Ethnopharmacological relevance: Diabetes is a serious metabolic disease which imposes a heavy burden on the society. It may also bring about a variety of complications if the blood glucose level is not well controlled. Yuye Decoction (YYD) is an ancient herbal medicinal formulation of China and has been widely used in Traditional Chinese medicine to treat patients with diabetes for thousands of years. There are seven medicinal herbs in YYD.
Aim of the study: The aim of the present review is to summarize and critically appraise data concerning medicinal plants used in YYD, its main active constituents and signaling pathways mediating its therapeutic effects on diabetes and diabetic complications.
Materials and methods: The search of papers published in the period 2009 to 2019 and recorded in PubMed was conducted using specific search terms.
Results: After screening, 88 studies were included. Among seven medicinal herbs in YYD formulation, six of them exhibited therapeutic effects on diabetes and its complications through different signaling pathways. Most (55.7%) of the studies were animal studies. Type 2 diabetes was studied in most (37.5%) of the research papers and diabetic nephropathy was the most (19.3%) studied diabetic complication. Focus was placed on Astragalus membranaceus (Fisch.) Bge. and Pueraria lobata (Willd.) Ohwi in the largest number of research papers.
Conclusion: YYD exerted a therapeutic effect on diabetes and a preventive effect on diabetic complications.
traditional chinese medicine, diabetes, phytochemical, mechanism, medicinal plants
ACC: Acetyl CoA Carboxylase; pNF-κB: Activated Phosphorylated Derivative; Acrp30: Adiponectin; (ATP)-binding: Adenosine Triphosphate; AGEs: Advanced Glycation End Products; UA1b: Albuminuria; PGC-1α: Alpha Susbunit of Peroxisome Proliferators-Activated Receptor-Gamma Coactivator-1; AMPK: AMP-Activated Protein Kinase; Arg-1: arginase-1; APS: Astragalus polysaccharides; ABCB11: ATP-binding cassette (transporter) B11; Bas: bile acids; BBB: Blood-Brain Barrier; BUN: blood urea nitrogen; BW: Body Weight; CAT: catalase; JNK: Jun N-terminal kinases; Col IV: CrCl: Collagen Type IV Creatinine Clearance; DCs: Dendritic Cells; DCM: Diabetic cardiomyopathy; DCI: Diabetic Cognitive Impairment; DPN: Diabetic Peripheral Neuropathy; DM: Diabetes (not specific); DN: Diabetic Nephropathy; DO: Diabetic Ophthalmopathy; DR: Diabetic Retinopathy; DVC: Diabetic Vascular Complications; EBPs: Enhancer Binding Protein; eNOS: Endothelial NOS Endothelial Nitric Oxide Synthase; ESRD: End Stage Renal Disease: FBG: Fasting Blood Glucose: FABP: Fatty Acid Binding Protein; F/B ratio: Firmicutes to Bacteroidetes Ratio; FN: Fibronectin; FOXO1: Forkhead Box Protein O1; GIN: glutamine; GSH: Glutathione; GIR: Glucose Infusion Rate; GLUT2: Glucose Transporter 2; GLUT4: Glucose Transporter 4; GSH: Glutathione; GPx: Glutathione Peroxidase; HbA1C: Hemoglobin; HGF: Hepatocyte Growth Factor; HMGB1: High Mobility Group Box 1; HOMA-IR: Homeostatic Model Assessment of Insulin Resistance; HUVECs: Human Umbilical Vein Endothelial Cells; HAT: hydroxyacetone; H2O2: Hydrogen Peroxide; IKKβ: IκB kinase β; INOs: Inducible Nitric Oxide Synthase; IGF: Insulin-like Growth Factor-1; IRS: Insulin Receptor Substrate (IRS); IL-1β: Interleukin-1β; IL-6: Interleukin-6; IL-10: Interleukin-10; IR: Insulin Resistance; IRS-1: Insulin Receptor Substrate-1; Ile: Isoleucine; MDA: Malondialdehyde; MnSOD: Manganese Superoxide Dismutase; MEKC: Micellar Electrokinetic Chromatography; MetS: Prediabetes & Metabolic Syndrome; mMVECs: Mouse Vascular Endothelial Cell; NO: nitric oxide; NF-κB: Nuclear-Factor Kappaβ; 2-NBDG: 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl) Amino)-2-Deoxyglucose; PPARa: Peroxisome Proliferators-Activated Receptor a; pInsR: Phospho-Insulin Receptor; PI3K: Phosphatidylinositol 3-kinase; NPCs: Primary Mouse Nonparenchymal Cells; PPAR: Proliferator-Activated Receptor γ; PCNA: Proliferating Cell Nuclear Antigen; PUE: Puerarin; ROS: Reactive Oxygen Species; Tregs: Regulatory T cells; sCr: Serum Creatinine; FPG: Serum Fasting Plasma Glucose; SUA: Serum Uric Acid; SIRT1: Silent Information Regulator 1; sICAM-1: Soluble Intercellular Cell Adhesion Molecule-1; SOD: Superoxide Dismutase; STZ: streptozotocin; TXNIP: Thioredoxin-Interacting Protein; TG: Triglyceride; TC: Total Cholesterol; TNF-α: Tumor Necrosis Factor-α; T1DM: Type 1 Diabetes; T2DM: Type 2 diabetes; UAE: Urinary Albumin Excretion; ACR: Urinary Albumin/Creatinine Ratio; UDP: Uridine Diphosphate; s-VCAM-1: vascular cell adhesion molecule-1; VEGF: Vascular endothelial growth factor; VAT: visceral adipose tissue.
Diabetes is a group of metabolic diseases characterized by hyperglycemia due to insulin resistance, absolute insulin deficiency and/or abnormal insulin secretion [1]. According to the WHO, more than 171 million people worldwide suffer from diabetes and the number of diabetic patients keeps escalating [2].
Diabetes may lead to a series of complications such as blindness, stroke, renal failure, nerve damage and limb amputation [3]. Persistent hyperglycemia brings about chronic damage to various tissues in the heart, eyes, kidneys and blood vessels and causes dysfunctions of these organs. These complications, including nephropathy, retinopathy, neuropathy, cardiomyopathy and cognitive impairment, are major causes of morbidity and mortality in diabetic patients. Consequently, diabetes has become a serious social health problem [4]. According to WHO, diabetes is expected to become the 7th leading cause of death in 2030 globally [5].
However, the current treatment of diabetes with western medicine still leaves much to be desired. There is a paucity of information available on effective treatment options for diabetic patients. Thus, the perspective of achieving good long-term metabolic control in diabetes is of central importance.6 Traditional Chinese medicine has a long history in treating diabetes and is widely popular in China. Many hospitals in China use traditional medicinal plants or a combination of western medicine with Chinese medicine to treat diabetes.
Yuye Decoction (YYD) is an ancient formulation and was first recorded in the book of Chinese medicine“yixue zhongzhong canxi lu (醫學衷中參西錄)” written by Zhang XiChun in 1909. It is widely used in Traditional Chinese medicine to treat diabetes. There are seven medicinal herbs that compose YYD, including Dioscorea opposite Thunb. (RD), Astragalus membranaceus (Fisch.) Bge. (AM), Anemarrhena asphodgfoides Bge. (RA), Schisandra chinensis (Turcz.) Baill. (SC), Trichosanthes kirilowii Maxim. (TK), Gallus gallus domesticus Brisson (GGD), and Pueraria lobata (Willd.) Ohwi (PL) (Table 1). In traditional Chinese medicine theory, YYD is beneficial to kidney function and improves the amount of body fluid. Thus, it can be used for relieving the symptoms of diabetes mellitus.
Table 1. Basic information of Yuye decotion
Medicinal herbs |
family |
Full scientific name |
Medicinal part |
Weight of herbs in ancient medica (qian)/equivalent g) |
Ratio |
Dioscorea opposite Thunb. |
Dioscoreaceae |
Dioscoreae Oppositae Rhizoma |
Rhizome |
10/30 |
20 |
Astragalus membranaceus (Fisch.) Bge. |
Fabaceae |
Astragali Membranacei Radix |
Root |
5/15 |
10 |
Anemarrhena asphodgfoides Bge. |
Asparagaceae |
Anemarrhenae Rhizoma |
Root |
6/18 |
12 |
Schisandra chinensis (Turcz.) Baill. |
Schisandraceae |
Schisandrae chinensis Fructus (SCF) |
Fruit |
3/9 |
6 |
Trichosanthes kirilowii Maxim. |
Cucurbitaceae |
Trichosanthis kirlowii Radix |
Root |
3/9 |
6 |
Gallus gallus domesticus Brisson |
Phasianidae |
Galli Gigerii Endothelium Corneum |
Corneal endothelium |
2/6 |
4 |
Pueraria lobata (Willd.) Ohwi |
Fabaceae |
Puerariae lobatae Radix |
Root |
1.5/4.5 |
3 |
However, as medicinal herbs are usually mixed with other herbs and are seldom used alone in traditional Chinese medicine, it is hard to evaluate the effect of individual herbs on treating diabetes and its complications. Therefore, the aim of this review is to provide a comprehensive coverage of the individual herbs of Yuye Decoction regarding their active components and functional mechanisms for treating diabetes and its complications. It also provides an overview for researchers who intend to perform randomized control trials on YYD in the future.
The search was done by using the specific search terms listed in Table 2 to gather information in PubMed regarding the use of YYD and its individual components in the treatment of diabetes respectively. After a preliminary search, articles related to YYD and its component herbs and published from 2009 to 2019 were screened. Articles whose topics matched diabetes and its complications were included. The basic information for each article such as country, experimental design (human, animal, cell based, chemical test) and results were extracted. Irrelevant or repeated articles were excluded.
Table 2. Strings used in search
Formula or medicinal herbs |
strings |
Yuye decoction (YYD) |
yu ye tang OR yuyetang OR Yuye decotion |
Dioscorea opposita Thunb. (RD) |
shan yao OR shanyao OR Rhizoma Dioscoreae |
Astragalus membranaceus (Fisch.) Bge. (AM) |
huang qí OR huangqí OR Astragalus membranaceus |
Anemarrhena asphodgfoides Bge. (RA) |
zhi mu OR zhimu OR rhizoma anemarrhenae |
Schisandra chinensis (Turcz.) Baill. (SC) |
wu wei zi OR wuweizi OR Schisandra chinensis |
Trichosanthes kirilowii Maxim. (TK) |
tian hua fen OR tianhuafen OR Trichosanthes |
Gallus gallus domesticus Brisson (GGD) |
ji nei jin OR jineijin OR Gallus gallus domesticus |
Pueraria lobata (Willd.) Ohwi (PL) |
Ge gen OR gegen OR Pueraria lobata |
After preliminary screening, clinical trials and mechanistic studies were analyzed respectively. For all human studies, the details of the study were extracted. Mechanistic studies were divided into animal studies, cell-based studies, animals & cell-based studies and chemical tests. These studies were then further grouped into different categories such as diabetes (not specific), insulin and metabolic syndrome; Type 1 diabetes; Type 2 diabetes; diabetic ophthalmopathy; diabetic nephropathy; diabetic retinopathy; diabetic cardiomyopathy; diabetic vascular complications; diabetic peripheral neuropathy; and diabetic cognitive impairment.
Based on the key word search described in Table 2, 2978 articles were found in the PubMed database. Finally, 88 articles were included in our review after comprehensive screening. All 88 included studies came from Asia. Among them, most (73.8%) of the papers were from China (Tables 3 and 4).
Table 3. Results of literature search
Formula or medicinal herbs |
Total |
Not relevant |
Animals studies |
Cell-based studies |
Chemical studies |
Animals & cell-based studies |
RCT |
Cohort studies |
Animals & cohort studies |
Included |
Yuye decoction |
2 |
1 |
- |
- |
1 |
- |
- |
- |
- |
1 |
Dioscorea opposita Thunb. |
590 |
577 |
9 |
2 |
1 |
1 |
- |
- |
- |
13 |
Astragalus membranaceus (Fisch.) Bge. |
714 |
687 |
13 |
4 |
2 |
6 |
1 |
1 |
- |
27 |
Anemarrhena asphodgfoides Bge. |
118 |
112 |
3 |
1 |
1 |
1 |
- |
- |
- |
6 |
Schisandra chinensis (Turcz.) Baill. |
767 |
756 |
6 |
1 |
- |
4 |
- |
- |
- |
11 |
Trichosanthes kirilowii Maxim. |
171 |
167 |
1 |
- |
- |
1 |
- |
- |
1 |
3 |
Gallus gallus domesticus Brisson |
12 |
12 |
- |
- |
- |
- |
- |
- |
- |
0 |
Pueraria lobata (Willd.) Ohwi |
604 |
577 |
17 |
4 |
1 |
5 |
- |
- |
- |
27 |
Total |
2978 |
|
|
|
|
|
|
|
|
88 |
Table 4. Number of papers by countries
Country |
Number of papers |
China |
62 |
South Korea |
7 |
Taiwan |
9 |
Japan |
3 |
India |
5 |
Hong Kong |
2 |
Animal studies: Forty-nine animal studies investigated the effects of YYD on diabetes and its complications (Tables 5-7).
