Introduction: Diffuse intrinsic pontine glioma (DIPG) is a lethal brain tumor and leading cause of brain tumor–related death in children. Over the past few decades, clinical trials have shown no improvement in outcome. The purpose of this correspondence is to discuss the use of IV and oral Antineoplaston therapy (ANP {A10 + AS2-1}) in the treatment of a 3 and ½ month female with newly-diagnosed DIPG.
Materials and methods: The patient was enrolled as a special exception according to BT-11, a Phase II protocol utilizing IV and oral Antineoplastons A10 and AS2-1 (ANP). Her tumor response to ANP was measured by MRIs of the brain.
Results: At presentation to the Burzynski Clinic (BC), the patient demonstrated prominent Cushingoid features, limited lateral deviation of the left eye and incomplete closure of the left eyelid. There was partial paresis of the left side of the face. There were no long tract signs. The infant’s cry was normal as were the protective reflexes of the throat. Motor reflexes were intact, including the grasp and sucking reflexes. Muscle tone was normal and there was no posturing or arching. Babinski was negative bilaterally. Following ANP, the patient achieved resolution of her clinical signs, a complete response (CR) and > 23 years survival.
Conclusions: ANP is an effective treatment for DIPG and for a variety of low- and high-grade brain tumors. Multiple Phase II protocols utilizing ANP have now been completed and its impact on the treatment of brain tumors has been widely published.
brain tumor, brain stem glioma, diffuse intrinsic brainstem glioma., diffuse midline glioma, H3-K27 mutant (DMG H3-K27M), antineoplaston therapy, phase II and III clinical studies
A-10: Antineoplaston; A10 (Atengenal); AE: Adverse event; ANP: Antineoplaston; ANP therapy: A10 (Atengenal) + AS2-1 (Astugenal); AS2-1: Antineoplaston AS2-1 (Astugenal); Astugenal: Antineoplaston AS2-1 (AS2-1); Atengenal: Antineoplaston A10 (A10); BC: Burzynski Clinic; BRI: Burzynski Research Institute; CSF: Cerebral Spinal Fluid; CR: Complete Response; DIPG: Diffuse intrinsic pontine glioma; DMG H3-K27M: Diffuse midline glioma, H3-K27 mutant; FLAIR: Fluid attenuated inversion recovery; FDA: Food and Drug Administration; IV: Intravenous; MRI: Magnetic Resonance Imaging (of the brain); OR: Objective Response; PD: Progressive Disease; PR: Partial Response; RT: Radiation Therapy; SD: Stable Disease; WHO: World Health Organization.
Diffuse intrinsic pontine glioma (DIPG) is a malignant pediatric tumor with a median age at diagnosis of 6–7 years. Current treatment options are limited and prognosis is dismal—with less than 10% of patients surviving beyond 2 years from the time of diagnosis [1]. Surgery is not an option, the effects of radiation therapy (RT) are temporary, and no chemotherapeutic agent has demonstrated significant efficacy. Numerous clinical trials of new agents and novel therapeutic approaches have been performed over the course of several decades in efforts to improve the outcome of children with DIPG, but without success. The median survival for children with DIPG is less than one year from diagnosis and no improvement in survival has been realized in more than three decades [2,3].
The diagnosis of DIPG is based on characteristic magnetic resonance imaging (MRI) findings in the face of typical clinical findings such as abnormal or limited eye movements, diplopia, an asymmetric smile, clumsiness, difficulty walking, loss of balance, and weakness. Classic findings on clinical examination include the triad of multiple cranial neuropathies, long tract signs (hyperreflexia, clonus, increased tone, presence of a Babinski reflex), and ataxia.
On MRI, the tumor appears as an expansile brainstem mass. While there may be an exophytic component due to expansion of the tumor via the path of least resistance, the epicenter of a DIPG lies within the pons. DIPGs are hypo- or iso-intense on T1- weighted imaging, hyperintense on T2-weighted imaging, and frequently appear relatively homogeneous on fluid attenuated inversion recovery (FLAIR) sequences. Other MRI features of a typical DIPG include ventral involvement of the pons, and encasement of the basilar artery. Gadolinium contrast-enhancement is variable, but these lesions frequently do not enhance significantly at the time of diagnosis.
