The review summarizes results of clinical studies investigating the influence of a combination of plant extracts with aminoacids, functioning as NO-donors.
Prelox®, a combination of French maritime pine bark extract with L-arginine aspartate, enhanced activity of endothelial nitric oxide synthase (e-NOS) and reduced plasma concentrations of the e-NOS inhibitor ADMA (asymmetric dimethylarginine), thus enhancing bioavailability of nitric oxide.
Men with confirmed erectile dysfunction (ED) restored erectile function in clinical studies to normal scores following supplementation with Prelox® according to the questionnaire for erectile dysfunction. L-arginine aspartate alone was barely effective, placebo produced minor effects. The improvement of ED with Prelox® was not persistent, ED deteriorated again at the end of the supplementation period.
Prelox® was also effective in improving quantity and quality of spermatozoa in men with ED. Concentration, morphology and viability of spermatozoa were highly significantly improved (p<0.001) versus placebo.
Results from 7 clinical studies indicate a substantial impact of Prelox® on improvement of men’s virility and fertility. No unwanted effects were observed.
asymmetric dimethylarginine, erectile function, nitric oxide, Prelox®, Pycnogenol®, spermatozoa
Sexual health is closely connected to endothelial health. The endothelium produces a large array of factors to maintain vascular homeostasis. Amongst these substances, the endothelium-derived nitric oxide (NO), acting as vasodilator, has a pivotal role for erection as well as for function and quality of spermatozoa.
NO, produced from the substrate L-arginine by the endothelial nitric oxide synthase (e-NOS), acts both as a neurotransmitter in the nonadrenergic, noncholinergic nerve terminals in the penis and as a vasodilator, produced by endothelial cells and sinusoids of penile arteries [1,2].
NO interacts with guanylate cyclase in vascular smooth muscle cells to produce cGMP. cGMP triggers vascular relaxation and vasodilation, thus enhancing blood flow into penile arteries and sinusoids and entrapment of pressurized blood in corpora cavernosa.
The general age-related decline of endothelial function reduces the bioavailability of NO [3,4]. The impaired generation of sufficient quantities of NO represents a pathophysiological reason for men’s declining erectile quality. The diminished production of NO with increasing age is caused by a fading activity of e-NOS. There is a clear decrease of e-NOS activity with age in spermatozoa of subjects with erectile dysfunction (ED) [5]. Supplementation with Prelox®, (Horphag Research Ltd), consisting of French maritime pine bark extract, (Pycnogenol®, Horphag Research Ltd) and L-arginine aspartate, restored e-NOS activity to high values and, subsequently, also erectile function to normal values [5].
e-NOS activity is inhibited by the asymmetric dimethylarginine (ADMA) which acts as a strong competitive inhibitor for the formation of NO from L-arginine [6].
ADMA accumulates in endothelial cells and impairs vascular relaxation [7]. High ADMA levels are found in patients with hypercholesterolemia, hypertension, chronic renal failure, diabetes and heart failure [8-12]. Elevated ADMA plasma concentrations were found also in patients with early atherosclerosis and accompanying erectile dysfunction as well as in patients with arteriogenic ED [13,14].
ADMA plasma concentrations increase with age as observed in the study of Stanislavov et al., [5] Figure 1., in accordance with the observations of Sydow [15]. Supplementation of patients with ED with Prelox reduced the ADMA levels significantly (Figure 1).
The reduction of ADMA concentrations is mirrored in the same study by the corresponding increase of e-NOS activity, leading to improvement of erectile dysfunction [5].
Reason for the observed decrease of ADMA levels is most probably the reinstated metabolisation of ADMA by the enzyme dimethylarginine dimethylaminohydrolase II (DDAH II) which reduces and regulates ADMA concentrations in tissues expressing e-NOS [16]. This could be demonstrated by transfer of the DDAH II gene to endothelial cells which reduced the ADMA concentrations and increased e-NOS activity and NO production [17]. DDAH activity is disturbed by TNF-alpha, oxidized LDL and homocysteine [18,19].
As DDAH activity is reduced by these substances producing or indicating oxidative stress, DDAH activity was partially restored by antioxidative vitamin E which decreased ADMA concentration [20]. Pycnogenol, main constituent of Prelox, exerts a wide range of free radical scavenging and anti-oxidative actions in men [21,22]. Therefore, the reduction of oxidative stress during treatment with Pycnogenol containing Prelox may lead, via regeneration of DDAH II activity, to lower ADMA concentrations and, in turn, to enhancement of e-NOS activity.