Table 5. Number of papers related to diabetic or its complications (DM: Diabetes (not specific); T1DM: Type 1 diabetes; T2DM: Type 2 diabetes; DO: Diabetic ophthalmopathy; DN: diabetic nephropathy; DR: diabetic retinopathy; DCM: diabetic cardiomyopathy; DVC: diabetic vascular complications; IR: insulin resistance; DPN: diabetic peripheral neuropathy; MetS: prediabetes & metabolic syndrome; DCI: diabetic cognitive impairment)
Formula or medicinal herbs |
DM |
T1DM |
T2DM |
DR |
DN |
DO |
DCM |
DVC |
IR |
DPN |
MetS |
DCI |
Total |
Yuye decoction |
1 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
1 |
Dioscorea opposita Thunb. |
2 |
3 |
6 |
- |
- |
- |
- |
1 |
1 |
- |
- |
- |
13 |
Astragalus membranaceus (Fisch.) Bge. |
2 |
- |
8 |
2 |
6 |
- |
4 |
- |
- |
- |
3 |
2 |
27 |
Anemarrhena asphodgfoides Bge. |
- |
- |
2 |
- |
1 |
1 |
- |
- |
- |
- |
- |
2 |
6 |
Schisandra chinensis (Turcz.) Baill. |
- |
- |
8 |
- |
2 |
- |
1 |
- |
- |
- |
- |
- |
11 |
Trichosanthes kirilowii Maxim. |
1 |
- |
- |
- |
2 |
- |
- |
- |
- |
- |
- |
- |
3 |
Gallus gallus domesticus Brisson |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
0 |
Pueraria lobata (Willd.) Ohwi |
1 |
2 |
9 |
- |
6 |
1 |
4 |
1 |
2 |
1 |
- |
- |
27 |
Total |
7 |
5 |
33 |
2 |
17 |
2 |
9 |
2 |
3 |
1 |
3 |
4 |
88 |
Table 6. Different studies related to diabetic or its complications
Studies Type |
DM |
T1DM |
T2DM |
DR |
DN |
DO |
DCM |
DVC |
IR |
DPN |
MetS |
DCI |
|
Animal studies |
1 |
5 |
16 |
1 |
14 |
2 |
4 |
- |
1 |
- |
2 |
3 |
49 |
Cell-based studies. |
1 |
- |
3 |
1 |
1 |
- |
3 |
- |
1 |
1 |
- |
1 |
12 |
Animals & cell-based studies |
1 |
- |
11 |
- |
1 |
- |
2 |
2 |
1 |
- |
- |
- |
18 |
Animals & cohort studies |
1 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
1 |
Chemical studies |
3 |
- |
2 |
- |
1 |
- |
- |
- |
- |
- |
- |
- |
6 |
RCT |
- |
- |
1 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
1 |
Case-control studies |
1 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
1 |
Total |
8 |
5 |
33 |
2 |
17 |
2 |
9 |
2 |
3 |
1 |
2 |
4 |
88 |
Table 7. Applications of herbal TCM and/or monomers in diabetes and its complications
Animal studies |
Astragalus membranaceus (Fisch.) Bunge |
Extract |
Topic |
Duration |
Model |
Pathways |
Results |
NIL (Jian, W, et al. 2016) |
Diabetic Retinopathy |
24 weeks |
STZ rats |
Through regulating multiple factors involved in the DR pathological pathway. |
attenuating the increase in erythrocyte aggregation, plasma viscosity, and acellular vessel and pericyte loss; reversing the hyper-activation of AR, the hyper-expression of VEGF, ICAM-1, and ET-1, and the hypo-expression of PEDF and occludin in the retinas of STZ-treated rats |
Polysaccharide AERP
(Liu, Y, et al. 2019) |
Cognitive Dysfunction |
10 weeks |
db/db mice |
Through altering the gut microbiota |
Alleviating the hyperglycemia, tissue impairment; inhibiting cognitive impairment; Modulating the composition of metabolites like SCFAs |
Refined-JQ (JQ-R) (Li-hui Gao, et al. 2014) |
Prediabetes |
4 months |
HFD-C57 mice |
Through activating the AMPK signaling pathway |
Reducing BW, TC, HOMA-IR; Enhancing the glucose tolerance; Improving insulin response; Activating liver glycogen syntheses; Improving GIR; Increasing the levels of phosphorylated AMPKαand phosphorylated ACC. |
Astragaloside (Wang, Y, et al. 2015) |
Diabetic Nephropathy |
10 weeks |
KKAy mice |
Through inhibiting TGF-β/SMADS signaling pathway. |
Down-regulating TGF-β1, SMAD2/3, α-SMA expression; alleviating renal interstitial fibrosis. |
Astragaloside IV (Gui, D, et al. 2013) |
Diabetic Nephropathy |
8 weeks |
STZ rats |
Through inhibiting NF-κβ mediated inflammatory genes expression |
Ameliorating UA1b, renal histopathology and podocyte foot process effacement; decreasing the serum levels of TNF-a, MCP-1 and ICAM-1; decreasing a1-chain type IV collagen mRNA. |
Astragaloside IV (Wang, Z. S, et al. 2015) |
Diabetic Nephropathy |
8 weeks |
STZ rats |
Through decreasing ER stress |
Reducing UAE, sCr, BUN; preventing the mesangial matrix expansion and increase in mean mesangial induced by STZ; Preventing the phosphorylation of eIF2α, PERK and JNK; Inhibiting the expression of GRP78 and ORP150; Inhibiting the TM-induced apoptosis of podocytes, concomitant with decreased CHOP expression and cleaved caspase-3. |
Astragaloside IV (Zhang, N. et al. 2011) |
Metabolic Syndrome |
3 weeks |
fructose-fed rats |
Through the NO/cGMP pathway |
Reducing blood pressure and TG levels; Improving glucose tolerance and endothelium-dependent vasorelaxation. |
NIL (Ruonan Zhai, et al. 2019) |
Diabetic Nephrology |
12 weeks |
STZ rats |
Through inhibiting oxidative stress |
Upregulating nephrin, α-dystroglycan, Bcl-xl; Downregulating Bax and Nox4; Ameliorating diabetic podocyte injury. |
Astragalus polysaccharide (Liu, M, et al.2010) |
T2DM |
8 weeks |
KKAy mice |
Through regulating insulin signaling in insulin-resistant skeletal muscle |
Ameliorating hyperglycemia and IR; Restoring insulin-induced protein kinase B Ser-473 phosphorylation and glucose transporter 4 translocation in skeletal muscle. |
Astragalus polysaccharide (Chen, W, et al.2009) |
Diabetic Cardiomyopathy |
10 weeks |
STZ hamsters |
Through suppressing the local cardiac chymase–Ang II system |
Ameliorating myocardial collagen deposition via suppression of chymase–MMP activation; Lowering levels of myocardial collagen type I and ratio of collagen type I/III; Suppressing cardiac MMP-2 and ProMMP-2 activities; Inhibiting heart chymase activation. |
Astragalus polysaccharide (Gu et al. 2016) |
Diabetic Memory Impairment |
8 weeks |
STZ rats |
Through glucose and lipid metabolism. |
Decreasing FPG, HbA1c, and insulin levels; Reversing memory impairment in the diabetic model; Lowering hippocampal MDA concentration. |
NIL
(Chen et al. 2017) |
T2DM |
100 days |
STZ rats |
Through improving βcell function and reducing insulin resistance. |
Ameliorating impairments in glucose tolerance & insulin release function; Reducing serum lipid levels; Decreasing the expression of NO and MDA ; Increasing the expression of SOD and GSH-px; Restoring the impaired insulin signaling; Upregulating pInsR expression, IRS tyrosine phosphorylation, PI3K, GLUT4 expression; Downregulated the serine phosphorylation of IRS. |
Astragalus polysaccharides (Chen et al. 2018) |
Diabetic Cardiomyopathy |
15 weeks |
db/db diabetic mice |
Through the cardiac PPARa-mediated regulatory pathways. |
Improving the myocyte TG accumulation & cardiac dysfunction; Normalizating energy metabolic derangements in diabetic hearts; Repressing the activation of PPARa target genes involved in myocardial fatty acid uptake and oxidation in diabetic hearts; Reversing PPARa mediated suppression of genes involved in glucose utilization of diabetic hearts. |
Trichosanthes kirilowii |
Trichosanthes kirilowii lectin (Jiandong et al. 2019) |
Diabetic Nephropathy |
8 weeks |
STZ rat |
Through inhibiting Notch signaling. |
Attenuating STZ induced damages in renal function and structure; Increasing TNF-α & iNOS production; Suppressing IL-10 & Arg-1 production; Inhibiting induced inflammation by STZ; Blocking the polarization of macrophage into M1 type; Suppressing expression of Notch1, NICD1, Hes1. |
Schisandra chinensis (Turcz.) Baill. |
NIL (Hong et al. 2018) |
T2DM |
10 weeks |
db/db mice |
Through suppressing lipid synthesis, oxidative stress, inflammation. |
Decreasing plasma and hepatic TG & TC concentrations; Downregulating Hepatic expressions for fatty acid & TC synthesis; Upregulating beta-oxidation & TC export. Improving glucose tolerance; Increasing expression levels of antioxidative enzymes; Decreasing inflammatory cytokines, oxidative stress, leptin, insulin levels. |
Acidic polysaccharide (Du et al., 2019) |
T2DM |
8 weeks |
STZ rats |
Through protecting against β-cells apoptosis. |
Decreasing FBG, TG, TC, LDL-C, MDA levels; Increasing insulin, HDL-C levels and SOD activity; Improving the pathological changes in
pancreatic islet; Inhibiting the up-regulation of phosphorylated JNK, BAX and Cleaved Caspase-3 proteins; Increasing Bcl-2 protein expression. |
Ethanol extracts (Zhang et al. 2012) |
Diabetic Nephropathy |
9 weeks |
STZ rats |
Through inhibiting the epithelial to mesenchymal transdifferentiation. |
Lessening degree of fibrosis; Lowering the expressions of FN, α-SMA and PAI-1; Inhibiting the endothelial-myofibroblast transition
|
Schisandrae chinensis oil (An et al.2015) |
T2DM |
8 weeks |
STZ rats |
Through improving pancreatic β-cell function |
Decreasing FBG, TC, TG levels, pancreatic MDA; Increasing SOD & CAT activities; Enhancing protein expression of Bcl-2, PDX-1, GLUT-2, GCK; Upregulating expression of anti-apoptotic genes; Increasing expression of glucose metabolism; Delaying islet cell apoptosis. |
Schisandra chinensis fruit extract (Zhang et al. 2012) |
diabetic nephropathy |
7 weeks |
STZ rats |
Through preserving podocyte integrity by suppressing EMT. |
Decreasing UAE & ACR; Attenuating glomerulosclerosis and protected against podocyte loss and integrity of the slit diaphragm; Preventing the EMT of podocytes. |
A water-soluble polysaccharide
(Niu et al .2017) |
T2DM |
28 days |
STZ rats |
Through antioxidant effect |
Scavenging effect on superoxide anion free radical, hydroxyl radical, DPPH free radical; Increasing body weight; Improving the glucose tolerance; Reducing FBG; Elevating levels of FINS and value of ISI; Reducing the MDA content; Increasing GSHPX, CAT, SOD activities. |
Dioscorea opposita Thunb |
Yam dioscorin, dipeptide NW (Wu et al. 2018) |
T2DM |
135-day |
C57BL/6J mice |
Through impaired glucose tolerance controls |
Lowering TC & low-density lipoprotein; Lowering TG contents; Reducing total visceral lipid contents; Lowering blood glucose levels. |
Diosgenin (Sato et al., 2014) |
T1DM |
2 days |
STZ rats |
Through activating the muscular GLUT4 signaling pathway |
Increasing Serum DHEA level; Decreasing blood glucose level; Increasing GLUT4 translocation and Akt & PKC phosphorylation; Correlations were observed between blood glucose level, GLUT4 translocation level and muscular sex steroid hormone level 150 min after the administrations. |
NIL (Zhi-Hong et al. 2014) |
T2DM |
8 weeks |
STZ rats |
Through inhibiting polyol pathway |
Decreasing blood glucose, insulin levels, adipose tissue weight; Improving glucose tolerance; Lowering plasma TG, TC, liver TG levels; Inhibiting the activity of AR; Restoring adiponectin expression in serum. |
Allantoin (Go, H. K. et al.2015) |
T1DM |
31 days |
STZ rats |
Through modulating oxidative stress. |
Decreasing blood glucose; Decreasing HbAlc, TC, low-density lipoprotein; Increasing insulin, GLP-1, C-peptide; Ameliorating antioxidant stress; Decreasing MDA; Increasing SOD; Reducing GSH |
DOTP-80
(Fan, Y., et al. 2015) |
Diabetes |
18 days |
alloxan-induced mice |
Through preventing oxidative damage of pancreatic β-cell. |
Stimulating an increase in glucose disposal; Had strong hypoglycemic activity; Increasing the levels of antioxidant enzymes (SOD) activity |
Ethanol extract
(Cheng, Q, et al. 2015) |
T2DM |
10 weeks |
CD-1 (ICR) mice |
Through improving glucose and lipid metabolism. |
Improving glucose intolerance & normalize lipid profile; Increasing peripheral and hepatic insulin sensitivity; Decreasing serum free fatty acid level; Enhancing hepatic glucokinase activity & glycogen content; Improving serum antioxidant activity; Decreasing fatty deposits in the liver of mice. |
Allantoin
(Niu, C. S, et al. 2010) |
T1DM |
3 days |
STZ rats |
