemic cardiac output and perfusion pressures alters the equilibrium between myocardial oxygen supply and demand, which leads to ischemia and possible infarction [27]. In acute respiratory distress syndrome, RV afterload increases secondary to circulating vasoconstrictors, increased sympathetic tone and microvascular obstruction [34,35]. Some cardiac conditions such as congenital heart diseases in adults and acquired valvular heart disease place substantial volume loads on the RV aggravating the effect of pressure overload. RV volume overload is more detrimental than pressure overload and has a more pronounced effect on the LV systolic function [27]. RV failure may also occur in the setting of normal RV afterload usually secondary to MI. Right ventricular MI occurs because of disorders of the right coronary artery or the left circumflex artery in dominant circulation [36].
Clinical signs and symptoms of RHF are due to systemic venous congestion, low cardiac output and/or RV dysfunction [11,13]. Symptoms are non-specific and vary depending on the underlying cause and the presence of comorbidities (Table 3).
Table 3. Clinical signs of right ventricular failure
Clinical signs
|
Description
|
Signs of systemic congestion |
Jugular venous distension, hepatojugular reflux peripheral edema, pericardial effusion, congestive hepatomegaly, splenomegaly, ascites, anasarca. |
Signs of RV dysfunction |
Third heart sound, systolic murmur of tricuspid regurgitation, hepatic pulse, signs of concomitant LV dysfunction Paradoxical pulse |
Signs of low cardiac output |
Hypotension, tachycardia, cool extremities, central nervous system abnormalities, and oliguria |
Source: Harjola et al., 2016, p. 230 [11]
Patients with cardiac conditions leading to increased RV afterload may present with dyspnea, light-headedness and syncope while those with RV infarction and pulmonary embolism may present with chest discomfort [36]. In acute-on-chronic RV failure, patients present with right-upper quadrant discomfort due to hepatic congestion and peripheral edema. Physical examination may reveal signs of RV heart failure including systemic hypotension, tachycardia, elevated jugular venous pressure, RV third sound, tricuspid regurgitation, and signs of elevated pulmonary arterial pressure including accentuated sound of pulmonary valve pressure [27]. Hepatomegaly and peripheral edema, coexisting LV failure or valvular defect may be present in patients with acute-on-chronic RV failure [35,37]. In patients with acute RV failure and exacerbations of long-standing pulmonary disease such as chronic obstructive pulmonary disease, symptoms may combine with those of chronic cor pulmonale presenting a diagnostic and therapeutic challenge. Further, therapeutic mechanical ventilation for underlying pulmonary disorders may aggravate RV failure [27].
Diagnosis of RHF requires a high index of suspicion especially for patients with elevated risks such as those in circulatory shock, pre-existing pulmonary hypertension, adults with congenital heart disease or a recent deep vein thrombosis [36]. The Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the ESC provides expert consensus on the specific guidelines for the assessment and diagnosis of RHF. The guidelines recommend RHF diagnosis should include inquiry into past medical history, physical examination such as electrocardiogram and assessment of biomarkers (although they are non-specific) and cardiac imaging including routine chest x-rays, echocardiography, cardiac magnetic resonance imaging (CMRI) or invasive hemodynamic assessment using pulmonary artery catheter [11]. The prime goal of pre-hospital and emergency department triage is to evaluate acuity and urgency of clinical situation including seeking the etiology of RV failure and prioritizing the exclusion of etiologies such as pulmonary embolism that may require specific treatment [11]. Triage should focus on past medical history and physical examination. Past medical history is critical in the inquiry about the presence of coronary artery disease (CAD), emphysema or chronic bronchitis, history of deep venous thrombosis (VT), recurrent abortions, autoimmune diseases especially scleroderma and systemic lupus erythematosus (SLE), and chronic infections such as HIV, tuberculosis and schistosomiasis [13].
Physical examination should include the assessment of biomarkers. Although RHF has no specific biomarkers, clinical utility of B-type natriuretic peptides (BNP) and cardiac troponin assessment should be based on clinical context of the presenting acute RV failure. Elevated levels of BNP and troponin reflect stress and injury in different RHF scenarios as well as possess high sensitivity in the early diagnosis of RV failure and myocardial injury respectively in patients with acute pulmonary embolism (PE) as well as are associated with unfavorable prognosis in RV failure secondary to pulmonary arterial hypertension [38,39]. Electrocardiography in patients with pulmonary hypertension reveals signs of RV hypertrophy – right axis deviation, prominent R wave in lead V1 and dominant S wave in lateral lead V5 or V6 indicating left bundle block branch, and P pulmonale, and elevated ST in V3R and V4R suggesting RV infarction, which occurs in 50% of inferior myocardial infarction [13,36]. Chest x-ray may be routinely performed but it is poorly visualized in RHF because of its anatomical location and unpredictable dilation pattern, which limits its utility in detecting RV failure. However, inferential diagnosis may be made based on detectable enlarged main pulmonary artery, distended azygous vein and oligemia of a lobe, all indicating pulmonary embolus and RV failure [36,40].
Cardiac MRI is considered the gold standard of assessing RV systolic function in RHF. However, because of high cost and limited availability in the emergency department or intensive care unit, echocardiography is the most used first-line non-invasive cardiac imaging for the assessment of RV size, function and load [41,42]. It is also useful for excluding extrinsic causes of acute RV failure, especially for patients with pulmonary tamponade requiring immediate treatment, detect preclinical disease and predict prognosis. Moreover, echocardiography allows the quantification of pulmonary artery systolic pressure by the trans-tricuspid pressure gradient, which is more reliable compared to invasive measurements [43]. The ability to visualize the right heart using apical four-chamber RV-focused view and subcostal view provide accurate estimates of RV hypertrophy, sphericity (the LV D-shape) and the degree of dilatation [43]. With advances in ultrasound techniques, echocardiography diagnostic assessment of RV is shifting from qualitative assessment of the global and segmental RV function to quantitative evaluation [44].
The 2015 updated guidelines from the American Society of Echocardiography and the European Association of Cardiovascular Imaging acknowledge the absence of a single reliable measure of RV systolic function using conventional echocardiography. The guidelines propose a number of surrogate quantitative echocardiographic parameters for the assessment of the global RV function [45]. The main quantitative parameters include fractional area change (FAC), tricuspid annular plane systolic excursion (TAPSE), Doppler tissue imaging (DTI)-derived S’ velocity of the tricuspid annulus or RV index of myocardial performance (RIMP) [45-48] (Table 4).