In addition to this DDAH II based mechanism, the L-arginine aspartate component of Prelox may contribute to restore e-NOS activity by a competitive displacement of ADMA from the L-arginine receptor, by providing a surplus of L-arginine.
The bioavailability of NO is not only important for erectile function, but also for spermatogenesis and sperm maturation [23]. Normal spermatozoa contain e-NOS. Increased e-NOS activity and sperm motility are positively correlated [24,25]. Aberrant distribution of e-NOS in spermatozoa, deviating from the normal postacrosomal and equatorial localization, was linked to low mobility [24]. Spermatozoa lysates from patients with ED, supplemented with Prelox, oxidized L-arginine to citrulline with a significantly higher rate, indicating an enhanced activity of e-NOS [24]. Consequently, quantity and quality of semen improved highly significantly because of the higher bioavailability of NO [24].
The enhancement of e-NOS activity could be related both to the decrease of the inhibiting ADMA inside spermatozoa (Figure 1) and to the displacement of ADMA from the L-arginine binding region of e-NOS by high concentrations of L-arginine.
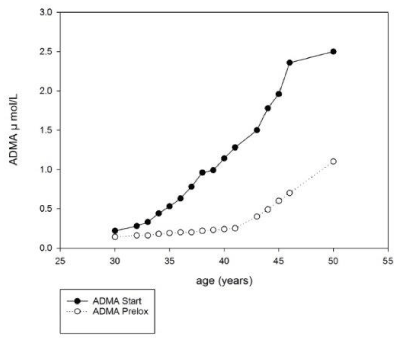
Figure 1. ADMA plasma concentrations of patients with ED at start of the study and following 1 month supplementation with Prelox.
The first hint that Pycnogenol, one component of Prelox, could improve erectile function was reported in a placebo-controlled, double-blind study with 21 patients [26]. Men with ED according to the International Index of Erectile Dysfunction (IIEF-S) and moderately high cholesterol (average 5.41 mmol/L) were treated with 120mg Pycnogenol per day for 3 months. ED improved from month to month. At 3 months, erectile function was increased from 12.6 (moderate) to 16.8 (mild) ED (p=0.019). Under placebo, ED deteriorated over the period of 3 months to severe ED (score 8.9). Total cholesterol decreased to 4.98 mmol/L (p=0.07), placebo has no effect.
The synergistic effects of Pycnogenol and L-arginine aspartate were shown by testing L-arginine aspartate separately and than acting in concert with Pycnogenol in a pilot study [27]. 40 men with confirmed ED received for a period of 1 month 1.7g L-arginine aspartate a day, resulting in a normalization of ED in just 2 men. The subsequent addition of 80 mg Pycnogenol to 1.7g L-arginine asparate in the next month increased the success rate unexpectedly to 80% (32 men with normal erection). A further increase of the erectile function was achieved by the combination of 120 mg/day Pycnogenol plus the 1.7g arginine aspartate on the third month, providing 92% of the patients a restored normal ED.
Based on these exploratory studies the formulation of Prelox was established to contain 20mg Pycnogenol and 700mg L-arginine aspartate per tablet. Formulations with higher dosages are not feasible because of size limitations.
An exploratory study with 37 men with mild ED, taking 4 Prelox tablets a day for 6 weeks, produced an increase of 10% of the IIEF scores [28]. 70% of the participants reported that erections are easier to sustain and to initiate, 65% reported an increase of morning erections.
To objectivate the findings from open studies, a double-blind, placebo-controlled, cross-over study was performed [5]. 50 patients with mild to moderate ED were treated for 1 month with 4 placebo tablets or 4 tablets Prelox a day. Patients answered to the questionnaire of IIEF and reported sexual wellness in diaries. Intake of Prelox for 1 month increased e-NOS activity, doubled intercourse frequency and restored ED to normal. In treatment periods with Prelox, IIEF scores were nearly doubled, placebo showed no significant effect. Testosterone levels were significantly (p<0.05) elevated by Prelox, to a smaller extent also by placebo.