Through increasing β-endorphin secretion from the adrenal gland. |
Decreasing plasma glucose levels in a dose related manner; Enhancing β-endorphin release from the isolated adrenal medulla of STZ-diabetic rat in a dose-related manner; Increasing radioactive glucose uptake in isolated skeletal muscle; Increasing GLUT4 mRNA & protein levels in muscle. |
DB extract
(Kim, S. et al. 2012) |
Early-stage obesity-induced insulin resistance |
7 weeks |
HFD mice |
Through activating the insulin signaling cascade leading to GLUT4 translocation |
Reversing HFD-induced elevations in plasma glucose & insulin levels, HOMA-IR and oral glucose tolerance test values. Up-regulating the level of p-AKT protein; Down-regulating the levels of p-ERK and p-S6K1 proteins in the adipose tissues; Reversing the HFD-induced decrease in the plasma membrane GLUT4 level; Improving glucose metabolism. |
Dioscorea opposita Thunb polysaccharide-zinc (Zhang, Y, et al. 2018) |
T2DM |
42 days |
STZ rats |
Through ameliorating lipid levels and oxidative stress. |
Decreasing the glucose and insulin levels; Reducing MDA contents; increasing SOD and T-AOC activities significantly in liver; Decreasing the levels of TCHO, TG and LDL-C in serum; Increasing HDL-C level. |
Rhizoma Anemarrhenae |
Sarsasapogenin
(Yao‐Wu Liu, et al. 2018) |
Diabetic nephropathy |
9 weeks |
STZ rats |
Through inhibiting NLRP3 inflammasome activation and AGEs-RAGE interaction |
Ameliorating renal dysfunction; Decreasing UA1b, kidney weight index, SUA, FN, Col IV levels. Decreasing IL‐18, NLRP3, activated caspase 1 levels, AGEs, RAGE levels in the renal cortex of diabetic rats. |
Ethanol extract
(Xuan Li, et al. 2013)) |
Diabetic ophthalmopathy |
12 weeks |
STZ rats |
Through Inhibiting AGE accumulation, polyol pathway activation and ROS overproduction. |
Increasing activities of SOD and GSH-Px in serum; Decreasing MDA, AGE levels in serum and sorbitol concentration in the lens in ERA-treated DO rats; Decreasing E/P ratio; Alleviating pathological changes of lens & retina; Ameliorating subnormal growth of pericytes induced by high glucose. |
Total saponins from Rhizoma Anemarrhenae (Liu, Y. W, et al. 2012) |
Diabetes-associated cognitive decline |
7 weeks |
STZ rats |
Through a sum of reduction of Aβ accumulation and inflammation in brain |
Increasing A(1–40), A(1–42), TNF- levels in temporal cortex and hippocampus of diabetic rats with cognition impairment; Improving the learning ability of diabetic rats; Reducing A(1–40), A(1–42) and TNF- levels in cortex as well as A(1–40) level in hippocampus; Inhibiting the elevation of TNF- level in serum; Decreasing FBG; increasing the body weight. |
Pueraria lobata (Willd.) |
PTY-2 extract
(Tripathi, Y. B, et al. 2017) |
Diabetic Nephropathy |
20 days |
STZ rats |
Through degrading the ECM accumulated in kidney tissue |
Lowering FBG, serum urea, sCr, UA1b levels; Increasing CrCl; Decreasing glomeruli tubular necrosis; Decreasing basement membrane thickening and less ECM deposition; Reducing Mmp-9 activity and expression. |
Puerarin
(Wu, K, et al. 2013) |
T1DM |
14 days |
STZ rats |
Through elevating insulin expression and maintaining metabolic homoeostasis. |
Reducing glycemia; Increasing serum insulin concentration; Improving dyslipidemia; Alleviating the STZ-lesioned pancreas tissue; Up-regulating intrapancreatic protein levels of IRS-1 & IGF-1; Increasing endogenous mRNA levels of skeletal muscle insulin receptor (InsR) and PPARa. |
PTY-2r
(Rashmi, S, et al. 2018) |
Diabetic Nephropathy |
20 days |
STZ rats |
Through suppressing oxidative stress and apoptosis. |
Raising the activity of antioxidant enzymes; Suppressing oxidative stress and apoptosis; Preventing urinary albumin excretion in a
dose-dependent manner. |
PTY-2r
(Shukla, R, et al. 2017) |
Diabetic Nephropathy |
20 days |
STZ rats |
Through inhibiting the expression of HIF-1a and VEGF. |
Decreasing Blood glucose, urine protein, sCr, and urea level; Decreasing the expression of HIF-1a & VEGF; Increasing the expression of nephrin in a dose-dependent manner. |
Puerarin
(Chen, X. F, et al. 2018) |
T2DM |
4 weeks |
STZ rats |
Through preventing the accumulation of intramyocellular lipids. |
Alleviated dyslipidemia; Decreased the accumulation of intramyocellular lipids by upregulating the expression of a range of genes involved in mitochondrial biogenesis, oxidative phosphorylation, detoxification of ROS, oxidation of fatty acids in the muscle of diabetic rats; Decreasing the trafficking of fatty acid translocase/CD36 to the plasma membrane to reduce the uptake of fatty acids by myocytes. |
NIL
(Gao, K, et al. 2018) |
T2DM |
8 weeks |
STZ rats |
Through altering features of the metabolite profiles and the gut microbiota. |
Modulating blood glycemic level; Enriching Bacteroidetes; Acting through TP53, AKT1, PPARA proteins. |
Flos Puerariae Extract
(Yu, W, et al.2014) |
Diabetic Cardiomyopathy |
10 weeks |
STZ rats |
Through inhibiting JNK and P38 MAPK signaling pathway. |
Normalizing glucose and weight profile; Preserving myocardial structure; Reducing apoptotic cardiac cell death; Reversing elevated markers of oxidative stress; Inhibiting increased Bax/Bcl-2 ratio & Caspase-3 expression; Suppressing JNK and P38 MAPK activation in the heart. |
PTY-2(Shivani Srivastava, et al. 2018) |
T1DM |
10 days |
STZ rats |
Through downregulating β cells apoptosis. |
Enhancing the size and number of islet cells along with the plasma level of GLP-1, GIP, pancreatic expressions of GLP-1R, GIP-R, Bcl2, and insulin. |
NIL (Wang, W, et al. 2018) |
T2DM |
8 weeks |
STZ rats |
Through the tight correlation between BAs and glucose-lipid metabolism status. |
Downregulating the elevated levels of HAT, desmosterol, lathosterol, LysoPC(18:1(9Z)), LysoPE(16:0/0:0); Upregulating the circulating concentrations of various primary BAs in the primary bile acid pathway, including CA, CDCA, TCA, GCA, TCDCA, taurine; Exerting hypoglycemic effects predominantly by altering the metabolism of BAs. |
PTY-2r
(Shukla, R, et al. 2018) |
Diabetic Nephropathy |
20 days |
STZ rats |
Through downregulating PKC-a & NF-κB pathway. |
Decreasing the expression of iNOS and inflammatory cytokines (IL-6 and TNF-a) ; Lowering the expression of PKC-a; Decreasing the expression of variation in NF-κB expression and pNF-κB. |
Puerariae flos extract (KUBO, Koshi, et al. 2012) |
T2DM |
8 weeks |
TSOD mice |
Through promoting catabolization/excretion of cholesterol in the liver. |
Suppressing body weight gain & visceral fat accumulation; Alleviating the abnormal glucose tolerance & hyperinsulinemia; Increasing Acrp30; Suppressing liver enlargement, fatty degeneration, anti-inflammatory effect; Increasing gene expression for cholesterol synthesis rate-limiting enzyme HMG-CoA reductase, cholesterol catabolization enzyme Cyp7A1, bile salt export pump ABCB11, low-density lipoprotein receptor. |
Puerarin (ZHANG, Duzhen, et al. 2019) |
Diabetic Cataract |
12 weeks |
STZ rats |
Through inhibiting the nrf2/Ho-1 signaling pathway. |
Reducing blood glucose levels and the incidence of cataract in STZ-induced diabetic rats; Reducing oxidative stress; Restoring the levels of MDA & GSH, GPx; Decreasing the expression levels of retinal VEGF & IL-1β; Increasing the mrna expression levels of nrf2 and Ho-1. |
Puerarin
(GUO, Bao‑Qiang, et al. 2018) |
Diabetic Cardiomyopathy |
4 weeks |
STZ rats |
Through upregulating VEGFA/Ang-1 and suppressing apoptosis. |
Reducing the myocardial infarct area; Increasing left ventricular developed pressure in diabetic rats with myocardial I/R; Reducing oxidative stress, inflammation, NF-κB protein expression; Activating the protein expression levels of VEGFA and Ang-I; Increasing NO production, phosphorylated- eNOS protein expression and caspase-3 activity. |
Puerarin (XU, Xiaohui, et al. 2016) |
Diabetic Nephropathy |
8 weeks |
STZ rats |
Through attenuating SIRT1/FOXO1 pathway for renal protection. |
Ameliorating FBG, BUN, Scr, 24-hour urine protein levels; Down-regulating IL-6, TNF-α, ROS in kidney; Increasing the activities of MnSOD and CAT; Improving kidney tissue damage; Up-regulating SIRT1, FOXO1, PGC-1α expressions; Down-regulating the protein expression of NF-κB. |
RPFC (CHEN, Zhengyue, et al. 2017) |
Diabetic Nephropathy |
9 weeks |
STZ rats |
Through inhibiting the PI3K/AKT pathway in the kidney. |
Decreasing blood glucose; Amelorating Glomerulus mesangial matrix expansion, renal capsule constriction, renal tubular epithelial cell edema; Reducing protein levels of PI3K, AKT, α-SMA, collagen IV. |
NIL (ZHAO, Jindong, et al. 2019) |
T2DM |
8 weeks |
GotoKakizaki rats |
Through improving glucose & lipid levels and modulating the gut microbiota. |
Reducing the FBG gain and a shif in the structure of the gut microbiota; Decreasing weight, FBG level, TC; Decreasing gut microbiota, Bacteroidetes, F/B ratio, Allobaculum, Desulfovibrionaceae; Enriching Lactobacillus. |
Puerarin (DONG, Songtao, et al. 2018) |
T2DM |
28 days |
STZ rats |
Through upregulating uridine diphosphate (UDP)-glucuronosyltransferase activity. |
Altering the pharmacokinetics of puerarin by the metabolic changes in diabetes; Upregulating UDP-glucuronosyltransferase activity; Enhancing puerarin clearance; Altering the hepatic and intestinal gene and protein expressions of Ugt1a1 and Ugt1a7. |
Cell-based studies |
Astragalus membranaceus (Fisch.) Bunge |
Extract |
Topic |
Model |
Pathways |
Results |
Astragaloside IV (Chen, X, et al. 2019) |
Diabetic Nephropathy |
Renal tubular epithelial cells (HK-2) |
Through blocking the mTORC1/p70S6K signaling pathway. |
Reducing EMT features in HK-2 cells; Inhibiting mTORC1/p70S6K pathway activation; Downregulating expression of snail & twist; Reducing secretion of FN and Col IV. |
Astragalus polysaccharide (Ruixin Zhang, et al. 2018) |
T2DM |
3T3-L1 preadipocytes |
Through activating AMPK. |
Increasing preadipocytes proliferation in a dose dependent manner; Increasing PCNA content; Enhancing intracellular lipid accumulation and mRNA expression of PPAR γ, CCAAT/EBPs α, FABP ; Increasing 2-NBDG uptake; Elevating both mRNA and protein content of Glut4; Enhancing tyrosine phosphorylation of IRS 1 and phosphor-Akt content; Increasing phosphorylated AMPK content in the APS treated cells. |
Astragalin
(Ke, M, et al. 2012) |
Diabetic Retinopathy |
Müller cells |
Through antioxidant activity |
Decreasing the overexpression of VEGF in Müller cells; Alleviating the effects caused by high glucose; Alleviating endoplasmic reticulum stress. |
Astragalus polysaccharide (Sun, S, et al. 2017) |
Diabetic Cardiomyopathy |
H9C2 cell |
Through inhibiting expression of proapoptotic proteins of extrinsic and intrinsic pathways. |
Inhibiting high glucose-induced H9C2 cell apoptosis; Decreasing the expressions of caspases and the release of cytochrome C from mitochondria to cytoplasm; Modulating the ratio of Bcl-2 to Bax in mitochondria. |
Schisandra chinensis (Trucz.) Baill |
SCPP11
(Jin, D, et al. 2016) |
T2DM |
buffalo rat liver cells (BRL cells) |
Through up-regulating the expression of GLUT-4 |
Improving the glucose consumption in BRL cells; Increasing the protein expression of Akt, p-AMPK, GLUT-4 in BRL cells; Enhancing the mRNA expression levels of IRS-1, PI3K, Akt, GLUT-4, AMPK, PPAR- in BRL cells. |
Dioscorea opposita Thunb |
Allantoin (Jörg Schweizer, et al. 2012) |
Diabetes |
C2C12 cell line |
Through activating I2BR to increase glucose uptake into cells. |
Increasing AMPK phosphorylation dose-dependently in C2C12 cells; Increasing glucose uptake in C2C12 cells. |
