The aim of the study was to investigate the response of somatosensory cortex neurons to ventral posteromedial nucleus (VPM) stimulation in WAG/Rij rats, at the age when spike-and wave discharges (SWD), which is an EEG sign of absences epilepsy (AE) in these animals, is already formed. Extracellular primary somatosensory cortex (S1) single unit responses to solitary electric stimulation of VPM were recorded in 7 - 8-month-old WAG/Rij rats. The evoked unit activity in somatosensory cortex in response to afferent stimuli was found to include four components: primary (short-latency) excitation and inhibition and secondary (long-latency) excitation and inhibition. It was fond that in 7 – 8-month-old WAG/Rij rats the secondary activation in response to afferent stimulation is represented mostly by multi-component high-frequency phase reaction (alternations of neuron’s responses and periods of inhibition). The frequency of phase discharges is compatible with the frequency of SWD. It is suggested that phase reaction may be considered as a neuronal equivalent of epileptic activity and the SWD in WAG/Rij rats may be provoked by short excitation of the VPM.
neuron, somatosensory cortex, VPM, absence epilepsy, WAG/Rij rats
Rats of WAG/Rij line are genetically predisposed to absence epilepsy It is supposed that the trigger zone of spike-wave discharges (SWD) in these animals is the somatosensory cortex (vibrissae representation) [Sitnikova E., van Luijtelaar G. 2004]. It is believed that the process of reverberation between neocortex and thalamic nuclei underlies generalization of SWD [1]. Earlier we found that stimulation of the posterior thalamic nucleus (PO) in WAG/Rij rats at the age of 7-8 months, when absence epilepsy is already formed, causes a phase response (alternation of short discharges with inhibitory pauses) in somatosensory cortex neurons. We interpreted this activity as the neural equivalent of a SWD [2]. However, the same reaction was found after vibrissae stimulation. Due to the fact that sensory signal delivery to the cortex from the vibrissae is mediated both by the PO and the ventro-posteromedial thalamic nucleus (VPM), the question arose whether excitation of the VPM is able to cause the same phase discharge in the cortex in the WAG/Rij rats as stimulation of the PO.
The aim of the present study was to investigate the response of somatosensory cortex neurons to VPM stimulation in WAG/Rij rats when the SWD has already been formed.
In an acute experiment under urethane anesthesia (150 mg/100 g animal weight) on 6 WAG/Rij rats aged 7-8 months, the response of 60 primary somatosensory cortex (S1) neurons to electrical stimulation of ventral posteromedial nucleus (VPM) was studied. All experiments were performed in accordance with the protocol for the use of laboratory animals of the Institute of Higher Nervous Activity and Neurophysiology, Russian Academy of Sciences, based on the European Community Council Directive (86/609/EEC).
The structure of the neuronal response (Excitation, inhibition), the latencies of individual response components, their duration and the frequency of impulse activity of each excitatory component were analyzed. This allowed us to describe in sufficient detail the responses of S1 neurons to afferent stimulation.
Recording of unit activity: Using glass microelectrodes filled with 2 M NaCl solution (tip diameter ≤ 1 μm, impedance 8-10 mOm), we recorded extracellular activity of single neurons of S1 somatosensory cortex. The recording electrode was oriented at the coordinates AP +2.5; L 5.5 and inserted vertically. In this case, it entered the S1 somatosensory cortex tangentially at an angle of 450. Based on morphometric studies [3], it was calculated that layer IV of the cortex in this case occupied the space at a depth of 550 to 1400 µm. Registration of neuronal activity was performed in this range.
VPM stimulation: The stimulating bipolar electrode was inserted stereotactically into the VPM in the coordinates AR -3.6; L 2.6; H 6 mm. Stimulation was performed with a series of 10 single rectangular current pulses of 0.5 µA, in duration 0.2 ms. The inter-stimulus interval was 10 s.
Analysis of evoked neuronal activity: Impulse unit activity was recorded for 4 seconds - 2 seconds before and 2 seconds after the stimulus. Peristimulus histograms with bin resolution from 1 to 16 ms were constructed to analyze reactions based on the results of 10 stimuli.
The analysis was performed using a computer program, created at our request by L-Card. Based on the comparison of the average frequency of action potentials in the background and after stimulation, the program distinguished the suspected reaction zones (excitation/inhibition) by the criterion of 30% increase or decrease in the average frequency of discharges. After establishing a significant difference in the frequency of action potentials in the estimated reaction zone compared to the background (nonparametric Wilcoxon criterion was used), we calculated the latency (the beginning of the reaction zone from the moment of stimulation), reaction duration, and the frequency of action potentials in the zone of reaction.