In further study with 124 men the effect of Prelox was assessed over a period of 6 months [29]. Men with mild to moderate ED participated on a randomized, double-blind, placebo-controlled, parallel arm trial. Medication consisted of 4 tablets Prelox or 4 tablets placebo a day. Men completed IIEF questionnaires in monthly intervals. The erectile domain of IIEF scores increased from 15 to 25 after 3 months and to 27 after 6 months, whereas placebo enhanced scores from 15 to 19.1 and 19 after 3 and 6 months. The effects of Prelox were stat. sign. better compared to placebo (p<0.05). Testosterone levels were sign. (p< 0.05) elevated vs placebo from 15.9 to 18.9 mmol/L.
A further double-blind, placebo-controlled, parallel group comparison investigated the effect of a combination of 60 mg Pycnogenol and 690 mg arginine (as arginine aspartate) a day on 23 Japanese men [30]. Following 8 weeks of supplementation, the total IIEF score increased, but did not reach stat. significance (p<0.1). However, scores related to hardness and intercourse satisfaction increased significantly (p<0.05). Testosterone levels increased by 28 pg/ml (p<0.05), but not by placebo.
Another combination of 80 mg Pycnogenol, 1.92g L-arginine, 1.2g citrulline and 40mg French oak wood extract (Prelox®R) a day was tested on 50 men with confirmed erectile dysfunction (ED scores 11-17) [31]. L-citrulline was added because it increases plasma L-arginine concentrations more effectively than L-arginine itself [32]. French oak wood extract has been shown to decrease tiredness and to increase mental energy [33].
The study was performed as a randomized, placebo-controlled, double-blind, cross-over study. Sexual wellness was evaluated by the IIEF questionnaire. Treatment over a period of 1 month enhanced all scores for sexual response significantly (p<0.05), in accordance with the studies with Prelox.
No adverse effects were reported in all of the referred studies. Clinical chemistry was not changed to negative outcomes, but cholesterol levels were decreased significantly (p<0.05) in some of the studies [5,28,31]. Slightly elevated blood pressure was lowered to normal values [28-30].
A prospective, non-randomized, clinical study with 19 subfertile men revealed an improvement of sperm morphology by 38% and improvement of mannose receptor binding by 19% following treatment with 200 mg/day Pycnogenol for 90 days [34]. These results indicated that Pycnogenol could play a part in improvement of quality of sperms.
Another prospective, non-randomized clinical study with 50 infertile men with subnormal testosterone levels was initiated to test the effect of Prelox in combination with testosterone on fertility of men. Patients received 120 mg Pycnogenol a day plus 3g arginine aspartate together with 40mg testosterone undecanoate [35]. Following the 11 months treatment period, ejaculate volume, sperm concentration, mobility and morphology were sign. improved (p<0.0001 – 0.01). Most importantly, 22 spouses achieved pregnancy. The treatment improved significantly erectile function to normal in 76% of men. Activity of e-NOS in sperm lysate increased continuously during treatment.
To verify these findings, the improvement of seminal parameters by Prelox was controlled in a randomized, double-blind, placebo-controlled, cross-over trial [24]. Results showed a highly significant (p<0.001) improvement of all seminal parameters.
Morphology of sperms was improved significantly (p<0.01) by reducing number of sperms with head, neck and tail defects and cytoplasmatic droplets, increasing the number of normal spermatozoa from 36.8% to 63.45% (p<0.001). However, the seminal quality was going back to infertile values at the end of the study at 16 weeks.
The influence of Prelox®R on male fertility was tested with 50 subfertile men. Sperm quality was tested in monthly intervals during the randomized, double-blind, placebo-controlled, cross-over study [36]. Subjects received either 2 tablets Prelox®R or 2 placebo twice daily during test periods and no medication during the wash-out period of 1 month. The supplementation with Prelox®R enhanced sperm volume and concentration of spermatozoa. Vitality, morphology and motility of spermatozoa were significantly improved (p<0.001) e-NOS activity in spermatozoa lysate increased significantly during treatment (p<0.001).Results were summarized in Table 1. The supplementation with Prelox®R produced not only more, but healthier spermatozoa. Prelox®R was well tolerated, no unwanted effects were reported.