Dioscorea polysaccharide (Lee, B. H, et al. 2011) |
T2DM |
FL83B cells |
Through inhibiting insulin resistance via the activation of JNK. |
Increasing glucose uptake & GLUT2 expression of insulin-resistant cells; Stimulating IRS tyrosyl phosphorylation; Increasing p-Akt level to alleviate insulin resistance; Attenuating JNK and IR caused by TNF-R induction; Elevating the levels of p-IRSTyr and p-AktSer to improve insulin sensitivity in the TNF-R-induced FL83B cells |
Anemarrhena asphodgfoides Bge. |
Sarsasapogenin (Zhang, M. Y, et al. 2017) |
Diabetes-associated Cognitive decline |
HT-22 cells |
Through activating PPARγ and subsequent downregulating BACE1. |
Elevating Aβ expression and Aβ42 level in HG-treated HT-22 cells; Increasing BACE1 protein, mRNA levels, enzymatic activity; Reversing reduced nuclear PPARγ levels; Suppressing HG-induced decreases in cell viability of HT-22 cells. |
NIL
(FONG, Chi Chun, et al. 2011) |
Diabetic Cardiomyopathy |
H9c2 cells |
Through up-regulating IRS/AKT and JNK pathways as well as inhibiting TNF and p38 pathways. |
Promoting H9c2 cell viability & cell proliferation; Stimulating GM-CSF, CNIF, b-NGF; Suppressing TIMP-1 expression; Stimulating three interleukin subclasses IL-1, 1X, 6; Down-regulating expression of pro-inflammatory factors TNF- & IFN- ; Up-regulating anti-apoptosis related genes Cdkn2c & Ppp3ca, several cardiovascular disease suppressers, anti-inflammatory mediators; Down-regulating pro-apoptotic related genes Caspase and Tnf- . |
Kakkalide (ZHANG, Dongyan, et al. 2013) |
Endothelial Insulin Resistance |
Human umbilical vein endothelial cells (HUVEC) |
Through facilitating insulin PI3K/Akt/eNOS signaling. |
Inhibiting ROS overproduction; Restoring mitochondrial membrane potential; Inhibiting ROS-associated inflammation in endothelium; Inhibiting TNF-a & IL-6 production & gene expression; Suppressing the phosphorylation of c-Jun N-terminal kinase and IjB kinase b/nuclear factorjB; Positively regulating serine/tyrosine phosphorylation of IRS-1. |
Puerarin (LIAN, Dawei, et al. 2019) |
Diabetic Cardiomyopathy |
mMVEC line |
Through inhibiting Nlrp3 inflammasome activation. |
Decreasing Nlrp3 protein; Exerting anti-oxidation & ROS scavenged effects; Decreasing TXNIP protein & TXNIP binding to Nlrp3; Decreasing HMGB1 release from mMVECs; Decreasing expression of the tight junction proteins ZO-1/ZO-2; Recovering the gap junction protein; Decreasing monolayer cell permeability in endothelial cells. Inhibiting high glucose induced Nlrp3 inflammasome formation and activation. |
Puerarin (XUE, Bing, et al. 2017) |
Diabetic Peripheral Neuropathy |
Schwann cells (SCs) |
Through inhibiting apoptosis and oxidative stress. |
Incubating SCs with intermittent high glucose for 48 h decreased cell viability; Increasing the number of apoptotic cells; Suppressing activation of apoptosisrelated proteins including PARP and caspase-3; Downregulating bcl-2; Upregulating intracellular distribution of bax from cytosol to mitochondria; Inhibiting the elevation of intracellular ROS and mitochondrial depolarization. |
Animal studies and Cell-based studies |
Astragalus membranaceus (Fisch.) Bunge |
Extract |
Topic |
Duration |
Model |
Pathways |
Results |
The saponins of A. membranaceus (Quan Liu, et al. 2017) |
T2DM |
10 weeks |
KKAy mices/L6 myotubes |
Through the insulin-dependent PI3K-AKT signaling pathway. |
Decreasing fasting insulin levels; Improving the plasma lipid profiles; Increasing activity of SOD; Decreasing MDA & iNOS levels; Elevating the insulin-stimulated glucose uptake with upregulated phosphorylation of AKT; Improving the phosphorylation levels of NF-κB p65, inhibitor of NF-κB (IκB α), c-Jun N-terminal kinase (JNK1/2) and extracellular-signal-regulated kinases (ERK1/2). |
Astragalus polysaccharides (Jie Sun, et al. 2019) |
T2DM |
12 weeks |
C57BL/6 mice |
HepG2/IR cell |
|
Through activating hepatic insulin signaling. |
Improving body weight & blood glucose/lipid levels; Recovering liver functions; Regaining insulin sensitivity; Improving the excessive and proapoptotic ER stress response; Inhibiting autophagy; Modulating the insulin-initiated phosphorylation cascades. |
Astragaloside IV (Bin Lenga, et al. 2018) |
Diabetes |
8 weeks |
STZ rats/HUVECs |
Through inhibiting the TLR4/NF-κB signaling pathway. |
Improving aortic endothelial function; Increasing eNOS expression & NO production; Decreasing the content of IL-6 and TNF-α and the expressions of VCAM-1, ICAM-1, TLR4, nuclear NF-κB p65. |
Astragalus polysaccharide (Zou, F, et al. 2009) |
T2DM |
8 weeks |
STZ rats/C2C12 cells |
Through an AMP activated protein kinase (AMPK)-dependent pathway |
Improving the hyperglycemia status, insulin sensitivity, glucose uptake, activation level of AMPK; Alleviating glucose toxicity in cultured mouse cells by the activation of AMPK. |
Astragalus polysaccharide (Chen, W, et al. 2015) |
Diabetic Cardiomyopathy |
16 weeks |
MHC-PPARα transgenic male mice/H9c2 cells |
Through down-regulating the cardiac PPARα-mediated regulatory pathways. |
Preventing myocardial triglyceride accumulation & cardiac dysfunction; Reducing free fatty acids utilization; Increasing glucose uptake; Downregulating the PPARα gene regulatory pathway involved in FFA-oxidation; Normalizing the suppression of PPARα target genes involved in glucose uptake and oxidation. |
NIL (Hui, C, et al. 2017) |
T2DM |
8 weeks |
ob/ob mice/
T cells |
Through improving abnormal immune and metabolic homeostasis. |
Normalizing glucose and insulin level; Increasing the expression of Acrp30; Diminishing fat accumulation & lipogenesis; Promoting glucose uptake; Decreasing Ile, adenosine, TC; Increasing GIn levels in liver and VAT of ob/ob mice; Promoting the shift of pro-inflammatory to anti-inflammatory cytokines; Suppressing T lymphocytes proliferation; Enhancing Tregs differentiation; Inhibiting DCs maturation; Attenuating DCs-stimulated T cells proliferation and secretion of IL-12p70 cytokine from DCs; Promoting the interaction of DCs with Tregs. |
Trichosanthes kirilowii Maxim. |
Trichosanthes kirilowii lectin (Jiandong Lu, et al. 2018) |
Diabetic Nephropathy |
8 weeks |
STZ rat/ HK-2 cells |
Through inhibiting the LOX1/NFκB /caspase-9 signaling pathway. |
Increasing the viability of HG-treated HK-2 cells; Inhibiting cell apoptosis; Attenuating STZ-induced histopathological damage & the inflammatory response in rat kidney tissues; Inhibiting the phosphorylation of IKKβ and NF-κB inhibitor protein (IκBα); Reducing the nuclear translocation of NF-κB (p65); Inhibiting the binding of p65 to the CASP9 gene in HG-treated HK-2 cells; Suppressing transcription of the CASP9 gene; Inhibiting luciferase activity in cells co-transfected with p65 and a wild-type capase-9 construct instead of mutated caspase-9 constructs. |
Schisandra chinensis (Trucz.) Baill |
Gomisin N (GN)
(Jung, D. Y., et al. 2017) |
T2DM |
6 weeks |
HFD obese mice/ C2C12 myoblasts |
Through activating AMPK. |
Enhancing the phosphorylation of AMPK/ACC, Akt; Promoting glucose uptake in C2C12 myotubes; Increasing the expression of mitochondria biogenesis & fatty acid oxidation genes in C2C12 myotubes; Decreasing levels of fasting blood glucose & insulin; Improving glucose tolerance; Rescuing decreased phosphorylation of AMPK and Akt; Stimulating expression of mitochondria biogenesis genes in skeletal muscle of HFD mice. |
Water extracts of schisandrae chinensis (Park, S, et al. 2009) |
T2DM |
8 weeks |
Px rats/ NCI-H716 cells |
Through enhancing insulinotropic actions. |
Improving glucose tolerance in an oral glucose tolerance test in Px rats; Increased cell mass by hyperplasia; Elevating IRS2 and PDX-1 expression in the islets. |
Gomisin N (Arulkumar, N, et al. 2018) |
T2DM |
6 weeks |
HFD obese mice/ HepG2 |
Through inhibiting CB1R-induced ER stress and improving insulin resistance & gluconeogenesis. |
Reversing 2-AG-mediated effects; Improved 2-AG-mediated impairment of insulin signaling; Inhibiting 2-AG-induced intracellular triglyceride accumulation & glucose production in HepG2 cells by downregulating lipogenesis & gluconeogenesis genes; Reducing HFD-induced increase in FBG & insulin levels; Downregulating HFD-induced expression of CB1R, ER stress markers, ceramide synthesis gene, gluconeogenesis genes in livers. |
NIL (Jing Tian, et al. 2018) |
Diabetic cardiomyopathy |
2 months |
db/db mice/ H9C2 cardiomyocytes |
Through improving mitochondrial lipid metabolism. |
Recovering diabetes-induced myocardial hypertrophy and diastolic dysfunction; Restoring mitochondrial structure & function; Enhancing SIRT1 & p-AMPKα protein levels; Decreasing expression of acetylated-PGC-1α & uncoupling protein 2 protein; Restoring depletion of NRF1 & TFAM levels in diabetic hearts and H9C2 cardiomyocytes. |
Dioscorea opposita Thunb |
Dispo85E (Peng, K. Y, et al. 2011) |
Diabetic Vascular Complications |
8 weeks |
AGEs-induced diabetic mice/ NPCs |
Through enhancing the clearance of AGEs by HGF-induced autophagic-lysosomal pathway. |
Enhancing endocytosis & degradation activity of AGEs in hepatic NPCs; Positively correlating HGF expression level with clearance capacity of the AGEs in NPCs; Increasing hepatic HGF messenger RNA expression levels; Decreasing serum AGEs level in diabetic mice; Improving the function of retina and kidneys |
Anemarrhena asphodgfoides Bge. |
Rhizoma Anemarrhenae extract (TFA) (Jun Han, et al. 2015) |
T2DM |
7 days |
STZ rats/ Murine 3T3-L1 preadipocytes & LKB1-deficient Hela cells |
Through mediating the activation of AMPK. |
Enhancing glucose-lowering effects of exogenous insulin administration i; Reducing FBG, serum insulin levels; Increasing the size and the number of insulin-producing beta cells in KK-Ay mice; Improving glucose infusion rate; Increasing phosphorylation of AMPK and its downstream target, ACC in 3T3-L1 cells; Activating AMPK in a LKB1-independent manner. |
Pueraria lobata (Willd.) Ohwi |
Pueraria lobata root extract (SUN, Ran, et al. 2019) |
T2DM |
not mention |
STZ rats/ HepG2 cells |
Through inhibiting PTP1B. |
Exhibiting high PTP1B inhibitory activity with IC50; Increasing the glucose uptake by two times; Decreasing blood glucose (AUC). |
NIL (ZHAO, Wenwen, et al. 2019) |
Diabetic Vascular Injury |
7 weeks |
STZ rats/ HUVECs |
Through reducing oxidative stress, |
Decreasing serum levels of insulin, NO, H2O2, MDA, sICAM-1, s-VCAM-1; Increasing SOD & CAT levels; Improving pathological alterations of aorta; Inhibiting increased expression of ICAM-1, VCAM-1, NOX2, and NOX4 in aorta; Suppressing HG-induced endothelial ROS formation, ICAM-1, VCAM-1, NOX4 expression, monocyte-endothelial adhesion. |
NIL (YEO, Jiyoung, et al. 2011) |
T2DM |
3 weeks |
db/db mice/ C2C12 cells |
Through alleviating ER stress. |
Inhibiting TNF-a-stimulated IKKb/NF-κB signaling; Attenuating ER stress in HepG2 cells; Reducing FBG & HbA1c levels; Improving postprandial glucose levels; Enhancing insulin sensitivity; Decreasing plasma free fatty acid, TG, TC. |
Puerarin (CHEN, Xiufang, et al. 2018) |
T2DM |
4 weeks |
STZ rats/ L6 skeletal muscle cells |
Through improving insulin sensitivity. |
Enhancing μ-opioid receptor expression & phosphorylation; Increasing insulin-stimulated glucose transporter 4 translocation to the plasma membrane in the skeletal muscle of diabetic rats. |
Puerarin (HUANG, Fang, et al. 2012) |
Endothelial Insulin Resistance |
not mention |
not mention/ HUVECs |
Through inhibiting inflammation and attenuating endothelial insulin resistance. |