Table 4. Echocardiographic parameters in the assessment of right ventricular failure
Parameters
|
Definition
|
Pericardial fluid |
> 5 mm in diastole |
RV wall thickness |
> 5mm |
Inferior vena cava diameter/ inspiration collapse |
> 21 mm/< 50% (suggests high RA pressure) |
Tricuspid regurgitation-peak systolic velocity |
> 2.8 m/s (by TR) |
Tricuspid annular plane systolic excursion |
< 17 mm |
RV dilation (RVEDD/LVEDD)/RV basal diameter |
> 1.0/> 41 mm |
RV fractional area change |
< 35% |
Ventricular interdependence septal shift |
D shaped LV |
Systolic S’ velocity of tricuspid annulus |
< 9.5 cm/s (by DTI) |
Longitudinal strain of RV free wall |
< 20% |
RV index of myocardial performance |
> 0.54 (by DTI) |
3D RV ejection fraction |
< 45% |
DTI: Doppler Tissue Imaging
Source: Harjola et al., 2016, p. 231 [11]
The assessment of RV ejection fraction (RVEF) using two-dimensional (2D) echocardiography is currently not recommended due to unacceptably high levels of inaccuracy. However, only RVEF provides adequate assessment of the true RV global pump function and 3D echocardiography is the only echocardiography modality capable of reliably assessing RV volumes and RVEF from end-diastolic and end-systolic volume measurements. 3D echocardiography measurement of RVEF are accurate, reproducible and correlates well with cardiac MRI in both adults and children [11].
Other imaging modalities such as cardiac computed tomography (CT) are useful for detecting and excluding congenital heart diseases, arrhythmogenic right ventricular dysplasia, myocarditis and constrictive pericarditis (pericardial thickening). Pulmonary CT angiography should be considered when chronic thromboembolic pulmonary hypertension is suspected (CTEPH) [13]. For patients with unexplained diagnosis or unresponsive to therapy, invasive hemodynamic assessment using pulmonary artery catheter may be considered. It provides continuous and accurate information about the right and left atrial pressure, cardiac output and pulmonary vascular resistance from intermittent repetitive follow-up [11]. These parameters allow the diagnosis of pulmonary artery hypertension (PAH) and constrictive pericarditis as well as the distinction between intrinsic RV failure, RV failure secondary to increased pressure overload, and RV failure in the setting of diseases of the left-sided heart [36]. For patients admitted to the intensive care unit (ICU), diagnosis of RHF is complicated by the lack of pothognomic clinical signs. Organs affected by RVF-induced congestion are the liver and kidneys manifesting as a decrease in urine and creatine clearance respectively [40]. Despite the value of invasive hemodynamic assessment or monitoring, the ESC recommends it should be used for the shortest time possible.
Meta-analysis of echocardiographic diagnosis
The traditional cardiac imaging assessment of RV dysfunction has centered on qualitative markers – the shape, position and motion of the inter-ventricular septum (IVS), visual estimation of the size of the RV relative to that of the LV, and the assessment of wall motion abnormalities [49]. In RHF patients, overload conditions resulting from flattening of the IVS causes alterations in the crescent-shaped RV of the normal heart. The LV takes a D-shape suggesting RV volume overload if the flattening of the IVS occurs only during diastole or RV pressure overload if the IVS flattening persists during systole. At advance stage of RHF, altered RV shape persists during the entire cardiac cycle [49]. To improve diagnostic accuracy of RHF, the 2015 updated guidelines from the American Society of Echocardiography and the ESC recommend the inclusion of quantitative assessment of RV geometry and function [45] but which have been difficult to determine due to the complex and unusual nature of the RV anatomy [49]. Cardiac MRI has emerged as the gold standard for visualizing and assessing RV function (RV ejection fraction [RVEF]). In addition, technical advances in echocardiography have been helpful in enabling the evaluation of the RV function and volumes as well as the measurement of pulmonary artery pressure. Since echocardiography is widely available, relatively inexpensive and has no side effects, it is the imaging modality of choice for non-invasive assessment of the morphology and function of the RV in clinical practice [45,49]. However, the accuracy of quantitative measures such as TAPSE and RVFAC is not well established. This meta-analysis combines findings from echocardiographic studies assessing TAPSE and RVFAC, and compares the findings to CMRI derived RVEF to determine diagnostic accuracy of the two echocardiography-parameters.
Search strategy and study selection: A systematic search for studies was conducted in the PubMed, EMBASE and MEDLINE online databases from inception to May 2018. The search strategy for relevant literature included indexing terms “right ventricular failure”, “right ventricular dysfunction”, “right-sided heart failure”, ‘right heart failure and “pulmonary hypertension”. The eligibility criteria for studies were: (a) prospective or retrospective clinical trials involving patients suspected with RV failure; and (b) reported quantitative measures of RV dysfunction (TAPSE and RVFAC) obtained using echocardiography, and cardiac MRI-defined RVEF obtained from the same patient. The search was limited to studies examining humans and published in peer-reviewed journals. There was no restriction on language or publication year. Studies were excluded if they recruited patients with congenital heart disease, case reports, review articles, commentaries and editorials. To minimize bias, two observers independently reviewed all the qualifying studies and any disagreement resolved by consensus. A hierarchical strategy based on a review of title, abstract and full text review was used to screen and include pertinent studies. In addition, bibliographies of the included studies and review articles were screened to identify studies not identified by the initial online search. For duplicate studies using the same patient population, the study with the most complete data on RV function was included. Data extracted from the included studies were (a) study characteristics – first author and publication year, patient population (sample, mean age and sex), and quantitative diagnostic outcomes (TAPSE, RVFAC and CMRI-RVEF) (Table 5)
Table 5. Characteristics of included studies
Author [Ref #] |
Year |
Study Design |
Patient Population |
Sample |
Mean Age yrs.(SD) |
Female (%) |
TAPSE (mm) |
RVFAC (%) |
CMRI RVEF (%) |
Arnould et al. [50] |
2009 |
Prospective |
Mixed EF ≤ 45% |
19 |
NR |
NR |
19(6) |
33(11) |
29.5(10.3) |
Pavlicek et al. [51] |
2011 |
Prospective |
RVEF > 50% |
129 |
44(19) |
30 |
20(6) |
41(13) |
61(7) |
|
|
|
RVEF > 30-49% |
67 |
41(18) |
34 |
17(6) |
33(11) |
44(3) |
|
|
|
RVEF < 30% |
27 |
53(20) |
15 |
13(5) |
23(8) |
26(2) |
Sato et al. [52] |
2012 |
Prospective |
PH |
37 |
53(15) |
70 |
19(4) |
31(17) |
38(11) |
Yang et al. [53] |
2013 |
Prospective |
PAH |
30 |
30(10) |
80 |
16.3(2.7) |
28.9(7.0) |
27(12) |
Focardi et al. [54] |
2014 |
Prospective |
Mixed RVEF ≤ 45% |
63 |
43(17) |
52 |
23.2(4.6) |
55.4(11) |
58.8(8.2) |
Lemarie et al. [55] |
2015 |
Prospective |
AMI |
135 |
55(11) |
13 |
12.8(4.5) |
43.8(9.6) |
54.8(6.2) |
Zhou et al. [56] |
2015 |
Prospective |
Mixed EF ≥ 45% |
72 |
55.5(16.2) |
49 |
19.6(5.4) |
42.9(9.2) |
53.6(11.6) |
Spruijt et al. [57] |
2017 |
Retrospective |
PH |
96 |
53(16) |
72 |
18.8(4.0) |
35.8(11.4) |
41.4(15.2) |
AMI: Acute Myocardial Infarction; CMRI: Cardiac Magnetic Resonance Imaging; MI: Myocardial Infarction; PH: Pulmonary Hypertension; RVFAC: Right Ventricular Fractional Area Change; TAPSE: Tricuspid Annular Plane Systolic Excursion.