Table 1. Improvement (p<0.001) of seminal quality following supplementation with Prelox®
Parameter |
Before treatment |
After treatment |
Ejaculate volume (mL) |
2.6 |
3.3 |
Concentration of spermatozoa (x106/mL) |
39.05 |
65.3 |
Total number of spermatozoa (x106) |
103.6 |
217.5 |
Percentage of vital spermatozoa (eosin test) |
63.45 |
77.15 |
Intact membrane (%) (hypoosmotic test) |
51.53 |
68.3 |
Rapid progressing spermatozoa (%) |
58.5 |
55.9 |
Immotile spermatozoa (%) |
46.25 |
33.7 |
Normal morphology (%) |
36.8 |
63.45 |
Head defects (%) |
22.95 |
12.2 |
Neck defects (%) |
12.65 |
8.4 |
Cytoplasmatic droplets (%) |
14.4 |
6.25 |
Ref: Stanislavov R, Nikolova V, Rohdewald P. Improvement of seminal parameters with Prelox(R): A randomized, double-blind, placebo-controlled, cross-over trial. Phytother Res 2009; 23: 297-302.
The results of our investigations highlight the importance of the pathway DDMA – ADMA – e-NOS – NO for human sex life and fertility and the improvement of sexual health by Prelox® or Prelox®R.
Besides the beneficial effects of Prelox on sexual functions, the improvement of endothelial health could provide preventive effects in case of impaired vascular functions, associated to ADMA-connected diseases as hypertension, atherosclerosis, hypercholesterinemia or chronic renal failure.
Notice: Prelox®R is now marketed as New Prelox®
- Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ (1992) Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic noncholinergic neurotransmission. N Engl J Med 326: 90-94. [Crossref]
- Burnett AL, Lowenstein CJ, Bredt DS, Chang2021 Copyright OAT. All rights reserv physiologic mediator of penile erection. Science 257: 401-403. [Crossref]
- Carr A, Frei B. 2000. The role of natural antioxidants in preserving the biological activity of endothelium-derived nitric oxide. Free Rad Biol Med 28: 1806-1814. [Crossref]
- Herrera MD1, Mingorance C, Rodríguez-Rodríguez R, Alvarez de Sotomayor M (2010) Endothelial dysfunction and aging: an update. Ageing Res Rev 9: 142-152. [Crossref]
- Stanislavov R, Nikolova V, Rohdewald P (2008) Improvement of erectile function with Prelox: a randomized, double-blind, placebo-controlled, cross-over trial. Int J Impot Res 20: 173-180. [Crossref]
- Tsikas D, Böger RH, Sandmann J, Bode-Böger SM, Frölich JC (2000) Endogenous nitric oxide synthase inhibitors are responsible for the L-arginine paradox. FEBS Lett 478: 1-3. [Crossref]
- Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, et al. (2007) Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem 282: 879-887. [Crossref]
- Böger RH, Sydow K, Borlak J, Thum T, Lenzen H, et al. (2000) LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ Res 87: 99-105. [Crossref]
- Hasanoglu A, Okur I, Oren AC, Biberoglu G, Okta S, et al. (2011) The levels of asymmetric dimethylarginine, homocysteine and carotid intima-media thickness in hypercholesterolemic children. Turk J Pediatr 53: 522-527. [Crossref]
- Perticone F, Sciacqua A, Maio R, Perticone M, Galiano Leone G, et al. (2010) Endothelial dysfunction, ADMA and insulin resistance in essential hypertension. Int J Cardiol 142: 236-241. [Crossref]
- Aldámiz-Echevarría L, Andrade F (2012) Asymmetric dimethylarginine, endothelial dysfunction and renal disease. Int J Mol Sci 13(9): 11288-11311.
- Sibal L, Agarwal SC, Home PD, Boger RH (2010) The Role of Asymmetric Dimethylarginine (ADMA) in Endothelial Dysfunction and Cardiovascular Disease. Curr Cardiol Rev 6: 82-90. [Crossref]
- Elesber AA, Solomon H, Lennon RJ, Mathew V, Prasad A, et al. (2006) Coronary endothelial dysfunction is associated with erectile dysfunction and elevated asymmetric dimethylarginine in patients with early atherosclerosis. Eur Heart J 27: 824-831. [Crossref]
- Paroni R, Barassi A, Ciociola F, Dozio E, Finati E, et al. (2012) Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and L-arginine in patients with arteriogenic and non-arteriogenic erectile dysfunction. Int J Androl 35: 660-667. [Crossref]
- Sydow K, Fortmann SP, Fair JM, Varady A, Hlatky MA, et al. (2010) Distribution of asymmetric dimethylarginine among 980 healthy, older adults of different ethnicities. Clin Chem 56: 111-120. [Crossref]
- Leiper JM, Santa Maria J, Chubb A, MacAllister RJ, Charles IG, et al. (1999) Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem J 343: 209-214.