Inhibiting IKKb/NF-kB activation; Decreasing TNF-a & IL-6 production; Downregulating relative gene overexpression; Attenuating PA-induced phosphorylation of IRS-1 at S307; Ameliorating insulin-mediated tyrosine phosphorylation of IRS-1; Increasing insulin-mediated NO production |
Chemical studies |
Astragalus membranaceus (Fisch.) Bunge |
Extract |
Topic |
Pathways |
Results |
Astragalosides (Motomura, K, et al. 2009) |
Diabetic Nephropathy |
Through inhibiting AGEs |
Inhibiting the formation of both CML and pentosidine; Astragaloside V had the strongest inhibitory effect among all if the isolated compounds. |
Refined-JQ (JQ-R) (Chang, Y. X, et al. 2015) |
Diabetes |
Through a potent anti-diabetic activity. |
Scavenging free radicals; inhibiting α-glucosidase, aldose reductase, α-amylase and lipase. |
Yuye Decoction |
NIL (Liu, J, et al. 2014) |
Diabetes |
Through the temperature-correlated mobility scale |
Achieving the optimization of the system conditions for the MEKC separations in the temperature-correlated mobility scale by correcting
for viscosity changes; Monitoring the influence of the operating temperature in a more distinct way. |
Dioscorea opposita Thunb. |
NIL (Yin-Shiou Lin, et al. 2016) |
T2DM |
Through DPP-IV inhibitions. |
Lowering the area under the curve (AUC0−120) of blood glucose and DPP-IV activity; Elevating the AUC0−120 of blood insulin. |
Anemarrhena asphodgfoides Bge. |
Mangiferin (Aihua Lin, et al. 2019) |
T2DM |
Through establishing a rapid, reliable, sensitive LC/MS-MS method |
The tissue distribution study results showed that mangiferin displayed rapid and wide distribution in plasma and tissues and it
could not cross the BBB. |
Pueraria lobata (Willd.) Ohwi |
Pueraria Lobata extracts (DENG, Wenji, et al.2019) |
Diabetes |
Through alleviating the oxidative stress & improving the pancreatic function. |
Producing significant hypoglycemic effects; Providing outstanding intestinal permeability and transepithelial transport aptness |
Human studies |
Astragalus membranaceus (Fisch.) Bunge |
Extract |
Topic |
Study design/duration |
No. of participants (intervention ⁄ control) |
Age; Duration of disease; Extra information; Ethnicity |
Intervention |
Results |
Drop-out |
3 mg of a mixture of plants in powder form (Chao, M, et al., 2009) |
T2DM |
Randomized Controlled/
3 months |
43(23/20) |
Age range: 18–70 years; FPG >_ 7 mmol/l and/or OGTT 2 h >_11.1 mmol/l; Body mass index (BMI) of 23–35 kg/m2; Two FPG concentrations between 7.0 and 10.0 mmol/l
within a month |
Each patient received seven tablets prepared for TCM or placebo group three times daily before meals for 3 months. |
Improving glucose disposal rate in the TCM group as compared to that in the placebo group (P< 0.05); Improving other metabolic related components like fasting plasma glucose, postprandial plasma glucose, glycated hemoglobin, systolic blood pressure, diastolic blood pressure, body mass index, retinol binding protein were improved in TCM group, but no statistical differences were detected between the two groups; No severe side effect was found in TCM group. |
2 |
Integrative TCM use (Lien, A. S. Y. et al., 2016) |
Diabetic Ketoacidosis |
Case-control design |
2024(416/1608) |
Samples from the registry for catastrophic illness patients from the National Health Insurance Research Database (NHIRD); The incidence of DKA and the annual costs of emergency visits and hospitalizations were evaluated for all causes |
Patients with T1DM in 2000–2011 were designated as cases (TCM users) and controls (non-TCM
users). |
The most common Chinese herbal formula and single herb is Liu-wei-di-huang-wan
(Six-ingredient pill of Rehmannia) and Astragalus membranaceus (Fisch.) Bunge respectively; A 33% reduction in DKA incidence for all TCM users and 40% reduction for users receiving TCM treatment for more than 180 days were found compared with non-TCM users; No significant differences between TCM users and non-users were found in the frequency and medical costs of emergency visits and hospitalizations. |
NIL |
Animal studies and cohort studies: Trichosanthes kirilowii Maxim. |
Extract |
Topic |
Duration |
Model |
Pathways |
Results |
Trichosanthes kirilowii Maxim. (TK) (Lo, H. Y, et al. 2017) |
Diabetes |
Not mention |
STZ rat |
Through stimulating IR kinase activity. |
The most common disease that TK was treated for was diabetes. TK was the most frequently used Chinese medicinal herb in type 2 diabetic patients in Taiwan, followed by Astragalus mongholicus, Salvia miltiorrhizae, Dioscoreae opposita, Scrophularia ningpoensis, Ophiopogonis japonicus, Pueraria lobata, Atractylodes lancea, Dendrobium nobile, Rehmannia glutinosa. TK displayed hypoglycemic effects and enhanced the clearance of glucose in a dose-dependent manner in diabetic mice; Interacting with IR; Activating the kinase activity of IR. |
Diabetes (not specific), insulin and metabolic syndrome: Fan et al. showed that a polysaccharide DOTP-80 from Dioscorea opposita Thun roots had potent hypoglycemic activity [7]. Another study demonstrated that Discorea batatas extract could ameliorate insulin resistance in mice which were fed a high-fat diet [8]. In fructose-fed rats, a daily dose of 2 mg/kg astragaloside for 3 weeks improved metabolic syndrome and endothelial dysfunction [9]. In a cohort study, it was found that Huang-qi (Astragalus membranaceus) was one of the common Chinese medicines which could reduce the risk of diabetic ketoacidosis in diabetic patients [10].
Type 1 diabetes (T1D): Insulin is a hormone produced by the beta cells in the pancreas. It is important to transfer glucose into cells where glucose will be stored and further used for energy production. In type 1 diabetes, however, the pancreatic beta cells produce little or no insulin [11].
It was suggested that acute administration of diosgenin, a compound of Dioscorea, could reduce hyperglycemia with increased muscular steroidogenesis in type 1 diabetes rats [12]. Allantoin, another active constituent in Dioscorea batatas, could improve the function of β-cells to maintain normal plasma insulin and glucose levels in rats [13]. Another study also showed that allantoin could increase GLUT4 gene expression in muscle by increasing β-endorphin secretion from the adrenal glands in diabetic rats [14]. It was found that puerarin isolated from Pueraria lobata (Wild.) could promote insulin expression and ameliorate metabolic functions in streptozotocin (STZ)- induced diabetic mice [15]. Another study revealed that the aqueous extract of Pueraria tuberosa tubers could protect against STZ-induced diabetes by down-regulating β cell apoptosis [16].
Type 2 diabetes (T2D): Type 2 diabetes is the most common type of diabetes. It is characterized by insulin resistance in which the human body cannot fully respond to insulin. As insulin cannot exert its action properly, the blood glucose level keeps rising. Finally, the pancreas will be exhausted, and hyperglycemia will result [17].
Chen et al. showed that Jia-Wei-Jiao-Tai-Wan (JWJTW), which contains Astragalus membranaceus, could ameliorate T2D by improving β cell function and reducing insulin resistance in diabetic rats [18]. Astragalus polysaccharide (APS) is an important bioactive component of Astragalus membranaceus. It was reported that APS could regulate part of the insulin signaling in insulin-resistant skeletal muscle in KKAy mice [19].
Hong et al. stated that Schisandra chinensis fruit-supplemented Korean rice cookie called dasik (RCD) had lipid-lowering and antidiabetic effects [20]. It was found that an acidic polysaccharide from Schisandra chinensis had a therapeutic effect on T2D rats by regulating apoptosis-related protein expression to alleviate the injury from oxidative stress [21]. A water-soluble polysaccharide (SSPW1) from Schisandra chinensis had antioxidant activities and anti-diabetic effect on T2D rats [22]. Another study disclosed that Schisandrae chinensis oil could improve pancreatic β-cell function by enhancing the antioxidant potential of the pancreas [23].
Wu et al. demonstrated that, after treatment of C57BL/6 mice on a high-fat diet with yam dioscorin for 135 days, weight gain was reduced, and impaired glucose tolerance was improved [24]. A daily dose of 8.02 g /kg of a functional formula diet including Rhizoma dioscorea, for 10 weeks improved insulin sensitivity, hepatic glucokinase activity and antioxidant activity [25]. Carassius auratus Complex Formula, which also contains Rhizoma dioscorea, inhibited the polyol pathway in T2D mice [26]. It was shown that D. opposita Thunb polysaccharide-zinc inclusion complex could reduce blood glucose and insulin levels in T2D rats [27].
Kubo et al. reported that Puerariae flos extract alleviated metabolic diseases in western diet-loaded and spontaneously obese mice representing an animal model of type 2 diabetes [28]. Puerarin (PUE) is a natural isoflavonoid isolated from the root of Pueraria lobata. Previous research had shown that PUE promoted fatty acid oxidation by increasing mitochondrial oxidative capacity and biogenesis in skeletal muscle of diabetic rats [29]. More recent studies confirmed that upregulation of UDP-glucuronosyltransferases 1a1 and 1a7 are involved in altered PUE pharmacokinetics in T2D rats [30]. Qijian mixture, a new traditional Chinese medicine (TCM) formula containing Pueraria lobata could alleviate T2D by altering metabolite profiles and gut microbiota [31]. Ge-Gen-Jiao-Tai-Wan (GGJTW) formula, which is composed of Pueraria montana var. lobata (Willd.), showed a hypoglycemic effect via the tight correlation between BAs and glucose-lipid metabolism status [32]. It was suggested that another Chinese Herbal Formula called Shenzhu Tiaopi Granule elicited metabolic improvement in T2D rats by modulating the gut microbiota [33].
Diabetic nephropathy: Diabetic nephropathy (DN) is one of the major complications of diabetes and is the major leading cause of end stage renal disease (ESRD). It is a progressive disease characterized by rising urinary albumin excretion and declining renal functions [34].
Astragaloside IV (AS-IV) is derived from Astragalus membranaceus, a widely used herbal medicine in China. Wang et al. showed that AS-IV attenuated proteinuria in STZ rats by inhibiting endoplasmic reticulum stress [35]. Another study found that AS-IV ameliorated renal injury in STZ rats by inhibiting NF-κB -mediated inflammatory genes expression [36]. According to Wang et al., AS-IV administered to diabetic mice at a dose of 40 mg/kg daily for 10 weeks could delay the renal fibrosis process by influencing the TGF-β/SMADS signaling pathway and down-regulating TGF-β1, SMAD2/3 and α-SMA expression [37]. It was suggested that a novel renoprotective compound, which is composed of Astragalus membranaceus and Panax notoginseng, could synergistically protect against podocyte injury in STZ-induced diabetic rats [38].
It was shown that Trichosanthes kirilowii lectin ameliorated STZ-induced kidney injury via modulating the balance between M1/M2 phenotype macrophage [39]. Zhang et al. observed that Schisandra chinensis fruit extract attenuated albuminuria and protected podocyte integrity in STZ-induced diabetic rats [40]. Another investigation revealed that an ethanol extract from Fructus Schisandrae chinensis prevented renal interstitial fibrosis [41].
Sarsasapogenin is a major sapogenin from rhizomes of Anemarrhena asphodeloides Bunge. It was shown that it could markedly ameliorate DN in rats via inhibiting NLRP3 inflammasome activation and AGEs–RAGE interaction [42].