Study characteristics and outcomes: The initial online search yielded 2439 potential articles. After strict application of the inclusion and exclusion criteria to full text articles, and the removal of duplicate studies or studies having insufficient data for extraction, eight studies were eventually included in this meta-analysis [50-57]. One study [51] separated patients into three categories based on RVEF values (> 50%; > 30-49%; < 30%) and each of the three subgroups were considered separately in the analysis. Seven studies [50-56] recruited a prospective cohort while the remaining study recruited a retrospective cohort [57]. Pooled data from the eight studies included 675 patients (weighted mean age = 47.5 years; female = 46%). In each of the include studies, patients underwent echocardiography imaging to assess TAPSE and RVFAC followed by cardiac MRI to measure RVEF. However, the period between TAPSE/RVFAC and RVEF was not reported in many of the included studies. The weighted mean for TAPSE = 17.87 mm (SD = 3.19); RVFAC = 36.78% (SD = 9.22); and RVEF = 43.41% (13.24). Mean correlation from the eight studies show that RVFAC has a stronger correlation (r = 0.86) with cardiac MRI-defined RVEF compared with TAPSE, with a mean correlation (r = 0.43).
Discussion:Cardiac MRI remains the gold standard for the quantitative assessment of RV volumes and ejection fraction in patients with congenital heart diseases. However, high cost and the lack of a wide availability has considerably limited its use. Thus, echocardiography remains the most widely available and most frequently used non-invasive imaging modality for assessing RV systolic function. Despite its wide usage, standard 2D echocardiography cannot measure accurately RVEF because of its pyramidal shape. The updated 2016 ECS guidelines proposed new surrogate echocardiography parameters to quantify RV function. These parameters, which include TAPSE, RVFAC, DTI-derived S’ velocity of the tricuspid annulus or RIMP have greatly improved the accuracy of detecting systolic RV function. The most common quantitative echocardiograph parameters include TAPSE and RVFAC. However, the accuracy of the two methods, and the determination of which of the two is more accurate remains unclear. Using CMRI-RVEF as the reference standard, the present meta-analysis examined the correlation between TAPSE and RVFAC with CMRI-RVEF. The weighted mean of TAPSE, RVFAC and CMRI-RVEF values (17.87 mm; 36.78%; 43.41%) were much higher than the reference values provided by the updated 2016 ECS guidelines (< 17 mm; < 25%, < 45%) respectively. The reason is the current meta-analysis included patients with depressed and preserved ejection fraction since the intention was to measure the correlation between TAPSE/RVFAC and CMRI-RVEF. The pooled data form the eight studies reveal RVFAC has a stronger correlation with CMRI-RVEF than TAPSE, suggesting it provides a more accurate measure of RV systolic dysfunction than TAPSE.
The positive correlation between TAPSE and RVFAC with CMRI-defined RVEF is consistent with previous studies and review articles. In a study of 34 subjects with MI, a history of PE and/or persistent dyspnea, Kjaergaard et al [58] report TAPSE has a significant correlation with CMRI-RVEF (r =0.48, p < 0.01). In a review of conventional to novel echocardiographic assessment of the RV systolic function, Kossaify et al. [59] report TAPSE presents an excellent correlation with RVEF as calculated with radionuclide ventriculography or cardiac MRI as well as is non-geometric and less dependent on acoustic window. In a very recent meta-analysis, Lee et al. [60] find both RVFAC and TAPSE have a correlation with CMRI-RVEF (r =0.56 and r = 0.40, p=0.018) respectively with RVFAC reporting a statistical significant correlation. Based on reports that CMRI-RVEF is the reference standard for the assessment of RV systolic function, the study concluded that RVFAC provides a more accurate assessment of RV systolic function than TAPSE as well as is an independent predictor of morbidity and mortality in RHF patients.
Several theories have been advanced to support the superiority of RVFAC over TAPSE in the assessment of RV function [60]. TAPSE is a unidimensional measure while RVFAC is a two dimensional measure. TAPSE also has a relative load and angle-dependent, and is subject to cardiac translation and is the least user-dependent measure of RV function [59,60]. Since it is dependent on angle, TAPSE partially represents the global RV function. Thus, in cases of regional differences in RV function, it does not always present accurate information because it disregards the transverse contribution of RV free wall and the septum. TAPSE is also significantly depressed following cardiac surgery yet 3D echocardiography shows preserved RVEF, indicating post-operative changes in the geometry of the RV contraction may affect TAPSE [61,62]. However, the use of the ratio of TAPSE and systolic pulmonary artery pressure (>0.36) may improve prognostic risk stratification in RHF patients compared to TAPSE alone [63] and multiplying TAPSE value by the constant 2.9, could enable accurate echocardiographic measure of RVEF, which in comparison to CMRI-RVEF has no statistically significant difference [64]. On the other hand, RVFAC in the assessment of RV function includes both longitudinal fractional shortening and changes occurring in the transverse plane providing better estimated than TAPSE in situations of differences in regional RV function. However, RVFAC requires a better image quality of the RV to enable tracing of the RV endocardium to calculate RVFAC compared to TAPSE [60]. To improve the accuracy of TAPSE and RVFAC as surrogate measures of RV systolic function, there is need to examine inter-observer variability and its impact on accuracy.
In a summary, the present findings suggest that although echocardiography is a frequent imaging modality for assessing RV dysfunction, it lacks a single and widely accepted parameter. Echocardiography requires the use of multiple acoustic windows and techniques to improve diagnostic accuracy. The analyzed studies propose performing a comprehensive examination that should consider all the available patient information as well as both qualitative and quantitative assessment. Although newer modalities such as 3D echocardiography and speckle tracking echocardiograph may overcome challenges of the conventional echocardiograph, they are an ongoing research area. At present, RVFAC may provide a more accurate surrogate marker of RV systolic dysfunction compared to TAPSE since it assesses both longitudinal fractional shortening and alterations in the transverse plane.
Treatment algorithm
Effective treatment of RV failure requires a skilled multi-disciplinary team to assess and triage patients appropriately. Monitoring RHF patients depends on the clinical situation but the primary focus remains managing RV function, the consequences of RV failure and alleviating distressing physical and emotional symptoms. Clinical management of the underlying cardiac conditions and hemodynamic support remain the mainstay of the treatment of RHF [27]. The Heart Failure Association and the ESC proposed a six-step algorithm for the clinical management of RHF [11].
Step 1:Assess the severity of RHF through a series of tests: clinical evaluation, biochemical evaluation and cardiac imaging evaluation of RV function.
Step 2:Identify and treat triggering factors such as sepsis, arrhythmias drug withdrawals, and institute cause specific management such as percutaneous coronary intervention for RV infarction and reperfusion for acute and high-risk pulmonary embolism.
Step 3:Optimize fluid status through intravenous diuretics in case of volume overload or renal replacement therapy if diuretics therapy has less than optimal outcomes.
Step 4:Maintain arterial pressure using norepinephrine to treat low vascular resistance.
Step 5:Consider inotropes (levosimendan, dobutamine or phosphodiesterase III inhibitors) to reduce cardiac filling pressures.
Step 6:Further measures for afterload (pressure overload) reduction using inhaled nitric oxide or inhaled prostacyclin.