The standard of care for children with newly-diagnosed DIPG is focal RT, using a 1 cm margin to cover microscopic disease, to a total dose of 54–60 Gy administered over 6 weeks, usually in daily (Monday– Friday) 180–200 cGy fractions. About 75% of patients will have some improvement in neurological symptoms in response to RT, which appears to control tumor growth for a short period of time, prolonging survival by a mean of ~3 months [4]. Within 3–8 months after completion of RT, most children with DIPG will have clinical or radiographic evidence of disease progression. The pattern of failure is generally local. In one study, 25% of cases with disease progression involved the irradiated volume, while 75% involved the margin of the radiation field [5]. We present the case of a 3½ month old female, with DIPG, who had not received RT or chemotherapy prior to being seen at the Burzynski Clinic (BC).
ANP’s mechanism of action differs from that of RT or cytotoxic chemotherapy. Growth of normal cells is controlled by cell cycle progression genes (oncogenes) and by cell cycle arrest genes (tumor suppressor genes). In cancer, alteration of these control genes in malignant cells favors aggressive cell proliferation. Evidence suggests that ANP affects 112 genes in the tumor genome and functions as “molecular switches” which “turn on” tumor-suppressor genes and “turn off” oncogenes [6,7]. Hence, the antineoplastic action of ANP therapy in DIPG involves restoration of cell cycle control, induction of programmed cell death, and interference with cancer cell metabolism and nuclear transport
This 3½ month old female presented to a pediatrician because of decreased movement of the left eye and partial paresis of the left side of the face and mouth (Figure 1). On August 12, 1998, an MRI of the brain revealed a 3.2 x 2.5 x 4.5 cm mass within the brainstem, involving the pons, medulla, and the midbrain. There was a moderate mass effect on the 4th ventricle, but no hydrocephalus. The tumor was thought to be a brainstem glioma, likely originating in the pons. Surgery, RT, and chemotherapy were not considered viable treatment options because of the location of the tumor and the patient’s age. The infant was provided dexamethasone and Mylanta beginning August 15, 1998.
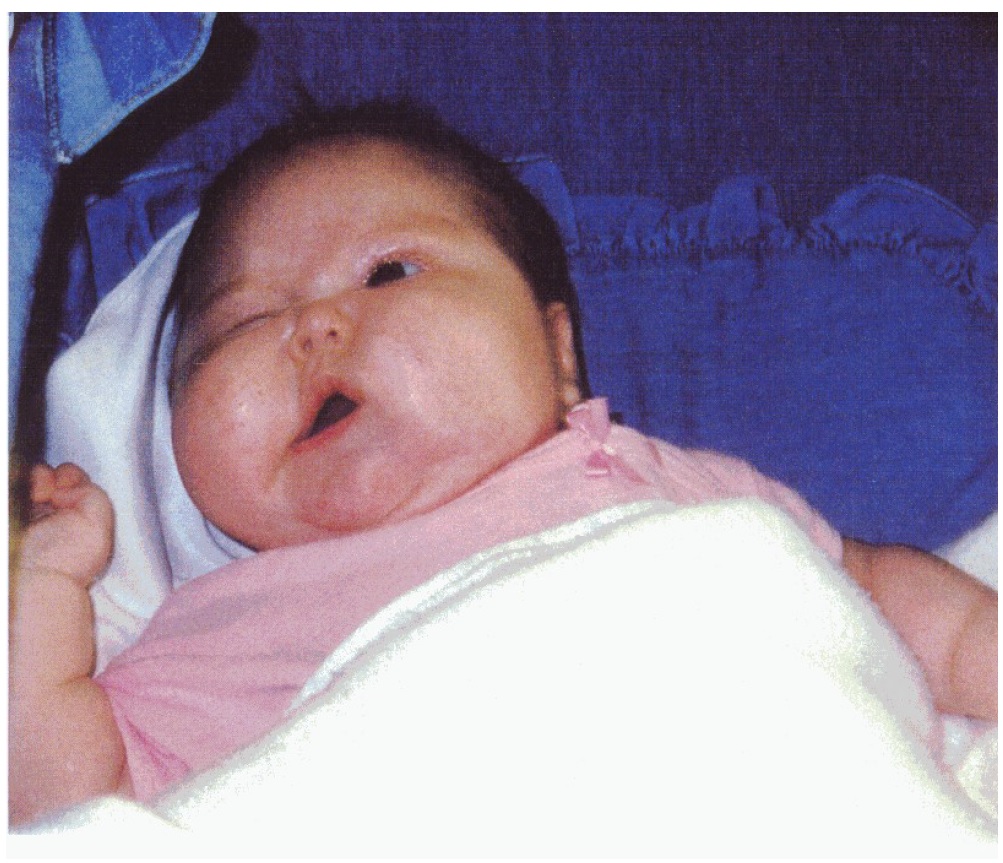
Figure 1: Pre-treatment female patient at ~ 3 and ½ months of age.