PTY-2 is an active fraction of tubers from Pueraria tuberosa. According to Yamini et al., it could attenuate diabetic nephropathy by upregulating matrix metalloproteinase-9 expression in the kidneys of diabetic rats [43]. In another study by Shukla et al., PTY-2 exerted antioxidant and antiapoptotic effects on DN. Later, the same group discovered that PTY-2 alleviated the kidney damage induced by chronic hyperglycemia and delayed the development of DN by suppressing the expression of HIF-1a and VEGF, thereby restoring the expression of nephrin [44]. It was also found that PTY-2 inhibited iNOS and IL-6 through suppressing the PKC-a and NF-κB pathway in treating DN [45]. Another study suggested that PUE protected against DN by attenuating oxidative stress [46]. A Radix Puerariae and Fructus Crataegi mixture could inhibit DN via decreasing of AKT/PI3K [47].
Diabetic retinopathy: Diabetic retinopathy induced by diabetes involves the retinal capillaries, arterioles and venules. It is accompanied by leakage or occlusion of the small vessels [48].
Jian et al. found that Fufang Xueshuantong capsules, which contain Astragalus membranaceus, could attenuate STZ-induced retinal lesions in rats [49].
Diabetic ophthalmopathy: Diabetic ophthalmopathy is a disease induced by diabetes. It impairs patients’ eyesight and even causes blindness [50].
It was shown that Anemarrhena asphodeloides rhizomes could counteract diabetic ophthalmopathy progression in STZ-induced diabetic rats [51]. Zhang et al. showed that PUE could prevent cataract development and progression in diabetic rats through the Nrf2/HO‑1 signaling pathway [52].
Diabetic cardiomyopathy: Diabetic cardiomyopathy (DCM) is a diabetes-related complication characterized by left ventricular (LV) hypertrophy, myocardial fibrosis, compromised myocardial function and is a leading cause of morbidity and mortality [53].
The study by Chen et al. revealed that Astragalus polysaccharides inhibited DCM in hamsters by suppressing heart chymase activation [54]. It was also demonstrated that Astragalus polysaccharides improved PPRAa-mediated lipotoxicity in DCM [55]. Yu et al. showed that Flos Puerariae Extract could prevent myocardial apoptosis by attenuating oxidative stress in STZ-Induced diabetic mice [56]. Recently, Guo et al. suggested that PUE reduced ischemia/reperfusion-induced myocardial injury in diabetic rats through upregulating vascular endothelial growth factor A/angiotensin‑1 and suppressing apoptosis [57].
Diabetic cognitive impairment: Diabetes and insulin resistance affect the central nervous system as well as the development of cognitive and memory impairments which diminish the quality of life of diabetic patients [58].
Astragalus polysaccharides (APS) are active constituents of Astragalus membranaceus. Research finding by Liu et al. demonstrated that APS could improve cognitive dysfunction by altering the gut microbiota in diabetic mice [59]. Dun et al. also found that APS could improve memory in rats with STZ-induced diabetes. This was associated with its effects on glucose and lipid metabolism, antioxidative activity and insulin resistance [60]. In addition, Liu et al. showed that total saponins from Rhizoma Anemarrhenae alleviated diabetes-associated cognitive decline in rats via reduction of amyloid-beta in the brain [61].
Cell-based studies: Twelve cell-based studies investigated the effects on diabetes and its complications (Table 7).
Diabetes (not specific), insulin and metabolic syndrome: Allantoin is an active principle of the yam. Allantoin could activate I2BR to enhance glucose uptake into cells. Hence it may be a new target for antidiabetic therapy [62]. Kakkalide is the predominant isoflavone extracted from the flowers of Pueraria lobata. Zhang et al. demonstrated that Kakkalide inhibited reactive oxygen species (ROS)-associated inflammation and ameliorated insulin-resistant endothelial dysfunction due to effects on insulin receptor substrate 1(IRS-1) function [63].
Type 2 diabetes: It was shown that Astragalus polysaccharide improved insulin sensitivity via AMPK activation in 3T3-L1 adipocytes [64]. The Schisandra polysaccharide also increased glucose consumption by up-regulating the expression of GLUT-4 in buffalo rat liver cells in the study of Jin et al. [65]. It was reported that Dioscorea polysaccharide manifested inhibitory effects on TNF-α-induced insulin resistance in mouse FL83B cells [66].
Diabetic retinopathy: Ke et al. showed that astragalin extracted from Astragalus membranaceus, attenuated the overexpression of VEGF in Müller cells and alleviated the effects caused by a high glucose level [67].
Diabetic nephropathy: Chen et al. suggested that Astragaloside IV ameliorated high glucose‑induced renal tubular epithelial‑mesenchymal transition by blocking mTORC1/p70S6K signaling in HK‑2 cells [68].
Diabetic cardiomyopathy: Astragalus polysaccharides could attenuate DCM by inhibiting the extrinsic and intrinsic apoptotic pathways in high glucose stimulated H9C2 cells [69]. Another study revealed that PUE inhibited high glucose-induced Nlrp3 inflammasome formation and activation by ROS-dependent oxidative pathway [70]. Besides, Danshen–Gegen decoction, which contains Pueraria lobata, had been proven to display a proliferative effect on rat cardiac myoblasts H9c2 via MAPK and insulin pathways [71].
Diabetic peripheral neuropathy: Diabetic peripheral neuropathy, which is one of the most debilitating complications of diabetes, is characterized by axonal degeneration, demyelination, and atrophy [72]. Xue et al. suggested that PUE may protect Schwann cells against glucose fluctuation-induced cell injury by inhibiting apoptosis and oxidative stress [73].
Diabetic cognitive impairment: It was showed that sarsasapogenin (Sar), an active component purified from Rhizoma Anemarrhenae, suppressed Aβ overproduction induced by a high glucose level in HT-22 cells [74].
Animal studies & cell-based studies:
Diabetes (not specific), insulin and metabolic syndrome: Astragaloside IV improved vascular endothelial dysfunction by inhibiting the TLR4/NF-κB signaling pathway in vivo and in vitro [75].
According to Huang et al., puerarin attenuated endothelial insulin resistance by inhibiting the inflammatory response in an IKKβ/IRS-1-dependent manner [76].
T2DM: It was reported that APS could potentially activate hepatic insulin signaling in vivo and in vitro [77]. Another study revealed that APS could alleviate glucose toxicity and restore glucose homeostasis in diabetic states by activating AMPK [78]. A Chinese herbal medicine preparation JQ-R, which contains Astragalus membranaceus, manifested anti-diabetic effects in vivo and in vitro [79]. Another decoction called Dangguiliuhuang (DGLHD) exerted anti-insulin resistance and antisteatotic effects by improving abnormal immune and metabolic homeostasis [80].
Gomisin N (GN) is a lignan derived from Schisandra chinensis. Jung et al. showed that GN exerted anti-hyperglycemic effects by AMPK activation [81]. Another study suggested that GN protected against hepatic cannabinoid type 1 receptor-induced insulin resistance and gluconeogenesis [82]. In Huang-Lian-Jie-Du-Tang supplemented with Schisandra chinensis and Polygonatum odoratum Druce, glucose tolerance was improved by potentiating insulinotropic actions in islets [83].
In the study of Han et al., Rhizoma Anemarrhenae extract ameliorated hyperglycemia and insulin resistance through activating AMP-activated protein kinase in vivo as well as in vitro [84]. The antidiabetic potential of Pueraria lobata root extract through promoting insulin signaling and inhibiting PTP1B was demonstrated by Sun et al. [85]. Besides, PUE acted on the skeletal muscle to improve insulin sensitivity in diabetic rats involving μ-opioid receptor [86]. In a multi-herbal extract including Pueraria lobata, Yeo et al. showed that PUE had therapeutic effects for treating type 2 diabetes in both cells and animal models [87].
Diabetic nephropathy: Trichosanthes kirilowii lectin alleviated DN by inhibiting the LOX1/NF-κB /caspase-9 signaling pathway both in vivo and in vitro [88].
Diabetic cardiomyopathy
A recent study by Chen et al. showed that APS repressed myocardial lipotoxicity in a PPARalpha-dependent manner in vitro and in vivo [89] Shengmai san, which includes Schisandra chinensis, was shown to alleviate diabetic cardiomyopathy by improving mitochondrial lipid metabolic disorder [90].
Diabetic vascular complications: The vascular complications of diabetes are the most serious manifestations of the disease. Dispo85E is the extract of rhizomes from Dioscorea alata L. It could enhance the clearance of advanced glycation end products (AGEs) through hepatocyte growth factor (HGF)-induced autophagic-lysosomal pathway for treating diabetic vascular complications [91]. Another study suggested that an aqueous extract of the pair of herbs Salvia miltiorrhiza Bunge-Radix Puerariae ameliorated diabetic vascular injury by inhibiting oxidative stress in STZ-induced diabetic rats [92].
Chemical studies
Diabetes (not specific), insulin and metabolic syndrome: A study by Liu et al. revealed a successful application of temperature-correlated mobility theory for separating the main lignans from Schisandra chinensis Fructus and its prescription Yuye Decoction in MEKC [93]. Jinqi Jiangtang Tablet, which is a traditional Chinese anti-diabetic formula containing Astragalus membranaceus, was demonstrated to scavenge free radicals and inhibit α-glucosidase, aldose reductase, α-amylase and lipase for treating diabetes [94]. Another study suggested that selenium-layered nanoparticles used for oral delivery of mulberry leaf and Pueraria lobata extracts expressed a better antihyperglycemic activity [95].
T2DM: Lin et al. conducted a tissue distribution study of mangiferin after intragastric administration of the mangiferin monomer, Rhizoma Anemarrhenae, and Rhizoma Anemarrhenae-Phellodendron decoctions in normal or type 2 diabetic rats by LC-MS/MS respectively. Results showed a lower mangiferin distribution in pancreas and intestine of diabetic rats administered with the same dose of the herb pair than that in normal rats [96]. Lin et al. reported that synthetic peptide derived from hydrolysis of yam dioscorin in silico exhibited dipeptidyl peptidase-IV inhibitory activity and improvements in oral glucose tolerance in normal mice [97].
Diabetic nephropathy: A study by Motomura et al. suggested that astragalosides isolated from Astragalus Radix inhibited the formation of advanced glycation end products and astragaloside V had the strongest inhibitory effect. Thus, it could be used to treat diabetic nephropathy [98].
Human studies
Randomized clinical trials (RCT): There was only one RCT in 88 included studies. This RCT study included 43 newly diagnosed type 2 diabetic patients, who had not used any antidiabetic drugs prior to the study. Then, they were randomly assigned into TCM and placebo groups. TCM mixture contains Astragalus mambranesceus. Results showed that TCM mixture could ameliorate insulin resistance in type 2 diabetes, so it is safe and effective for diabetic patients [99].
Case-control study design: A study by Lien et al. retrieved records of samples of patients from the registry for catastrophic illness patients in the National Health Insurance Research Database (NHIRD). Patients with T1DM in 2000–2011 were designated as cases (TCM users) and controls (non-TCM users) based on a frequency (1:4) matched case-control design. TCM treatment for patients with T1DM were then analyzed. The incidence of diabetic ketoacidosis and the annual costs of emergency visits and hospitalizations were also evaluated for all causes. Results showed that TCM may have a substantial positive impact on the management of TIDM [100].
A retrospective cohort study and an animal study: Lo et al. conducted a retrospective cohort study to analyze the usage of Chinese herbs in patients with type 2 diabetes in Taiwan and showed that Trichosanthes kirilowii Maxim. (TK) was the most frequently used Chinese medicinal herb. An animal study showed that TK protein enhanced the clearance of glucose in a dose-dependent manner [101].
Composition: According to the ancient medical literature “yixue zhongzhong canxi lu”, the composition of YYD is 30 g (Dioscorea opposite Thunb.); 15 g (Astragalus membranaceus (Fisch.) Bge.); 18 g (Anemarrhena asphodgfoides Bge.); 9 g (Schisandra chinensis (Turcz.) Baill.); 9 g (Trichosanthes kirilowii Maxim.); 6 g (Gallus gallus domesticus Brisson); and 4.5 g (Pueraria lobata (Willd.) Ohwi). The weight ratio of the herbs is 20:10:12: 6:6:4:3.
Dosage: In animals’ studies, the dose of herbs or formula administered ranged between 0.5 mg/kg and 12.15 g/kg per day. In RCT, the dose of formula administered was 9 mg/day. In all human, animal and cell studies, the dosage employed was not mentioned in only 4 out of 82 papers. The dose used was stated explicitly in 95.1% of the papers.
Duration: In all human and animal studies, 3 out of 70 papers did not mention the duration of the study. 95.7% of the papers stated the duration clearly. In animal studies, the duration of treatment ranged from 2 days to 100 days. In RCT, the duration of treatment I was 3 months.
Toxicity: No study indicated the toxicity of medicinal herbs or formulas. No toxicity in human trials has been reported.