- Feng M, Liu L, Guo Z, Xiong Y (2008) Gene transfer of dimethylarginine dimethylaminohydrolase-2 improves the impairments of DDAH/ADMA/NOS/NO pathway in endothelial cells induced by lysophosphatidylcholine. Eur J Pharmacol 584: 49-56. [Crossref]
- Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, et al. (1999) Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation 99: 3092-3095. [Crossref]
- Stühlinger MC, Tsao PS, Her JH, Kimoto M, Balint RF, et al. (2001) Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation 104: 2569-2575. [Crossref]
- Saran R, Novak JE, Desai A, Abdulhayoglu E, Warren JS, et al. (2003) Impact of vitamin E on plasma asymmetric dimethylarginine (ADMA) in chronic kidney disease (CKD): a pilot study. Nephrol Dial Transplant 18: 2415-2420. [Crossref]
- Rohdewald P (2002) A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther 40: 158-168. [Crossref]
- Rohdewald P (2015) Update on the clinical pharmacology of Pycnogenol®. Med Res Arch 3:1-11.
- Zini A, O’Bryan MK, Magid MS, Schlegel PN (1996) Immunohistochemical localization of endothelial nitric oxide synthase in human testis, epididymis, and vas deferens suggests a possible role for nitric oxide in spermatogenesis, sperm maturation, and programmed cell death. Biol Reprod 55: 935-941. [Crossref]
- Stanislavov R, Rohdewald P (2014) Sperm quality in men is improved by supplementation with a combination of L-arginine, L-citrullin, roburins and Pycnogenol®. Minerva Urol Nefrol 66: 217-223. [Crossref]
- Lewis SE, Donnelly ET, Sterling ES, Kennedy MS, Thompson W, et al. (1996) Nitric oxide synthase and nitrite production in human spermatozoa: evidence that endogenous nitric oxide is beneficial to sperm motility. Mol Hum Reprod 2:873-878. [Crossref]
- Durackova Z, Trebaticky B, Novotny V, Zitnanova I, Breza J (2003) Lipid metabolism and erectile function improvement by Pycnogenol®, extract from the bark of Pinus pinaster in patients suffering from erectile dysfunction – a pilot study. Nutr Res 23: 1189-1198.
- Stanislavov R, Nikolova V (2003) Treatment of erectile dysfunction with pycnogenol and L-arginine. J Sex Marital Ther 29: 207-213. [Crossref]
- Lamm S, Schönlau F, Rohdewald P (2003) Prelox® for improvement of erectile function: a review. Eur Bull Drug Res 11: 29-37.
- Ledda A, Belcaro G, Cesarone MR, Dugall M, Schönlau F (2010) Investigation of a complex plant extract for mild to moderate erectile dysfunction in a randomized, double-blind, placebo-controlled, parallel-arm study. BJU Int. 106: 1030-1033. [Crossref]
- Aoki H, Nagao J, Ueda T, Strong JM, Schoenlau F, et al. (2012) Clinical assessment of a supplement of Pycnogenol® and L-arginine in Japanes patients with mild to moderated erectile dysfunction. Phytother Res 26: 201-207. [Crossref]
- Stanislavov R, Rohdewald P (2015) Improvement of erectile function by a combination of French maritime pine bark and roburins with aminoacids. Minerva Urol Nefrol 67: 27-32. [Crossref]
- Schwedhelm E, Maas R, Freese R, Jung D, Lukacs Z, et al. (2008) Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol 65: 51-59. [Crossref]
- Országhova Z, Waczulíková I, Burki C, Rohdewald P, Durackova Z (2015) An effect of oak-wood extract (Robuvit®) on energy state of healthy adults – a pilot study. Phytother Res 29: 1219-1224. [Crossref]
- Roseff SJ (2002) Improvement in sperm quality and function with French maritime pine tree bark extract. J Reprod Med 47: 821-824. [Crossref]
- Stanislavov R, Nikolova V (2005) Prelox plus testosterone for achieving fertilization in previously infertile men. Eur Bull Drug Res 13: 7-13.
- Stanislavov R, Nikolova V, Rohdewald P (2009) Improvement of seminal parameters with Prelox®: A randomized, double-blind, placebo-controlled, cross-over trial. Phytother Res 23: 297-302. [Crossref]