Treatment of rv function
Volume optimization:Patients with RHF are often pre-load dependent but volume loading may cause the ventricles to over-distend resulting into increased RV wall tension, reduced contractility, increased ventricular interdependence, impaired LV filling and ultimately decreased cardiac output [11,27]. Hemodynamic support through volume optimization (volume loading with intravenous infusions) is usually indicated to control volume overload. The use of volume loading should depend on various factors mostly including baseline cardiovascular function of the patient, the degree of RV afterload and RV volume status. For patients with decompensated RV failure with no evidence of pulmonary edema and increased right-sided preload conditions, an initial trial of volume should be considered [27]. For patients with RV volume overload and central venous pressures of > 12 to 15 mm Hg, a dual therapy of vasopressors and inotropes without additional volume administration should be initiated [49]. Volume loading should be done cautiously guided by central venous pressure monitoring for low atrial pressures combined with the absence of elevated filling pressures [11]. Invasive pulmonary artery catheter helps to determine the ideal volume loading conditions [65]. Medication therapy using diuretics is the preferred initial option for most RHF patients especially those exhibiting symptoms of venous congestion but with maintained arterial blood pressure. Diuretics cause volume redistribution in the venous system leading to a rapid clinical improvement in RV volume overload [11,27].
Vasopressors and Inotropes:While waiting for primary therapy targeting the underlying cause of RV failure to take effect, supportive utility of vasopressors and inotropes is often necessary. Vasopressors alone or in combination with inotropes are also indicated in patients with acute RV failure with hemodynamic instability [46]. Vasopressors such as noradrenaline restore blood pressure, and improve coronary and cerebral perfusion. They improve systemic hemodynamics by improving ventricular systolic function and coronary perfusion without causing changes in pulmonary vascular resistance [47]. Inotropes such as dobutamine and milrinone enhance biventricular function and consequently cardiac output. Inotropes also possess potent vasodilatory effects improving RV afterload but may aggravate or precipitate systemic hypotension. Caution should be taken with the utility of inotropes and vasopressors since they may complicate proarrhythmic effects. However, when used successful together with vasopressors, inotropes retain their beneficial effects on cardiac output without causing hypotension and diminished systemic and coronary perfusion [27].
Mechanical Circulatory Support:Mechanical circulatory support may be required in some patients with RV failure to maintain coronary artery perfusion and preserve systemic blood pressure. It is commonly indicated for RHF patients diagnosed with RV myocardial infarction or acute pulmonary embolism, as well as, in RHF patients implanted with left ventricular assist device (LVAD) or following primary graft failure after cardiac transplantation [11]. Device selection usually depends on anticipated duration of mechanical support. RV assist devices such as intra-aortic balloon pump, extracorporeal membrane oxygenation (ECMO) or extracorporeal life support (ECLS) have been used to increase right coronary artery perfusion, reduce ischemia and allow weaning of vasopressors that cause adverse effects on pulmonary vascular resistance [20]. Device therapy improves cardiac hemodynamics as well as act as bridges to heart transplantation in patients with RV failure secondary to diseases intrinsic to the ventricles [66].
Therapeutic target of mechanical ventilation support in patients with acute RV failure is improving oxygenation and ventilation without aggravating RV impedance, venous return or diastolic function [27]. ECMO or ECLS have the potential for increased use in the short-term mechanical support. It is less expensive compared to other assist devices and could be inserted quickly, even percutaneously. Mostly, after five to ten days, the patient is weaned and ECMO explanted or therapy switched to intermediate or long-term device to avoid ECMO-associated complications such as infection, formation of thrombus around the cannulae, limb hypoperfusion or local infection. Right ventricular assist devices (RVAD) can be implanted percutaneously or surgically but only approved for use for up to four weeks since bleeding and thrombus formation are frequent complications. Despite reports of prolonged RV support with assist devices, options for long-term therapy are lacking, and thus, cardiac transplantation remains the ultimate treatment for refractory RV failure [67].
Treatment for underlying conditions
In addition to hemodynamic support, clinical management of RHF should include specific treatment of the underlying or comorbid conditions. The common conditions include pulmonary embolism (PE), pulmonary hypertension (PH) and RV infarction. Managements of PE is important since it is a frequent cause of RHF and RV failure is the principal determinant of early mortality in PE patients. Management of PE is important in RHF patients since it is a frequent cause of acute RHF and RV failure on the other hand the principal determinant of early morality in acute PE patients. The updated 2104 ESC guidelines on diagnosis and management of acute PE, patients with high-risk PE should receive reperfusion treatment mostly intravenous systemic thrombolysis [68]. Normotensive patients, those with low to intermediate risk of PE should receive hemodynamic monitoring for two to three days [69]. For high-risk PE patients contraindicated or non-responsive to thrombolysis therapy, or intermediate to high-risk PE patients with signs of developing hemodynamic decompensation and an elevated risk of systemic fibrinolysis, surgical pulmonary embolectomy may be considered [68].
In RHF patients with PAH and signs of venous and systemic congestion, diuretics should the first treatment option. If resistant to diuretics, renal replacement therapy may be considered but it is associated with dismal prognosis [70]. Close monitoring of fluid status by cardiac ultrasound or pulmonary artery catheter is necessary. Intravenous prostacyclin analogues are useful in reducing RV afterload but care must be observed to avoid systemic hypotension [68]. Inhaled nitric oxide or prostacyclin should be considered in patients intolerant to parenteral prostanoids due increased risk of hypotension [68,71]. Nitric oxide is promising for RV failure patients post orthotopic cardiac transplantation [72,73]. Balloon atrial septostomy may be useful to decompress the RV and may improve LV filling and cardiac output. It a high-risk procedure not recommended as an emergency procedure for patients with high RV filling pressures. In unresponsive patients, ECMO and RVADs may be considered as a bridge to recovery or lung transplant [68,71]. Treatment of RV infarction in RHF patients includes early myocardial reperfusion preferably concomitant with primary percutaneous coronary intervention or thrombolysis [74,75]. Complete reperfusion to the proximal right coronary artery and major RV branches results into immediate improvement and complete recovery of the RV function. RV preload treatment such as nitrates or diuretics may be harmful and volume optimization must be done with caution to prevent hemodynamic compromise. In non-responsive patients, inotropic support should be considered [75].
Meta-analysis of clinical management
Compared to the extensive research evidence supporting the management of LHF, management of RHF is not well supported by randomized controlled trials (RCTs). In addition, the evidence of RHF management is best established for patients with PAH yet in patients with RHF and PAH, it may be difficult to differentiate whether the beneficial effects of therapy are the result of changes in pulmonary vasculature or RV specific effects [71]. Moreover, since the prevalence of RHF is very small compared to LHF, investigating appropriate surrogate clinical outcomes has been an important research focus. Thus, the present meta-analysis seeks to combine research findings on clinical management of RV function in RHF patients to identify therapeutic effectiveness of hemodynamic support and volume optimization therapeutic approaches. The analysis was limited to inotropes, extracorporeal venous ultrafiltration and intravenous diuretics.