On October 13, 1998, this patient presented to the BC for evaluation. She had a Cushingoid appearance, was in no distress, and was well hydrated, alert, and active, weighing 19 pounds. The pupils were equal and reactive to light. Lateral deviation of the left eye was limited and closure of the left eyelid was incomplete. There was partial paresis of the left side of the face. There were no long tract signs. Babinski was negative bilaterally. Motor reflexes were intact, including the grasp and sucking reflexes. The infant’s cry was normal as were the protective reflexes of the throat. Muscle tone was normal and there was no posturing or arching.
On October 14, 1998, this 3 ½ month female was enrolled as a special exception, according to protocol BT-11, for administration of IV ANP. BT-11 was a Phase II study of Antineoplaston 10 (Atengenal) and Antineoplaston AS2-1 (Astugenal) for patients with brainstem gliomas [8]. The protocol was designed to determine an objective response (OR) to ANP therapy. Patients initially received ANP therapy via a subclavian catheter and an infusion pump. Oral ANP therapy was subsequently utilized.
Response to therapy was measured by MRIs with and without gadolinium enhancement. Tumor size was calculated as the product of the two greatest perpendicular diameters as determined by imaging. The response criteria were as follows: a complete response (CR) indicated complete disappearance of all enhancing tumor while a partial response (PR) indicated a > 50% reduction in tumor size. CR and PR required a confirmatory MRI performed at least four weeks after the initial finding. Progressive disease (PD) indicated a > 25 % increase in tumor size while stable disease (SD) did not meet the criteria for PR or PD. All MRIs were reviewed by an outside radiologist. Baseline MRI (October 13, 1988), on coronal images, showed an enhancing lesion measuring 10.73 cm2 while axial and sagittal images showed a non- enhancing lesion measuring 12.58 cm2 and a 14.70 cm2, respectively. The patient was treated with IV ANP therapy per BT-11 [8]. The dosages of A10 and AS2-1 were gradually increased to 9.42 g/kg/d and 0.56 g/kg/d, respectively. While on IV ANP therapy, the patient experienced the following adverse events (AEs), none of which were felt to be due to therapy: diarrhea, hematuria (x2), hyperchloremia, hyperglycemia, hypoglycemia, hypokalemia, urinary tract infection, and vomiting.
On February 22, 1999, the enhancing lesion seen on coronal imaging had disappeared while the non- enhanced axial and sagittal lesion now measured 8.12 cm2 and 12.04 cm2, respectively. On April 23, 1999, the enhancing lesion was again not visible, confirming that the patient had achieved a CR. On May 19, 2000, the axial and sagittal projections showed an 80% reduction in the size of the non-enhancing lesion, 2.28 cm2 and 3.45 cm2, respectively. Complete disappearance of tumor on the enhanced coronal imaging persisted (Figure 2 and Figure 3). On June 8, 2000, IV ANP therapy was discontinued and the patient began receiving oral ANP therapy. The outside radiologist commented, “Measurement of the unenhanced portion of the mass is difficult given the minimal change in signal associated on the T1 sequences…” He suggested that a PET scan be performed in the future to further quantitate the response. Subsequently, on April 8, 2004, whole body PET scan showed no hypermetabolic uptake in the brain parenchyma, especially the brainstem.