The quality of studies: All human, animal and cell studies stated the ratio of the individual herbs if a formula was used. All studies stated the origin, the extraction method and the composition of each constituent herb. However, some studies did not provide the name of pharmaceutical companies and the batch number of the concentrated tablets used in the experiments. There was only one RCT among all the studies examined. The follow-up study details were not stated in this randomized clinical trial. The method of randomization and the placebo detail were not stated clearly. There should be more well-designed RCT in the future in order to provide more and stronger evidence.
The statistics of YYD: There are seven medicinal herbs in YYD. There were research papers on all the herbs except Gallus gallus domesticus Brisson. Among them, the largest number of research papers (30.7%) were about Astragalus membranaceus (Fisch.) Bge. and Pueraria lobata (Willd.) Ohwi. T2DM was studied most (37.5%) of the research papers while DN was the most (19.3%) studied diabetic complication (Table 5).
Most, 49 out of 88 (55.7%) of the papers, reported animal studies. About 20.5% papers were animal & cell-based papers (Table 6). It is encouraging to do so as in vivo and in vitro studies gave more comprehensive insight on the signaling pathways involved. The protective effects against several diabetic complications are more impressive in in vivo and in vitro models.
The various medicinal herbs in YYD exhibit their antidiabetic activities through different signaling pathways which are illustrated in Figure 1. In compound level all medicinal herbs in YYD, except Gallus gallus domesticus Brisson because no research was done on it, can treat diabetes and its complications through different mechanisms. The chemical structures of some important compounds of different medicinal herbs in YYD are illustrated in Figure 2. Thus, YYD has strong evidences to treat diabetes and its complications. Since YYD and its components are devoid of toxic or allergic effects, in combination with western medicine they may serve as an alternative for mitigating diabetic complications. However, further investigations are necessitated before translation into clinical practice. Our review suggests that YYD and its herbs can improve diabetes and its complications through a diversity of signaling pathways. It may offer a new therapeutic avenue to treat diabetes and its complications. Besides, compelling evidence from well-designed RCT is needed in the future.
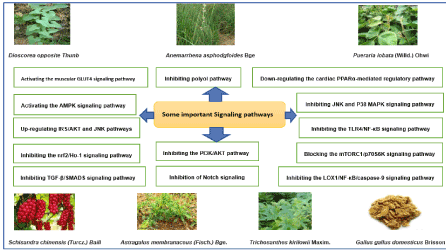
Figure 1. Medicinal plants used in treatment of diabetes and its complications with their mechanism of actions
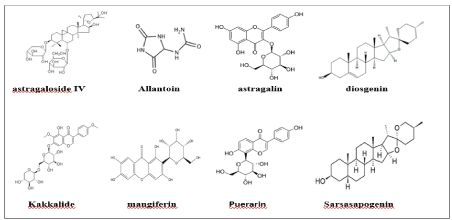
Figure 2. The chemical structures of some important compounds of different medicinal herbs in YYD
The authors have no conflict of interests to declare
The current study was funded by grants from Innovation and Technology Support Programme, Government of Hong Kong (Project code: UIM/321), and Seed Fund for Basic Research (Project code: 201611159232), University of Hong Kong. The founders had no role in the planning, analysis, or writing of this article.
Kalin YB ZHANG and Sydney CW TANG conceived and designed the study. Jack H WAN drafted manuscript. KH LAM, TH SONG, PS HO, Leanne L LEUNG, TL FONG, NC LAU, CH WONG and YY HUANG revised manuscript and check conferences. ZJ ZHANG and YG SHI supervised in the theory of Chinese medicine. George PH LEUNG, YG SHI, Calvin KF LEE, H WAN, TB NG and JF Wang reviewed and edited the manuscript. All authors read and approved the manuscript.
- Bell GI, Polonsky KS (2001) Diabetes mellitus and genetically programmed defects in β-cell function. Nature 414: 788-791. [Crossref]
- Wild S, Roglic G, Green A, Sicree R, King, et al. (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047-1053. [Crossref]
- Stehouwer CDA (2018) Microvascular dysfunction and hyperglycemia: A vicious cycle with widespread consequences. Diabetes 67: 1729-1741. [Crossref]
- Wang SL, Liu DS, Liang ES, Gao YH, Cui Y, et al. (2015) Protective effect of allicin on high glucose/hypoxia-induced aortic endothelial cells via reduction of oxidative stress. Experimental and Therapeutic Medicine 10: 1394-1400.
- Alwan AD, Galea G, Stuckler D (2011) Development at risk: addressing noncommunicable diseases at the United Nations high-level meeting. Bull World Health Organ 89: 546-546A.
- Wang Y, Xiang L, Wang C, Tang C, He X, et al. (2013) Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS One 8: e71144.
- Fan Y, He Q, Luo A, Wang M, Luo A, et al. (2015) Characterization and antihyperglycemic activity of a polysaccharide from Dioscorea opposita Thunb roots. International Journal of Molecular Sciences 16: 6391-6401. [Crossref]
- Kim S (2012) Extract from Dioscorea batatas ameliorates insulin resistance in mice fed a high-fat diet. Journal of medicinal food 15: 527-534. [Crossref]
- Zhang N, Wang XH, Mao SL, ZhaoF (2011) Astragaloside IV improves metabolic syndrome and endothelium dysfunction in fructose-fed rats. Molecules 16: 3896-3907. [Crossref]
- Lien ASY (2016) Integrative traditional Chinese medicine therapy reduces the risk of diabetic ketoacidosis in patients with type 1 diabetes mellitus. Journal of Ethnopharmacology 191: 324-330. [Crossref]
- VanBuecken DE, Greenbaum, CJ (2014) Residual C-peptide in type 1 diabetes: What do we really know? Pediatr Diabetes 15: 84-90. [Crossref]
- Sato K, Fujita S, Iemitsu M (2014) Acute administration of diosgenin or dioscorea improves hyperglycemia with increases muscular steroidogenesis in STZ-induced type 1 diabetic rats. The Journal of Steroid Biochemistry and Molecular Biology 143: 152-159. [Crossref]
- Go HK, Rahman M (2015) Antidiabetic effects of yam (Dioscorea batatas) and its active constituent, allantoin, in a rat model of streptozotocin-induced diabetes. Nutrients 7: 8532-8544. [Crossref]
- Niu, C. S, Chen, W, Wu, H. T, Cheng, K. C, Wen, Y. J, Lin, K. C, Cheng, J. T. (2010) Decrease of plasma glucose by allantoin, an active principle of yam (Dioscorea spp.), in streptozotocin-induced diabetic rats. Journal of Agricultural and Food Chemistry 58: 12031-12035. [Crossref]
- Wu K (2013) Anti-diabetic effects of puerarin, isolated from Pueraria lobata (Willd.), on streptozotocin-diabetogenic mice through promoting insulin expression and ameliorating metabolic function. Food and Chemical Toxicology 60: 341-347. [Crossref]
- Srivastava S (2018) Incretin hormones receptor signaling plays the key role in antidiabetic potential of PTY-2 against STZ-induced pancreatitis. Biomedicine and Pharmacotherapy 97: 330-338. [Crossref]
- Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813. [Crossref]
- Chen G, Yang X, Yang X (2017) Jia-Wei-Jiao-Tai-Wan ameliorates type 2 diabetes by improving β cell function and reducing insulin resistance in diabetic rats. BMC Complementary and Alternative Medicine 17: 507. [Crossref]
- Liu M (2010) Astragalus polysaccharide improves insulin sensitivity in KKAy mice: regulation of PKB/GLUT4 signaling in skeletal muscle. Journal of Ethnopharmacology 127: 32-37. [Crossref]
- Hong SH (2018) Hypolipidemic and antidiabetic effects of functional rice cookies in high-fat diet-fed ICR mice and db/db mice. Journal of Medicinal Food 21: 535-543. [Crossref]
- Du XX (2019) Hypoglycemic effect of acidic polysaccharide from Schisandra chinensis on T2D rats induced by high-fat diet combined with STZ. Biological and Pharmaceutical Bulletin 42: 1275-1281. [Crossref]
- Niu J (2017) In vitro antioxidant activities and anti-diabetic effect of a polysaccharide from Schisandra sphenanthera in rats with type 2 diabetes. International Journal of Biological Macromolecules 94: 154-160. [Crossref]
- An L (2015) Protective effect of Schisandrae chinensis oil on pancreatic β-cells in diabetic rats. Endocrine 48: 818-825. [Crossref]
- Wu G C (2018) 135-day interventions of yam dioscorin and the dipeptide asn-trp (nw) to reduce weight gains and improve impaired glucose tolerances in high-fat diet-induced c57bl/6 mice. Journal of Agricultural and Food Chemistry 66: 645-652.
- Cheng Q (2015) Anti‐diabetic effects of the ethanol extract of a functional formula diet in mice fed with a fructose/fat‐rich combination diet. Journal of the Science of Food and Agriculture 95: 401-408.
- Wang ZH (2014) Antidiabetic effects of Carassius auratus complex formula in high fat diet combined streptozotocin -induced diabetic mice. Evidence-Based Complementary and Alternative Medicine.
- Zhang Y (2018) Preparation and characterization of D. opposita Thunb polysaccharide-zinc inclusion complex and evaluation of anti-diabetic activities. International Journal of Biological Macromolecules 121: 1029-1036.
- Kubo K (2012) Puerariae flos alleviates metabolic diseases in Western diet-loaded, spontaneously obese type 2 diabetic model mice. Journal of Natural Medicines 66: 622-630.
- Chen XF (2018) Effect of puerarin in promoting fatty acid oxidation by increasing mitochondrial oxidative capacity and biogenesis in skeletal muscle in diabetic rats. Nutrition and Diabetes 8: 1.
- Dong S (2018) Upregulation of UDP-Glucuronosyltransferases 1a1 and 1a7 are involved in altered Puerarin pharmacokinetics in type II diabetic rats. Molecules 23: 1487.
- Gao K (2018) Effects of Qijian mixture on type 2 diabetes assessed by metabonomics, gut microbiota and network pharmacology. Pharmacological Research 130: 93-109.
- Wang W (2018) Metabolomics-based evidence of the hypoglycemic effect of Ge-Gen-Jiao-Tai-Wan in type 2 diabetic rats via UHPLC-QTOF/MS analysis. Journal of Ethnopharmacology 219: 299-318.
- Zhao, J, Li, Y, Sun, M, Xin, L, Wang, T, Wei, L, Yu, C, Liu, M, Ni, Y, Lu, R, Bao, T. (2019) The Chinese herbal formula Shenzhu Tiaopi granule results in metabolic improvement in type 2 diabetic rats by modulating the gut microbiota. Evidence-Based Complementary and Alternative Medicine 2019. [Crossref]
- Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, et al. (2004) Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 28: 164-76. [Crossref]
- Wang ZS (2015) Astragaloside IV attenuates proteinuria in streptozotocin-induced diabetic nephropathy via the inhibition of endoplasmic reticulum stress. BMC Nephrology 16: 44. [Crossref]
- Gui D (2013) Astragaloside IV ameliorates renal injury in streptozotocin-induced diabetic rats through inhibiting NF-κB-mediated inflammatory genes expression. Cytokine 61: 970-977. [Crossref]
- Wang Y (2015) Astragaloside effect on TGF-β1, SMAD2/3, and α-SMA expression in the kidney tissues of diabetic KKAy mice. International Journal of Clinical and Experimental Pathology 8: 6828-6834.
- Zhai R (2019) Astragalus membranaceus and Panax notoginseng, the novel renoprotective compound, synergistically protect against podocyte injury in streptozotocin-induced diabetic rats. Journal of Diabetes Research.
- Jiandong L, Yang Y, Peng J, Xiang M, Wang D, et al. (2019) Trichosanthes kirilowii lectin ameliorates streptozocin-induced kidney injury via modulation of the balance between M1/M2 phenotype macrophage. Biomedicine & Pharmacotherapy 109: 93-102.
- Zhang M (2012) Schisandra chinensis fruit extract attenuates albuminuria and protects podocyte integrity in a mouse model of streptozotocin-induced diabetic nephropathy. Journal of Ethnopharmacology 141: 111-118.
- Zhang Y, Zhang D, Zhang M (2012) Inhibition mechanism of compound ethanol extracts from wuweizi (fructus schisandrae chinensis) on renal interstitial fibrosis in diabetic nephropathy model mice. Journal of Traditional Chinese Medicine 32: 669-673.
- Liu YW (2018) Protective effects of sarsasapogenin against early stage of diabetic nephropathy in rats. Phytotherapy Research 32: 1574-1582.
- Tripathi YB, Shukla R, Pandey N, Pandey V, Kumar M, et al. (2017) An extract of Pueraria tuberosa tubers attenuates diabetic nephropathy by upregulating matrix metalloproteinase‐9 expression in the kidney of diabetic rats. Journal of Diabetes 9: 123-132.