Search criteria and sudy selection: A systematic search for literature using PubMed, EMBASE and MEDLINE was performed to identify pertinent studies investigating the clinical management of RHF. The online search focused on random controlled trials. Book chapters, meta-analysis, review articles and editorials were also scanned for additional studies. A combination of search terms used included right heart failure, right ventricular failure, right-sided heart failure, and specific therapies – volume optimization, inotropes, and extracorporeal venous ultrafiltration. The inclusion criteria included studies recruiting patients diagnosed with RHF; receiving HF therapy; and provided clinical outcomes of the therapy. There was no restriction on publication year, language and age of patients. Two reviewers assessed all the identified studies and inclusion of studies was based on consensus. Two investigators using a pre-defined data extraction form carried out data extraction independently. The following data was extracted: first author, study design, population (size, age and sex), intervention used, and intervention outcomes (Tables 6 and 7).
Table 6. Characteristics of included studies for inotropes (Hemodynamic Support)
1st Author |
Year |
Sample |
Class (Drug) |
Cardiac Output (liters/min) |
Mean Atrial Pressure (mm Hg) |
Pulmonary wedge pressure (mm Hg) |
Heart Rate (beats/min) |
Cardiac Index (L/min/m2) |
Myocardial Oxygen Consumption |
Akhtar et al. [76] |
1975 |
15 |
Dobutamine |
3.1 to 5.6 |
93 to 98 |
27.4 to 21.1 |
98.5 to 105.2 |
NR |
NR |
Grose et al. [77] |
1986 |
11 |
Dobutamine |
NR |
87 to 91 |
NR |
86 to 94 |
1.9 to 2.8 |
17.7 to 21.5 |
|
|
11 |
Milrinone |
NR |
NR |
NR |
87 to 92 |
1.9 to 2.5 |
18.3 to 17.5 |
Monrad et al. [78] |
1986 |
10 |
Dobutamine |
NR |
83 to 86 |
27.0 to 24.0 |
85 to 99 |
1.7 to 2.6 |
8.7 to 11.1 |
|
|
10 |
Milrinone |
NR |
NR |
26.0 to 19.0 |
86 to 89 |
1.8 to 2.7 |
8.8 to 7.6 |
Seino et al. [79] |
1996 |
54 |
Milrinone |
NR |
NR |
26.0 to 15.0 |
NR |
2.6 to 3.3 |
NR |
Follath et al. [80] |
2002 |
100 |
Dobutamine |
3.7 to 4.5 |
NR |
24.0 to 21.0 |
NR |
NR |
NR |
CHF: Congestive Heart Failure; NR: Not Reported
Table 7. Characteristics of included studies for diuretics (Volume Optimization)
1st Author [Ref#]
|
Publication Year
|
Sample Size
|
Therapy
|
Weight loss (kg)
|
Length of Stay (Days)
|
Net Fluid Loss (l)
|
Time (hrs.)
|
All-cause mortality (%)
|
Serum Creatine (mg/dl)
|
Costanzo et al. [81] |
2007 |
100 |
Ultrafiltration |
5.0 |
6.3 |
4.6 |
48 |
9.6 |
NR |
|
|
100 |
Intravenous Diuretics |
3.1 |
5.8 |
3.3 |
48 |
11.6 |
NR |
Libetta et al. [82] |
2007 |
5 |
Ultrafiltration |
9.7 |
NR |
NR |
NR |
NR |
0.3 |
|
|
5 |
Intravenous Diuretics |
7.7 |
NR |
NR |
NR |
NR |
0.0 |
Bartone et al. [83] |
2008 |
25 |
Ultrafiltration |
7.2 |
7.2 |
NR |
NR |
NR |
0.3 |
|
|
25 |
Intravenous Diuretics |
2.9 |
4.9 |
NR |
NR |
NR |
0.1 |
Rogers et al. [84] |
2008 |
9 |
Ultrafiltration |
2.2 |
NR |
2.3 |
48 |
NR |
0.01 |
|
|
10 |
Intravenous Diuretics |
1.9 |
NR |
5.8 |
48 |
NR |
0.11 |
Bart et al. [85] |
2012 |
94 |
Ultrafiltration |
7.4 |
NR |
NR |
96 |
17.0 |
0.23 |
|
|
94 |
Intravenous Diuretics |
7.1 |
NR |
NR |
96 |
14 |
-0.04 |
Hanna et al. [86] |
2012 |
19 |
Ultrafiltration |
4.7 |
4.5 |
5.2 |
NR |
21 |
0.0 |
|
|
17 |
Intravenous Diuretics |
1.0 |
9.6 |
2.2 |
NR |
24 |
0.2 |
Costanzo et al. [87] |
2016 |
110 |
Adjustable ultrafiltration |
10.7 |
6.0 |
12.9 |
48 |
NR |
0.13 |
|
|
114 |
Adjustable intravenous loop diuretics |
10.3 |
5.0 |
8.9 |
48 |
NR |
0.05 |
Study characteristics and outcomes: The online search and review of bibliographies yielded 2100 articles. After the application of the inclusion criteria to the full text of the studies, twelve studies published between 1975 and 2016 were included in the present meta-analysis. The studies included five studies [76-80] assessing treatment of RHF based on hemodynamic support using inotropes (dobutamine and/or milrinone) while the remaining seven studies [81-87] assessed the treatment of RHF based on volume optimization using diuretics (ultrafiltration or intravenous diuretics). Altogether, the twelve studies included 938 patients: 211 on hemodynamic support and 727 on volume optimization. Inotropes improve hemodynamic support by a mean increase of cardiac output by 1.65 liters/minute; increased mean heart rate (88.5 to 95.8 bpm); increased mean atrial pressure (87.6 to 91.6 mm Hg); reduced pulmonary wedge pressure (26.0-20.0 mm Hg); improved cardiac index (1.98-2.78); and improved myocardial oxygen consumption (13.4-14.4). Volume optimization using diuretics achieved a mean weight loss (5.78 kg); net fluid loss (5.65 l); and serum creatine (0.12 mg/dl) within 60 hours. Ultrafiltration achieved a better weight reduction (6.7 kg) than intravenous diuretics (4.9 kg). The mean length of stay in the hospital was 6.2 days
Discussion: The key therapeutic targets in the treatment of RHF are RV failure, underlying causes and heart failure [27]. The treatment of RV function is important to preserve coronary and pulmonary circulation [11,27]. The present meta-analysis assessed the clinical responses of the treatment of RV function to reduce volume overload using inotropes and extracorporeal venous ultrafiltration and/or the standard intravenous diuretic therapy. The findings reveal that inotropes exert a positive hemodynamics support. Hemodynamic improvement of >30% increase in cardiac output and > 25% or at least 4 mm Hg decrease in pulmonary wedge pressure (PWP) 24 hours after therapy [80]. Inotropes improved RV hemodynamics by increasing heart rate and atrial pressure, and reducing PWP ultimately causing an increase in cardiac output. Inotropes also improve cardiac index and myocardial oxygen consumption. These hemodynamic improvements were greatest within 25 hours after administration of inotropes strongly suggesting the effect of the drug action to improve RV congestion. The administration of standard intravenous diuretics or extracorporeal venous ultrafiltration optimizes RV volume by achieving mean reduction in body weight through fluid loss and causing a reduction in serum creatine concentration. However, extracorporeal venous ultrafiltration achieved better fluid loss and body weight loss compared to the conventional intravenous diuretics approach.