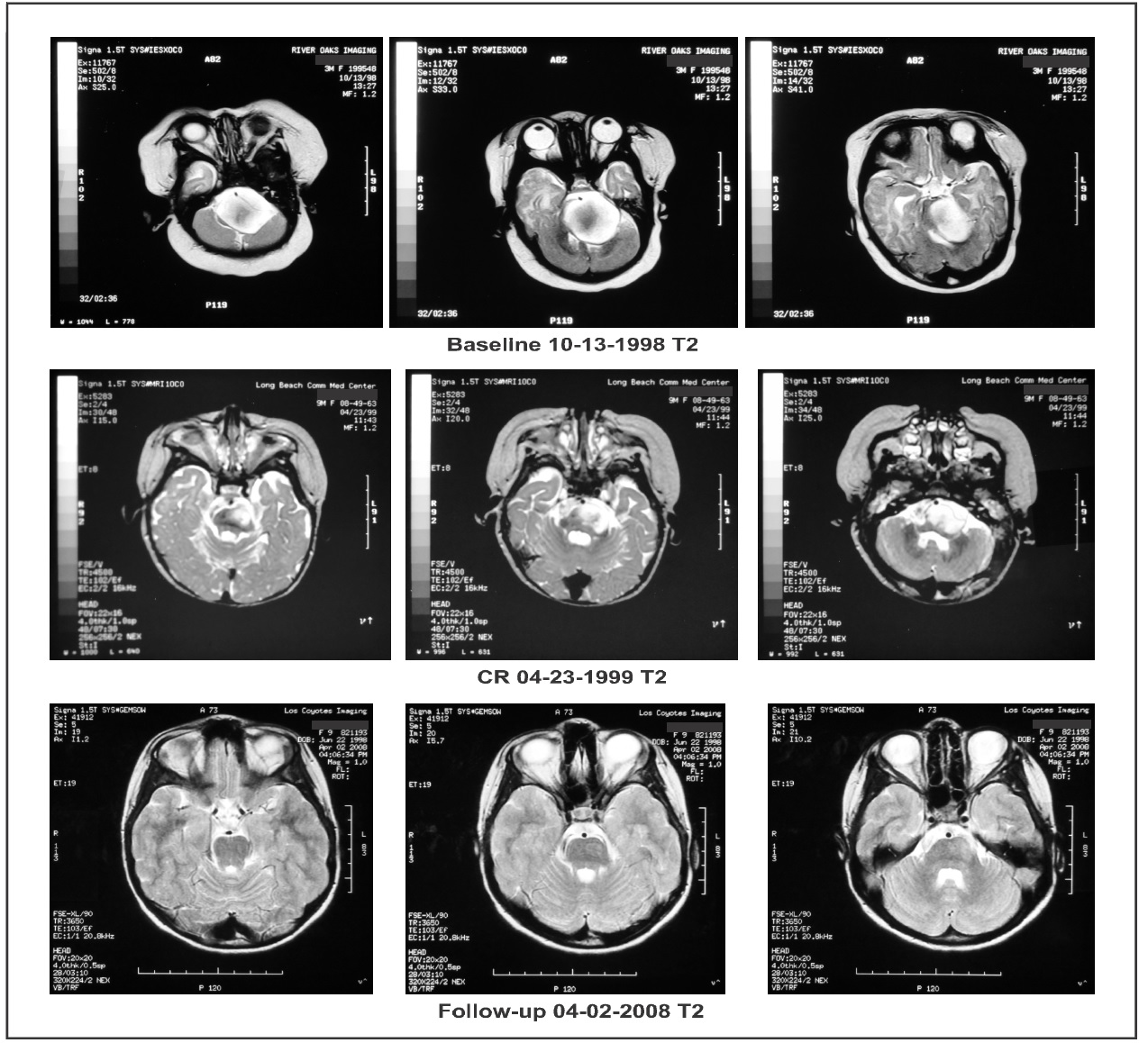
Figure 2. Axial brain magnetic resonance imaging (MRI) weighed in T2: “Baseline” demonstrates a high intensity contrast enhancing mass. “CR” shows a complete response (CR) of the high signal intensity mass following treatment. “Follow-up” shows persistence of the CR.