- Rashmi S, Somanshu B, Tripathi YB (2018) Antioxidant and antiapoptotic effect of aqueous extract of Pueraria tuberosa (roxb. ex willd.) dc. on streptozotocin-induced diabetic nephropathy in rats. BMC Complementary and Alternative Medicine 18: 156.
- Shukla R, Pandey N, Banerjee S, Tripathi YB (2017) Effect of extract of Pueraria tuberosa on expression of hypoxia inducible factor-1α and vascular endothelial growth factor in kidney of diabetic rats. Biomedicine & Pharmacotherapy 93: 276-285.
- Xu X, Zheng N, Chen Z, Huang W, Liang T, et al. (2016) Puerarin, isolated from Pueraria lobata (Willd.), protects against diabetic nephropathy by attenuating oxidative stress. Gene 591: 411-416.
- Chen Z, Yuan Y, Zou X, Hong M, Zhao M, et al. (2017) Radix Puerariae and Fructus Crataegi mixture inhibits renal injury in type 2 diabetes via decreasing of AKT/PI3K. BMC Complementary and Alternative Medicine 17: 454. [Crossref]
- Nentwich MM (2015) Diabetic retinopathy-ocular complications of diabetes mellitus. World Journal of Diabetes 6: 489. [Crossref]
- Jian W (2016) A combination of the main constituents of Fufang Xueshuantong Capsules shows protective effects against streptozotocin-induced retinal lesions in rats. Journal of Ethnopharmacology 182: 50-56. [Crossref]
- Cai J, Boulton M (2002) The pathogenesis of diabetic retinopathy: old concepts and new questions. Eye 16: 242-260. [Crossref]
- Li X, Cui X, Wang J, Yang J, Sun X, et al. (2013) Rhizome of Anemarrhena asphodeloides counteracts diabetic ophthalmopathy progression in streptozotocin‐induced diabetic rats. Phytotherapy Research 27: 1243-1250. [Crossref]
- Zhang, Duzhen LI (2019) Puerarin prevents cataract development and progression in diabetic rats through Nrf2/HO‑1 signaling. Molecular Medicine Reports 20:1017-1024. [Crossref]
- Joshi M, Kotha SR, Malireddy S, Selvaraju V, Satoskar AR, et al. (2014) Conundrum of pathogenesis of diabetic cardiomyopathy: Role of vascular endothelial dysfunction, reactive oxygen species, and mitochondria. Mol Cell Biochem 386: 233-249.
- Chen W, Li YM, Yu MH (2010) Astragalus polysaccharides inhibited diabetic cardiomyopathy in hamsters depending on suppression of heart chymase activation. J Diabetes Complications 24: 199-208.
- Chen W (2012) The critical role of astragalus polysaccharides for the improvement of ppraα-mediated lipotoxicity in diabetic cardiomyopathy. PLoS One 7: e45541.
- Yu W, Zha W, Guo S, Cheng H, Wu J, et al. (2014) Flos Puerariae extract prevents myocardial apoptosis via attenuation oxidative stress in streptozotocin-induced diabetic mice. PloS one 9: e98044.
-
- Guo BQ (2018) Puerarin reduces ischemia/reperfusion-induced myocardial injury in diabetic rats via upregulation of vascular endothelial growth factor A/angiotensin-1 and suppression of apoptosis. Molecular Medicine Reports 17: 7421-7427.
- Ott A, Stolk RP, Hofman A, van Harskamp F, Grobbee DE, et al. (1996) Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia 39: 1392-1397.
- Liu Y, Liu W, Li J, Tang S, Wang M, et al. (2019) A polysaccharide extracted from Astragalus membranaceus residue improves cognitive dysfunction by altering gut microbiota in diabetic mice. Carbohydrate Polymers 205: 500-512. [Crossref]
- Dun C (2016) Effects of Astragalus polysaccharides on memory impairment in a diabetic rat model. Neuropsychiatric Disease and Treatment 12: 1617.
- Liu YW (2012) Total saponins from Rhizoma Anemarrhenae ameliorate diabetes-associated cognitive decline in rats: involvement of amyloid-beta decrease in brain. Journal of Ethnopharmacology 139: 194-200.
- Chen MF (2012) Activation of imidazoline i-2b receptors by allantoin to increase glucose uptake into c2c12 cells. Hormone and Metabolic Research 44: 268-272
- Zhang D, Gao, X, Wang, Q, Qin, M, Liu, K, Huang, F, Liu, B. (2013) Kakkalide ameliorates endothelial insulin resistance by suppressing reactive oxygen species‐associated inflammation. Journal of Diabetes 5: 13-24.
- Zhang R, Qin X, Zhang T, Li Q, Zhang J, et al. (2018) Astragalus polysaccharide improves insulin sensitivity via AMPK activation in 3T3-L1 adipocytes. Molecules 23: 2711.
- Jin D (2016) Schisandra polysaccharide increased glucose consumption by up-regulating the expression of GLUT-4. International Journal of Biological Macromolecules 87: 555-562.
- Lee, B. H, Hsu, W. H, Pan, T. M. (2011) Inhibitory effects of dioscorea polysaccharide on TNF-α-induced insulin resistance in mouse FL83B cells. Journal of Agricultural and Food Chemistry 59: 5279-5285.
-
- Ke M (2012) The effect of astragalin on the VEGF production of cultured Müller cells under high glucose conditions. Bio-medical materials and engineering 22: 113-119. [Crossref]
- Chen X (2018) Astragaloside IV ameliorates high glucose‑induced renal tubular epithelial‑mesenchymal transition by blocking mtorc1/p70s6k signaling in hk‑2 cells. International Journal of Molecular Medicine 43: 709-716.
- Sun S (2017) The effect of Astragalus polysaccharides on attenuation of diabetic cardiomyopathy through inhibiting the extrinsic and intrinsic apoptotic pathways in high glucose stimulated H9C2 cells. BMC Complementary and Alternative Medicine 17: 310.
- Lian D (2019) Puerarin inhibits hyperglycemia-induced inter-endothelial junction through suppressing endothelial Nlrp3 inflammasome activation via ROS-dependent oxidative pathway. Phytomedicine 55: 310-319.
- Fong CC (2011) Danshen-Gegen decoction exerts proliferative effect on rat cardiac myoblasts H9c2 via MAPK and insulin pathways. Journal of Ethnopharmacology 138: 60-66.
- Vinik AI (2003) Diabetic autonomic neuropathy. Diabetes Care 26: 1553-1579.
- Xue B (2017) Puerarin may protect against Schwann cell damage induced by glucose fluctuation. Journal of Natural Medicines 71: 472-481.
- Zhang MY (2018) Sarsasapogenin suppresses Aβ overproduction induced by high glucose in HT-22 cells. Naunyn-Schmiedeberg's Archives of Pharmacology 391: 159-168.
- Leng B, Tang F, Lu M, Zhang Z, Wang H, et al. (2018) Astragaloside IV improves vascular endothelial dysfunction by inhibiting the TLR4/NF-κB signaling pathway. Life Sciences 209: 111-121.
- Huang F (2012) Puerarin attenuates endothelial insulin resistance through inhibition of inflammatory response in an IKKβ/IRS-1-dependent manner. Biochimie 94: 1143-1150.
- Sun J (2019) APS could potentially activate hepatic insulin signaling in HFD-induced IR mice. Journal of Molecular Endocrinology 63: 77-91. [Crossref]
- Zou F, Mao XQ, Wang N, LiuJ, Ou-Yang JP, et al. (2009) Astragalus polysaccharides alleviates glucose toxicity and restores glucose homeostasis in diabetic states via activation of AMPK. Acta Pharmacologica Sinica 30: 1607. [Crossref]
- Liu Q, Liu S, Gao L, Sun S, Huan Y, et al. (2017) Anti-diabetic effects and mechanisms of action of a Chinese herbal medicine preparation JQ-R in vitro and in diabetic KKAy mice. Acta Pharmaceutica Sinica B 7: 461-469. [Crossref]
- Cao H (2017) Immune and metabolic regulation mechanism of Dangguiliuhuang decoction against insulin resistance and hepatic steatosis. Frontiers in Pharmacology 8: 445. [Crossref]
- Jung DY (2017) Antidiabetic effect of gomisin N via activation of AMP-activated protein kinase. Biochemical and Biophysical Research Communications 494: 587-593. [Crossref]
- Nagappan A (2018) Protective effects of gomisin against hepatic cannabinoid type 1 receptor-induced insulin resistance and gluconeogenesis. International Journal of Molecular Sciences 19: 968.
- Park S (2009) Huang-Lian-Jie-Du-Tang supplemented with Schisandra chinensis Baill. and Polygonatum odoratum Druce improved glucose tolerance by potentiating insulinotropic actions in islets in 90% pancreatectomized diabetic rats. Bioscience, Biotechnology, and Biochemistry 73: 2384-2392.
- Han J (2015) Rhizoma Anemarrhenae extract ameliorates hyperglycemia and insulin
resistance via activation of AMP-activated protein kinase in diabetic
rodents. Journal of Ethnopharmacology 172: 368-376.
- Sun R (2019) Anti-diabetic potential of Pueraria lobata root extract through promoting insulin signaling by PTP1B inhibition. Bioorganic Chemistry 87: 12-15.
- Chen X (2018) Puerarin acts on the skeletal muscle to improve insulin sensitivity in diabetic rats involving μ-opioid receptor. European Journal of Pharmacology 818: 115-123. [Crossref]
- Yeo, J, Kang, Y. M, Cho, S. I, Jung, M. H. (2011) Effects of a multi-herbal extract on type 2 diabetes. Chinese Medicine 6: 10. [Crossref]
- Lu J, Peng J, Xiang M, He L, Wang D, et al. (2018) Trichosanthes kirilowii lectin alleviates diabetic nephropathy by inhibiting the LOX1/NF-κB/caspase-9 signaling pathway. Bioscience Reports 38.
- Chen W (2015) Astragalus polysaccharides repress myocardial lipotoxicity in a PPARalpha-dependent manner in vitro and in vivo in mice. Journal of Diabetes and its Complications 29: 164-175.
- Tian J (2018) Shengmai san alleviates diabetic cardiomyopathy through improvement of mitochondrial lipid metabolic disorder. Cellular Physiology and Biochemistry 50: 1726-1739.
- Peng KY (2011) Hepatocyte growth factor has a role in the amelioration of diabetic vascular complications via autophagic clearance of advanced glycation end products: dispo85e, an hgf inducer, as a potential botanical drug. Metabolism Clinical & Experimental 60: 888-892.
- Zhao W (2019) Aqueous extract of Salvia miltiorrhiza Bunge-Radix Puerariae herb pair ameliorates diabetic vascular injury by inhibiting oxidative stress in streptozotocin-induced diabetic rats. Food and Chemical Toxicology 129: 97-107. [Crossref]
- Liu J (2014) Application of temperature‐correlated mobility theory for optimizing the MEKC separation of the main lignans from Schisandra Chinensis Fructus and its prescription Yuye Decoction. Electrophoresis 35: 2907-2914. [Crossref]
- Chang YX (2015) The multi-targets integrated fingerprinting for screening anti-diabetic compounds from a Chinese medicine Jinqi Jiangtang tablet. Journal of Ethnopharmacology 164: 210-222.
-
- Deng W, Wang H, Wu B, Zhang X (2019) Selenium-layered nanoparticles serving for oral delivery of phytomedicines with hypoglycemic activity to synergistically potentiate the antidiabetic effect. Acta Pharmaceutica Sinica B 9: 74-86. [Crossref]
- Lin A, Li J, Li D, Jin H, Liu Y, et al. (2019) Tissue distribution study of mangiferin after intragastric administration of mangiferin monomer, Rhizoma Anemarrhenae, and Rhizoma Anemarrhenae-Phellodendron decoctions in normal or type 2 diabetic rats by LC-MS/MS. Journal of Chromatography B 1122: 18-28. [Crossref]
- Lin YS (2016) Synthesized peptides from yam dioscorin hydrolysis in silico exhibit dipeptidyl peptidase-IV inhibitory activities and oral glucose tolerance improvements in normal mice. Journal of Agricultural and Food Chemistry 64: 6451-6458. [Crossref]
- Motomura K (2009) Astragalosides isolated from the root of astragalus radix inhibit the formation of advanced glycation end products. Journal of Agricultural and Food Chemistry 57: 7666-7672. [Crossref]
- Chao M (2009) Improving insulin resistance with traditional Chinese medicine in type 2 diabetic patients. Endocrine 36: 268-274. [Crossref]
- Lien ASY (2016) Integrative traditional Chinese medicine therapy reduces the risk of diabetic ketoacidosis in patients with type 1 diabetes mellitus. Journal of Ethnopharmacology 191: 324-330.
- Lo HY (2017) Hypoglycemic effects of Trichosanthes kirilowii and its protein constituent in diabetic mice: the involvement of insulin receptor pathway. BMC Complementary and Alternative Medicine 17: 53. [Crossref]