The therapeutic value of inotropes, fluid removal or direct diuretic effects in improving RV hemodynamic and relieving RV congestion RHF patients is consistent with findings in previous studies and reviews. In a recent meta-analysis on treatment options for reducing RV volume overload in patients with acute decompensated HF, Ebrahim et al. [88] reported inotropes and vasodilators have a short-term hemodynamic benefit in improving biventricular filling pressures through reducing right atrial pressures and pulmonary wedge pressures. Although the mechanism underlying biventricular improvement (reduction in filling pressures) after administration of inotropes is unclear, the RV might sensitive to increased pressure overload such that drug-induced improvement in the LV filling pressure could translate into improved RV congestion [89]. Inotropes such as milrinone that possess both vasodilation and inotropic effects obtain a greater effect on relieving RV congestion than dobutamine that has only an inotropic effect. However, the 2016 ESC guidelines on the management of HF caution on the use of milrinone because of the risk of precipitating systemic hypotension [11]. In chronic HF patients, vasodilation produces a short-term net increase in cardiac output but increases cardiac contractility (workload) leading to increased energy utilization (increased oxygen consumption without an increase in supply) causing ischemia and arrhythmias [89].
Volume optimization using fluid removal or diuretics is also efficacious in relieving volume overload and RV congestion. Consistent with these findings, diuretics have been reported to reduce excessive RV preload to reduce RV dilatation and free wall tension to minimize the risk of developing RV ischemia and optimizing contractility [90]. When RV failure occurs in the setting of increased RV afterload, fluid removal (decreased intravascular volume) cause volume redistribution in the venous system leading to a rapid clinical improvement in RV volume overload [11,27,91]. Although the use of extracorporeal venous ultrafiltration is moderately effective in treating volume overload by reducing weight it is not associated with better reduction in serum creatinine and overcall all-cause mortality. In selected patients, extracorporeal venous ultrafiltration may also cause improvement in fluid loss but its use should not be universally recommended over standard intravenous diuretic therapy because of the lack of supporting evidence supporting its beneficial outcomes and its higher cost [89].
Right heart failure (RHF) is a progressive cardiac syndrome resulting from abnormalities in cardiac structure or function that impair RV filling and/or decreases RV output. The most frequent clinical presentations include fluid retention (peripheral edema), low cardiac output syndrome (fatigue, dyspnea, light-headedness and syncope), and atrial or ventricular tachyarrhythmias. The most common cause is pulmonary hypertension mostly resulting from LV failure. Other causes are tricuspid valve pathology and pericardial diseases. The main pathophysiologic mechanisms include reduced cardiac contractility, RV volume overload and RV afterload. Clinical signs and symptoms include dyspnea, light-headedness, syncope, and chest discomfort. Diagnosis of RHF requires a high index of suspicion. Electrocardiogram and biomarkers such as BNP are helpful in providing diagnostic clues but are non-specific. Cardiac MRI is the gold standard for assessing RV systolic function but its high cost and lack of wide availability limits its use. Echocardiography is the most commonly used imaging modality for assessing qualitative (hypertrophy, sphericity and the degree of dilatation) and surrogate quantitative markers (fractional area change tricuspid annular plane systolic excursion, S’ velocity of the tricuspid annulus or RV index of myocardial performance). Treatment targets managing RV function (afterload, volume overload and contractility) and RV failure using volume optimization (diuretics and extracorporeal venous ultrafiltration) vasopressors, inotropes and mechanical circulatory support. Treatment could also target the underlying conditions such as pulmonary embolism, pulmonary hypertension and RV infarction.
- Harvey W (1928) On the motion of the heart and blood in animals? Frankfurt am Main, 1628.
- Haddad F, Hunt SA, Rosenthal DN, Murphy DJ (2008) Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 117: 1436-1448. [Crossref]
- Starr I, Jeffers WA, Meade RH (1943) The absence of conspicuous increments of venous pressure after severe damage to the right ventricle of the dog, with a discussion of the relation between clinical congestive failure and heart disease. Am Heart J 26: 291-301.
- Taquini AC (1963) The right ventricle: some aspects of its hemodynamic behavior. Perspect Biol Med 239-247.
- Lee FA (1992) Hemodynamics of the right ventricle in normal and disease states. Cardiol Clin 10: 59-67. [Crossref]
- Grognola J, Gines F (2006) Right ventricular dysfunction. In Cardiovascular Failure. Pathophysiological Bases and Management. 4th Ed. pp.35-91
- Anastasiadis K, Westaby S, Antonitsis P (2015) The failing right heart. New York: Springer International Publishing.
- Cohn JN, Guiha NH, Broder MI, Limas CJ (1974) Right ventricular infarction. Clinical and hemodynamic features. Am J Cardiol 33: 209-214. [Crossref]
- Furey SA 3rd, Zieske HA, Levy MN (1984) The essential function of the right ventricle. Am Heart J 107: 404-410. [Crossref]
- Hunt SA (2005) ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 46: e1-e82.
- Harjola VP, Mebazaa A, Celutkiene J, Bettex D, Bueno H (2016) Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur J Heart Fail 18: 226-241.
- Greyson CR (2008) Pathophysiology of right ventricular failure. Crit Care Med 36: S57-65. [Crossref]
- Ibrahim BS (2016) Right ventricular failure. E-Journal of Cardiology Practice 14
- Hassanein M, Abdelhamid M, Ibrahim B, Elshazly A, Aboleineen MW, et al. (2015) Clinical characteristics and management of hospitalized and ambulatory patients with heart failure-results from ESC heart failure long-term registry-Egyptian cohort. ESC Heart Fail 2: 159-167. [Crossref]
- Lee FA (1992) Hemodynamics of the right ventricle in normal and disease states. Cardiol Clin 10: 59-67. [Crossref]
- Fogel MA, Rychik J (1998) Right ventricular function in congenital heart disease: pressure and volume overload lesions. Prog. Cardiovasc. Dis 40: 343-356.
- Burgess MI, Mogulkoc N, Bright-Thomas RJ, Bishop P, Egan JJ, et al. (2002). Comparison of echocardiographic markers of right ventricular function in determining prognosis in chronic pulmonary disease. J Am Soc Echocardiogr 15: 633-639.
- McLaughlin VV1, Presberg KW, Doyle RL, Abman SH, McCrory DC, et al. (2004) Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest 126: 78-92. [Crossref]
- Nagel E, Stuber M, Hess OM (1996) Importance of the right ventricle in valvular heart disease. Eur Heart J 17: 829-836. [Crossref]
- Goldstein JA (2002) Pathophysiology and management of right heart ischemia. J Am Coll Cardiol 40: 841-853. [Crossref]
- Mehta SR, Eikelboom JW, Natarajan MK, Diaz R, Yi C, et al. (2001) Impact of right ventricular involvement on mortality and morbidity in patients with inferior myocardial infarction. J Am Coll Cardiol 37: 37-43. [Crossref]
- Goor DA, Lillehei CW (1975) Congenital malformations of the heart: embryology, anatomy, and operative considerations. Grune & Stratton.
- Dell'Italia LJ (1991) The right ventricle: anatomy, physiology, and clinical importance. Curr Probl Cardiol 16: 653-720. [Crossref]
- Reiser PJ, Portman MA, Ning XH, Moravec CS (2001) Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am J Physiol Heart Circ Physiol 280: H1814-H1820.