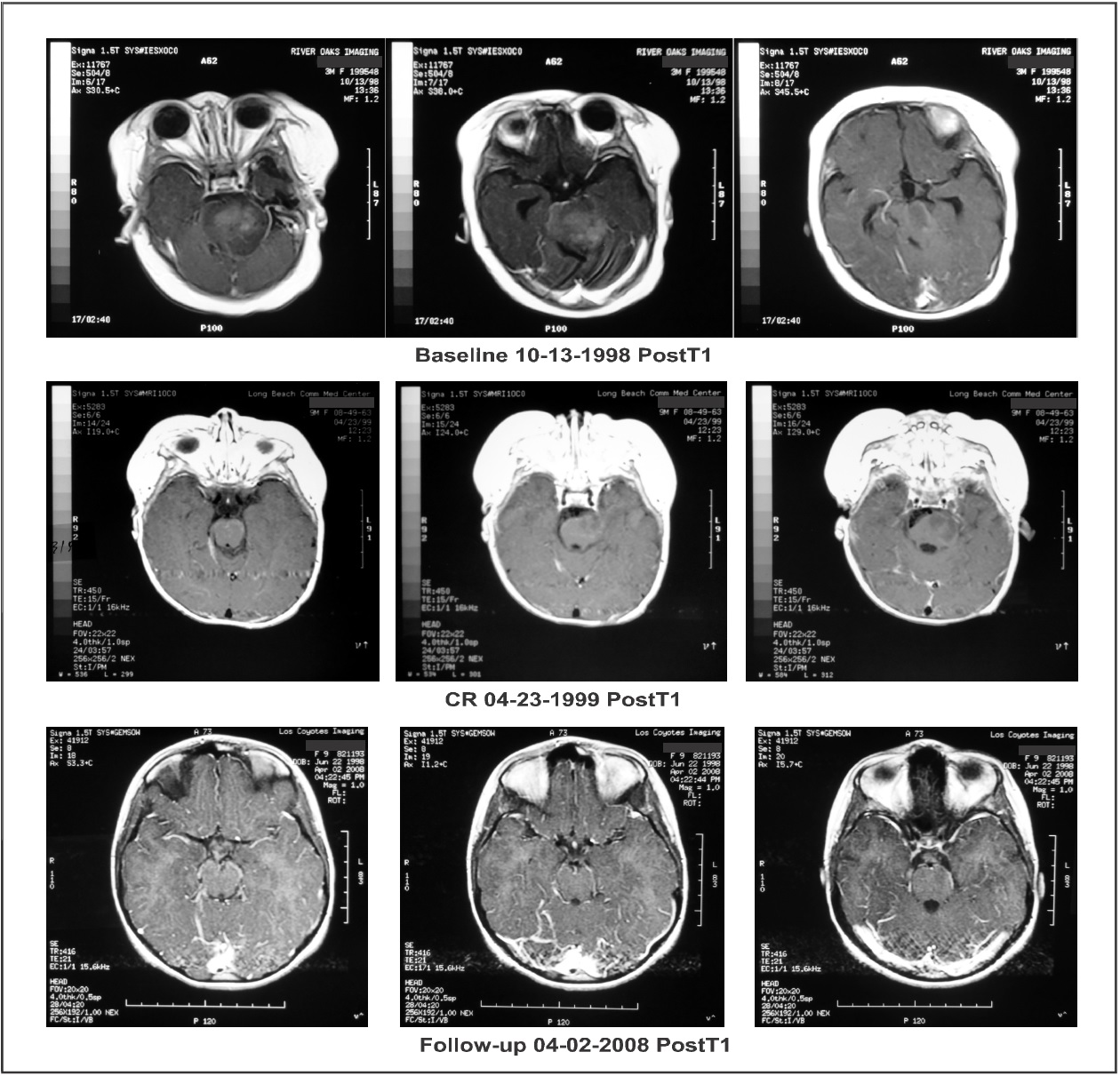
Figure 3: Axial brain magnetic resonance imaging (MRI) post contrast T1: “Baseline” demonstrates a contrast enhancing mass. “CR” shows a resolution of the contrast enhancing mass at a time corresponding with the beginning of complete response (CR). “Follow-up” shows complete resolution of the enhancing and low intensity signal mass.