Statistical significance of intergroup differences was determined using parametric t-test (all analyzed parameters had normal distribution - Shapiro-Wilk test).
Morphological control of stimulation electrode localization: After completing the electrophysiological part of the experiment,, the rats was sacrificed by chloroform, the brain was removed and fixed in buffered 4% formaldehyde (ph = 7.0) for 5 - 7 days. Then brains were kept in a 30 % sucrose solution in phosphate buffer (ph = 7.0) for cryoprotection (2–3 days, t = 4 °C). Serial coronal slices 25 μm thick were made with microtome cryostat (CTB 6, Medite GmbH ©, Germany), and stained with 0.1 % cresyl violet. In brain slices, the localization of the stimulating electrode tip in the VPM was determined according to the Paxinos rat brain atlas (Paxinos and Watson 1986). (Figure 1a).
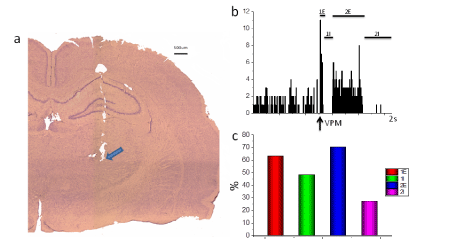
Figure 1. Responses of cortical somatosensory neurons to stimulation of VPM in Wag / Rij rats.
- Microscopic examination of the localization of the tip of the stimulating electrode in the VPM (indicated by the arrow).
- Cumulated peristimulus time histogram (10 trials). Trial epoch 2 sec, bin is 8 ms. Ordinate: number of spikes per bin. Arrow shows the moments of stimulation. Complex response, including primary excitation (1E), primary inhibition (1I), secondary excitation (2E) and secondary inhibition (2I).
- The frequency of the presence of activation and inhibition components of the response of somatosensory cortex neurons to VPM stimulation. (in %)
Statistics: Statistical significance of intergroup differences was determined using parametric t-test (all analyzed parameters had normal distribution - Shapiro-Wilk test).
The responses of somatosensory cortex neurons layer IV to VPM stimulation consisted of several activating and inhibitory components. We distinguished 4 components of the response according to the criteria of latency and form of response: primary excitation (1E), primary inhibition (1I), secondary excitation (2E), and secondary inhibition (2I) (Figure 1b).
The latency of primary excitation was 12.9 ± 2.5 ms, and the duration was 30.7 ± 19.8 ms. The latent period of secondary excitation was 272.2 ± 100.7 ms and the duration 310.1 ± 207.2 ms. For both measures, the primary and secondary responses were significantly different (t (13.1) - 45. P < 0.001; t (5.6), 45. P < 0.001, respectively). Primary inhibition occurred with a latency of 54.8 ± 44.4 ms, with a duration of 244.9 ± 219.9. The latency of secondary inhibition was 903.1 ± 271.2 ms, and the duration was 646.4 ± 250.9. The differences were significant (t (16.6) - 43. P < 0.001); t (6.7) - 43. P < 0.001, respectively).
the ratio of activation and inhibition components in response to VPM stimulation was different (Figure 1c). About 25% of neurons responded with a four-component response.
Secondary excitation manifested itself as two types of responses. In 36% of cases it was a tonic excitation of 340.4 ± 219.9 ms duration (Figure 1b). In 33% of the registered neurons a phase response was detected, including a group of 3 to 6 discharges, followed by periods of silence (Figure 2a).

Figure 2. Features of the phase response of neurons in the somatosensory cortex in WAG / Rij rats to VPM stimulation.
- secondary phase excitation. Peristimulus time histograms (10 trials). Trial epoch 2 sec, bin is 8 ms. Ordinate: number of spikes per bin. Arrow shows the moments of stimulation;
- ratio of the duration (in ms) of tonic and phase excitation components: Ordinate: duration of the response in ms.
* p < 0,001 according to the t test.
- ratio of the frequency of action potentials in the tonic and phase component of the response. Ordinate: frequency in Hz;
* p < 0,001 according to the t test
The duration of each discharge of the phase response was 91.1 ± 68.1 ms. The duration of the periods between activation phases ranged from 56 to 240 ms. The phase response differed significantly from the tonic response on two measures. The duration of the tonic discharge was significantly longer than the duration of the individual component of the phase response (t (59) = 5.83. P < 0.001) (Figure 2b). The frequency of action potentials in the phase response (36.86 ± 15.68 Hz) was significantly greater than the frequency of discharges in the tonic response (11.12 ± 2.7 Hz) - (t(26) = 6.05. P < 0,001) (Figure 2c).