- Lowensohn HS, Khouri EM, Gregg DE, Pyle RL, Patterson RE (1976) Phasic right coronary artery blood flow in conscious dogs with normal and elevated right ventricular pressures. Circ Res 39: 760-766.
- Lahm T, McCaslin CA, Wozniak TC, Ghumman W, Fadl YY, et al. (2010) Medical and surgical treatment of acute right ventricular failure. J Am Coll Cardiol 56: 1435-1446. [Crossref]
- Piazza G, Goldhaber SZ (2005) The acutely decompensated right ventricle: pathways for diagnosis and management. CHEST Journal 128: 1836-1852.
- Jardin F, Brun-Ney D, Auvert B, Beauchet A, Bourdarias JP (1990) Sepsis-related cardiogenic shock. Crit Care Med 18: 1055-1060. [Crossref]
- Vieillard-Baron A, Prin S, Chergui K, Dubourg O, Jardin F (2003) Hemodynamic instability in sepsis: bedside assessment by Doppler echocardiography. Am J Respir Crit Care Med 168: 1270-1276.
- Vieillard-Baron A, Schmitt JM, Augarde R, Fellahi JL, Prin S et al (2001) Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis. Crit Care Med 29: 1551-1555.
- Jardin F, Gueret P, Dubourg O, Farcot J, Margairaz A, et al (1985) Two-dimensional echocardiographic evaluation of right ventricular size and contractility in acute respiratory failure. Crit Care Med 13: 952-956.
- Stobierska-Dzierzek B, Awad H, Michler RE (2001) The evolving management of acute right-sided heart failure in cardiac transplant recipients. J Am Coll Cardiol 38: 923-931.
- Jardin F, Vieillard-Baron A (2006) Right ventricular function and positive pressure ventilation in clinical practice: from hemodynamic subsets to respirator settings. In Applied Physiology in Intensive Care Medicine 207-215. Springer, Berlin, Heidelberg.
- Lualdi JC, Goldhaber SZ (1995) Right ventricular dysfunction after acute pulmonary embolism: pathophysiologic factors, detection, and therapeutic implications. Am Heart J 130: 1276-1282.
- Goldhaber SZ, Elliott CG (2003) Acute pulmonary embolism: part I: epidemiology, pathophysiology, and diagnosis. Circ 108: 2726-2729.
- Kevin LG, Barnard M (2007) Right ventricular failure. Continuing Education in Anesthesia. Critical Care & Pain 7: 89-94.
- Palevsky HI, Fishman AP (1990) Chronic cor pulmonale. Etiology and management. JAMA 263: 2347-2353. [Crossref]
- Sztrymf B, Souza R, Bertoletti L, Jais X, Sitbon O, et al. (2010). Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. Eur Respir J 35: 1286-1293.
- Kurzyna M, Zylkowska J, Fijalkowska A, Florczyk M, Wieteska M, et al. (2008) Original article Characteristics and prognosis of patients with decompensated right ventricular failure during the course of pulmonary hypertension. Polish Heart Journal 66: 1033-1039.
- Mebazaa A, Karpati P, Renaud E, Algotsson L (2004) Acute right ventricular failure--from pathophysiology to new treatments. Intensive Care Med 30: 185-196. [Crossref]
- Mayo PH, Beaulieu Y, Doelken P, Feller-Kopman D, Harrod C, et al. (2009) American College of Chest Physicians/La Société de Réanimation de Langue Française statement on competence in critical care ultrasonography. Chest 135: 1050-1060. [Crossref]
- Lancellotti, P, Price S, Edvardsen T, Cosyns B, Neskovic AN, et al. (2014) The use of echocardiography in acute cardiovascular care: recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur Heart J Cardiovasc Imaging 16: 119-146.
- Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, et al. (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography: endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23: 685-713.
- Jurcut R, Giusca S, La Gerche A, Vasile S, Ginghina C, et al. (2010) The echocardiographic assessment of the right ventricle: what to do in 2010? Eur J Echocardiogr 11: 81-96. [Crossref]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, et al. (2015). Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr, 28: 1-39.
- Green EM, Givertz MM (2012) Management of acute right ventricular failure in the intensive care unit. Curr Heart Fail Rep 9: 228-235. [Crossref]
- Ghignone M, Girling L, Prewitt RM (1984) Volume expansion versus norepinephrine in treatment of a low cardiac output complicating an acute increase in right ventricular afterload in dogs. Anesthesiology 60: 132-135.
- Morelli A, Teboul JL, Maggior, SM, Vieillard-Baron A, Rocco M, et al. (2006). Effects of levosimendan on right ventricular afterload in patients with acute respiratory distress syndrome: a pilot study?. Crit Care Med 34: 2287-2293.
- Surkova E, Peluso D, Kasprzak JD, Badano LP (2016). Use of novel echocardiographic techniques to assess right ventricular geometry and function. Polish Heart Journal 74: 507-522.
- Arnould MA1, Gougnot S, Lemoine S, Lemoine J, Aliot E, et al. (2009) Quantification of right ventricular function by 2D speckle imaging and three dimensional echography. Comparison with MRI. Ann Cardiol Angeiol (Paris) 58: 74-85. [Crossref]
- Pavlicek M, Wahl A, Rutz T, de Marchi SF, Hille R, et al. (2011) Right ventricular systolic function assessment: rank of echocardiographic methods vs. cardiac magnetic resonance imaging. Eur J Echocardiogr 12: 871-880.
- Sato T, Tsujino I, Ohira H, Oyama-Manabe N, Yamada A, et al. (2012). Validation study on the accuracy of echocardiographic measurements of right ventricular systolic function in pulmonary hypertension. J Am Soc Echocardiogr 25: 280-286.
- Yang T, Liang Y, Zhang Y, Gu,Q, Chen G, et al. (2013). Echocardiographic parameters in patients with pulmonary arterial hypertension: correlations with right ventricular ejection fraction derived from cardiac magnetic resonance and hemodynamics. PloS one 8: e71276.
- Focardi M, Cameli M, Carbone SF, Massoni A, De Vito R, et al. (2015) Traditional and innovative echocardiographic parameters for the analysis of right ventricular performance in comparison with cardiac magnetic resonance. Eur Heart J Cardiovasc Imaging 16: 47-52. [Crossref]
- Lemarie J, Huttin O, Girerd N, Mandry D, Juilliere Y, et al. (2015). Usefulness of speckle-tracking imaging for right ventricular assessment after acute myocardial infarction: a magnetic resonance imaging/echocardiographic comparison within the relation between aldosterone and cardiac remodeling after myocardial infarction study. J Am Soc Echocardiogr 28: 818-827.
- Zhou X, Liu S, Qian Z, Lee JC, Goswami R, et al. (2015). Comparison of conventional echocardiographic parameters of RV systolic function with cardiac magnetic resonance imaging. J Cardiovasc Magn Reson 17: M10.