The patient’s residual non-enhancing mass had resolved. Subsequent MRI performed on April 2, 2008 showed persistence of the CR. The patient was ~5 years of age at the time of her last presentation to the BC on April 7, 2004. She weighed 47.5 lbs. The patient’s pupils were equal and reactive to light. The extraocular muscles were intact. There was lateral nystagmus of the right eye. The initial lateral deviation of the left eye, incomplete closure of the left eyelid, and the left-sided facial weakness had cleared. There was minimal weakness of the right-sided extremities and a slightly abnormal gait. As examined by finger-nose-finger and heel-to-shin exercises, the patient’s coordination was good. Motor reflexes were intact and equal bilaterally. Babinski was negative bilaterally.
On August 5, 2021, we received an email from the patient who is now 23 years old. She is doing very well, living a fully normal life, and has a four-year old son. Included in the email were photographs of the patient and her son. The patient also provided a signed consent allowing us to utilize her medical information, MRI images, and photographs in a manuscript for publication (Figure 4).

Figure 4: Twenty-three-year-old patient, after ANP therapy, holding her four-year-old son.
DIPGs represent 80% of all pediatric brain tumors that occur in the brainstem [9-11] while affecting 200-300 children in the USA every year. MRI has allowed classification of these tumors into distinct subsets of focal, dorsally exophytic, cervicomedullary, or DIPG based on imaging characteristics [9]. The prognosis for children with diffusely infiltrating DIPGs is significantly worse than that of other brainstem tumors.
Histologically, these tumors share features with anaplastic astrocytomas (grade III) or glioblastomas (GBM) (grade IV) [12]. New molecular understanding of pediatric high-grade gliomas has led to the reclassification of DIPG as one member of a family of diffuse gliomas occurring in the midline of the central nervous system that exhibit pathognomonic mutations in genes encoding histone 3 (H3 K27M). Histone H3F3A and HIST1H3B K27M mutations define two subgroups of DIPGs with different prognosis and phenotypes [13,14]. However, DIPG remains a clinically relevant term. Wild-type H3-K27M DIPGs have not yet been separately classified within the revised WHO classification, but show similar survival as mutant H3-K27M DIPGs [15].
Routine biopsy of children with suspected DIPG has been performed in Europe since 2003. [16] In their initial report detailing experience in 24 children, reversible morbidity was described by the investigators in two children (cranial nerve palsy, worsening hemiparesis) with no mortality. It was concluded that the procedure was safe in experienced hands using modern neurosurgical technique. Subsequently, within the pediatric neuro-oncology community, there has been movement toward routine biopsy of patients with suspected DIPG [16,17].
Antineoplaston (ANP) research began in 1967, when significant deficiencies were noticed in the peptide content of the serum of patients with cancer compared with healthy persons. Initially ANPs were isolated from the blood and later from urine [18]. Subsequent studies of the ANPs that were isolated demonstrated that Antineoplaston A10 and Antineoplaston AS2-1 were the most active ANPs. The chemical name of Antineoplaston A10 is 3-phenylacetylamino-2,6-piperidinedione. It consists of the cyclic form of L-glutamine connected by a peptide bond to phenylacetyl residue. When given orally, Antineoplaston A10 resists the attack of gastric enzymes. In the small intestine, under alkaline conditions, 30% is digested into phenylacetylglutamine (PG) and phenylacetylisoglutaminate (isoPG) in a ratio of approximately 4:1. The mixture of synthetic PG and isoPG in a 4:1 ratio, dissolved in sterile water constitutes Antineoplaston A10 IV injection. Further metabolism of Antineoplaston A10 results in phenylacetate (PN). Both metabolites PG and PN have anticancer activity. The mixture of PN and PG in a 4:1 ratio, dissolved in sterile water constitutes Antineoplaston AS2-1 IV injection [19]. The 3½ month female presented here responded to both IV and oral ANP therapy.