Thus, in WAG/Rij rats during the period when SWD has already been formed, the phase response of somatosensory cortex neurons can be induced not only by stimulation of PO [4], but also by stimulation of VPM. Consequently, the phasic neuronal response occurs in response to the sensory stimulus regardless of which sensory input the excitation reached the cortex.
The fact that the secondary phase component of the response to VPM stimulation differs from the tonic response in two respects suggests that it represents an independent response and is not the result of structuring the secondary tonic response. The duration of the periods between phase discharges ranges from 56 to 240 ms, which corresponds to a frequency of 17 Hz to 4 Hz, and is in the range of frequencies of SWD in WAG/Rij rats [5]. This testifies in favor of our assumption that this phase response can be the neuronal equivalent of the SWD. Another argument in favor of this assumption can be the similarity of the phase activity we recorded with the activity of thalamic and cortical neurons in GAERS rats during an epileptic seizure [6]. Rhythmic neuronal activity was found to be detected initially in the S2 somatosensory and insular cortical areas and later in the S1 somatosensory cortex [7-8]. Our results suggest that the phase response of S1 neurons to a solitary VPM stimulation appears with a latency of 272.2 ± 100.7 ms, which is significantly longer than the immediate neuronal response to this stimulation (12.9 ± 2.5 ms). Perhaps the later appearance of the phase component of the response reflects the spread of excitation between cortical areas. The duration of the period between discharges (98 ± 24.2 ms) allows for the possibility that the phase discharge reflects reverberation of thalamocortical excitation. In this case, the inhibitory pauses structuring the response phases are most likely due to return inhibition. However, the data we obtained allow us to doubt the presence of return inhibition in the cortex in our animals. As was found, primary excitation is very quickly interrupted by primary inhibition (Figure 1b). Assuming that the basis of this inhibition is return, it is unclear why the secondary tonic excitation is not limited as rapidly by it - our data indicate a significantly longer duration of the tonic component compared to the duration of the primary excitation (t(63) = 8.29. P < 0,001). However, we cannot exclude that the activity of some cortical neurons is regulated by the return inhibition, and these neurons generate a phase response, while the activity of other neurons is not regulated by the return inhibition, and their secondary activation is represented by the tonic response.
It remains unclear whether the phase response of neurons in the WAG/Rij rats is due to reverberation, or whether it results from a specific (e.g., pacemaker) activity of cortical neurons in response to sensory stimulation. Further research is expected to clarify this question.
- In WAG/Rij rats, during the period when SWD is already formed, electrical stimulation of VPM elicits a phase response in 33% of somatosensory cortex neurons.
- According to the main indicators, the phase response of cortical neurons corresponds to SWD, which allows us to consider it the neural equivalent of epileptic activity.
This work was supported by the Russian Foundation for Basic Research. Grant 20-013-00176 "Social factors of early ontogenesis.
There are no conflicts of interest to be disclosed.
- Bosnyakova D, Gabova A, Zharikovae A, Gnezditski V, Kuznetsova G, et al. (2007) Some peculiarities of time-frequency dynamics of spike-wave discharges in humans and rats. Clinical Neurophysiology 118: 1736-1743. [Crossref]
- Lüttjohann A, van Luijtelaar G (2015) Dynamics of networks during absence seizure’s on- and offset in rodents and man. Frontiers in Physiology 6. ID 16.
- Paxinos G, Watson C (2005) The Rat Brain in Stereotaxic Coordinates, 5th edition. Elsevier Academic Press, New York.
- Pinault D, O’Brien T (2005) Cellular and network mechanisms of genetically-determined absence seizures. Thalamus and Related Systems 3: 181-203. [Crossref]
- Shubina O, Teltsov L, Komusova O (2015) Cytological and morphometric features of the cortex of white rats. Modern problems of science and education (part 1).
- Sitnikova E, van Luijtelaar G (2004) Cortical control of generalized absence seizures: effect of lidocaine applied to the somatosensory cortex in WAG/Rij rats. Brain Research 1012: 127-137. [Crossref]
- Tsvetaeva D, Sitnikova E, Raevsky V (2019) Cortical Somatosensory Neurons in WAG/Rij Rats Transform Firing Evoked by Simulation of Posterior Thalamic Nucleus from Tonic to Phasic at Age of 6 Months. Bulletin of Experimental Biology and Medicine 168: 1-4.
- Zheng T, O’Brien T, Morris M, Reid C, JovanovskaV, et al. (2012) Rhythmic neuronal activity in S2 somatosensory and insular cortices contribute to the initiation of absence related spike and wave discharges. Epilepsia 53: 1948-1958. [Crossref]