- Spruijt OA, Di Pasqua MC, Bogaard HJ, van der Bruggen CE, Oosterveer F, et al. (2017) Serial assessment of right ventricular systolic function in patients with precapillary pulmonary hypertension using simple echocardiographic parameters: A comparison with cardiac magnetic resonance imaging. J Cardiol 69: 182-188. [Crossref]
- Kjaergaard J, Petersen CL, Kjaer A, Schaadt BK, Oh JK, et al. (2006) Evaluation of right ventricular volume and function by 2D and 3D echocardiography compared to MRI. Eur J Echocardiogr 7: 430-438.
- Kossaify A1 (2015) Echocardiographic Assessment of the Right Ventricle, from the Conventional Approach to Speckle Tracking and Three-Dimensional Imaging, and Insights into the "Right Way" to Explore the Forgotten Chamber. Clin Med Insights Cardiol 9: 65-75. [Crossref]
- Lee JZ, Low SW, Pasha AK, Howe CL, Lee KS, et al. (2018) Comparison of tricuspid annular plane systolic excursion with fractional area change for the evaluation of right ventricular systolic function: a meta-analysis. Open Heart 5: e000667. [Crossref]
- Maffessanti F, Gripari P, Tamborini G, Muratori M, Fusini L, et al. (2012) Evaluation of right ventricular systolic function after mitral valve repair: a two-dimensional Doppler, speckle-tracking, and three-dimensional echocardiographic study. J Am Soc Echocardiogr 25: 701-708.
- Tamborini G, Muratori M, Brusoni D, Celeste F, Maffessanti F, et al. (2009) Is right ventricular systolic function reduced after cardiac surgery? A two-and three-dimensional echocardiographic study. Eur J Echocardiogr 10: 630-634.
- Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti, L, et al. (2013). Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol 305: 1373-1381.
- Guzman-Sanchez CM, Pena-Huerta S, Harold G, Rocha-Munoz AD, Lazo-Monjaraz M (2017) Right ventricular ejection fraction obtained from TAPSE. Revista Mexicana de Cardiologia 28: 103-110.
- Goldhaber SZ (2000) The approach to massive pulmonary embolism. In Seminars in Respiratory and Critical Care Medicine 21: 541-548.
- McNeil K, Dunning J, Morrell NW (2003) The pulmonary physician in critical care 13: The pulmonary circulation and right ventricular failure in the ITU. Thorax 58: 157-162.
- Ducas J, Prewitt RM (1987) Pathophysiology and therapy of right ventricular dysfunction due to pulmonary embolism. Cardiovasc Clin 17: 191-202. [Crossref]
- Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, et al. (2014). 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Endorsed by the European Respiratory Society (ERS). Eur Heart J 35: 3033-3073.
- Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, et al. (2014) Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 370: 1402-1411. [Crossref]
- Sztrymf B, Prat D, Jacobs FM, Brivet FG, O'callaghan DS, et al (2013). Renal replacement therapy in patients with severe precapillary pulmonary hypertension with acute right heart failure. Respiration 85: 464-470.
- Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, et al. (2009) Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 30: 2493-2537.
- George I, Xydas S, Topkara VK, Ferdinando C, Barnwell EC, et al. (2006) Clinical indication for use and outcomes after inhaled nitric oxide therapy. Ann Thorac Surg 82: 2161-2169. [Crossref]
- Hoeper MM1, Granton J (2011) Intensive care unit management of patients with severe pulmonary hypertension and right heart failure. Am J Respir Crit Care Med 184: 1114-1124. [Crossref]
- Bowers TR, O'Neill WW, Grines C, Pica MC, Safian RD, Goldstein JA (1998). Effect of reperfusion on biventricular function and survival after right ventricular infarction. N Engl J Med 338: 933-940.
- Zehender M, Kasper W, Kauder E, Geibel A, Schonthaler M, Olschewski M, Just H (1994) Eligibility for and benefit of thrombolytic therapy in inferior myocardial infarction: focus on the prognostic importance of right ventricular infarction. J Am Coll Cardiol 24: 362-369.
- Akhtar N, Mikulic E, Cohn JN, Chaudhry MH (1975) Hemodynamic effect of dobutamine in patients with severe heart failure. Am J Cardiol 36: 202-205. [Crossref]
- Grose R, Strain J, Greenberg M, LeJemtel TH (1986) Systemic and coronary effects of intravenous milrinone and dobutamine in congestive heart failure. J Am Coll Cardiol 7: 1107-1113.
- Monrad ES, Baim DS, Smith HS, Lanoue AS (1986) Milrinone, dobutamine, and nitroprusside: comparative effects on hemodynamics and myocardial energetics in patients with severe congestive heart failure. Circ 73: 168-174.
- Seino Y, Momomura S, Takano T, Hayakawa H, Katoh K (1996) Multicenter, double-blind study of intravenous milrinone for patients with acute heart failure in Japan. Japan Intravenous Milrinone Investigators. Crit Care Med 24: 1490-1497. [Crossref]
- Follath F, Cleland JG, Just H, Papp JGY, Scholz H, et al. (2002). Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. The Lancet 360: 196-202.
- Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML., Bart BA, et al. (2007) Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 49: 675-683.
- Libetta C, Sepe V, Zucchi M, Pisacco P, Cosmai L, et al. (2007) Intermittent haemodiafiltration in refractory congestive heart failure: BNP and balance of inflammatory cytokines. Nephrol Dial Transplant 22: 2013-2019.
- Bartone C, Saghir S, Menon SG, Brosmer J, Kereiakes DJ, et al. (2008) Comparison of ultrafiltration, nesiritide, and usual care in acute decompensated heart failure. Congest Heart Fail 14: 298-301. [Crossref]
- Rogers HL, Marshall J, Bock J, Dowling TC, Feller E, et al. (2008) A randomized, controlled trial of the renal effects of ultrafiltration as compared to furosemide in patients with acute decompensated heart failure. J Card Fail 14: 1-5. [Crossref]
- Bart BA, Goldsmith SR, Lee KL, Givertz MM, O'Connor CM, et al. (2012) Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 367: 2296-2304. [Crossref]
- Hanna MA, Tang WH, Teo BW, O'Neill JO, Weinstein DM, et al. (2012) Extracorporeal ultrafiltration vs. conventional diuretic therapy in advanced decompensated heart failure. Congest Heart Fail 18: 54-63. [Crossref]
- Costanzo MR, Negoianu D, Jaski BE, Bart BA, Heywood JT, et al. (2016) Aquapheresis Versus Intravenous Diuretics and Hospitalizations for Heart Failure. JACC Heart Fail 4: 95-105. [Crossref]
- Ebrahim B, Sindhura K, Okoroh J, Sethi R, Hulten E, et al (2015). Meta-analysis of ultrafiltration versus diuretics treatment option for overload volume reduction in patients with acute decompensated heart failure. Arquivos brasileiros de cardiologia 104: 417-425.
- Mebazaa A, Karpati P, Renaud E, Algotsson L (2004) Acute right ventricular failure--from pathophysiology to new treatments. Intensive Care Med 30: 185-196. [Crossref]
- Mehmood M, Frank TA (2016) Treatment of right heart failure: is there a solution to the problem? European Society of Cardiology 14: 1-10.
- Ventetuolo CE, Klinger JR (2014) Management of acute right ventricular failure in the intensive care unit. Ann Am Thorac Soc 11: 811-822. [Crossref]