In this report, we have presented a 3 1/2 month old female with a newly-diagnosed DIPG, who received ANP therapy, had resolution of her tumor-induced signs and symptoms, has survived for >23 years and maintains a CR. ANP therapy has been utilized in in a variety of low- and high-grade brain tumors under the Burzynski Research Institute’s (BRI’s) IND # 43,742. Multiple Phase II protocols have been completed and the impact of ANP on the treatment of brain tumors has been widely published [19-51]. In conjunction with the FDA, two additional protocols of ANP therapy in DIPG patients, have been developed, a Phase II protocol and a Phase III protocol (ANP + RT versus RT alone). Both protocols will soon be accruing patients.
The authors express their appreciation to Carolyn Powers for preparation of the manuscript and to Ramiro Rivera, Mohamed Khan, Jennifer Pineda and Adam Golunski for their involvement.
- Warren K (2012) Diffuse intrinsic pontine glioma: poised for progress. Front Oncol 2: 205. [Crossref]
- Mandell L, Kadota R, Freeman C, Douglass E, Fontanesi J, et al. (1999) There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys 43: 959-964. [Crossref]
- Cohen K, Heideman, R, Zhou, T, Holmes, E, Lavey R, et al. (2011) Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro Oncol 13: 410-416. [Crossref]
- Haas-Kogan D, Banerjee A, Poussaint T, Kocak M, Prados M, et al. (2011) Phase II trial of tipifarnib and radiation in children with newly diagnosed diffuse intrinsic pontine gliomas. Neuro Oncol 13: 298-306. [Crossref]
- Grigsby P, Garcia D, Ghiselli R (1989) Analysis of autopsy findings in patients treated with irradiation for thalamic and brain stem tumors. Am J Clin Oncol 12: 255–258. [Crossref]
- Burzynski SR, Patil S (2014) The effect of Antineoplaston A10 and AS2-1 and metabolites of sodium phenylbutyrate on gene expression in glioblastoma multiforme. J Cancer Ther 5: 929-945.
- Burzynski SR, Janicki T, Burzynski G (2015) Comprehensive genomic profiling of recurrent classic glioblastoma in a patient surviving eleven years following antineoplaston therapy. Cancer Clin Oncol 4: 41-52.
- Burzynski SR, Janicki, T, Burzynski G, Marszalek A (2015) A phase II study of antineoplastons A10 and AS2-1 in patients with brainstem gliomas. The report on non-diffuse intrinsic pontine glioma (Protocol BT-11). J Cancer Ther 6: 334-344. [Crossref]
- Epstein F, Farmer JP (1993) Brain-stem glioma growth patterns. J Neurosurg 78: 408-412. [Crossref]
- Jansen MH, Veldhuijzen van Zanten SE, Sanchez Aliaga E, Heymans MW, Warmuth-Metz M, et al. (2015) Survival prediction model of children with diffuse intrinsic pontine glioma based on clinical and radiological criteria. Neuro Oncol 17: 160166. [Crossref]
- Langmoen IA, Lundar T, Storm-Mathisen I, Lie SO, Hovind KH (1991) Management of pediatric pontine gliomas. Childs Nerv Syst 7: 13-15. [Crossref]
- Smith MA, Freidlin B, Ries LA, Simon R (1998) Trends in reported incidence of primary malignant brain tumors in children in the United States. J Natl Cancer Inst 90: 1269-1277. [Crossref]
- Castel D, Philippe C, Calmon R, Le Dret L, Truffaux N, et al. (2015) Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol 130: 815-827. [Crossref]
- Karremann M, Gielen GH, Hoffmann M, Wiese M, Colditz N, et al. (2018) Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro Oncol 20: 123-131. [Crossref]
- von Bueren AO, Karremann M, Gielen GH, Benesch M, Fouladi M, et al. (2018) A suggestion to introduce the diagnosis of “diffuse midline glioma of the pons, H3 K27 wildtype (WHO grade IV)”. Acta Neuropathol 136: 171-173. [Crossref]
- Roujeau T, Machado G, Garnett MR, Miquelet C, Puget S, al. (2007) Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg 107: 1-4. [Crossref]
- Rutka J (2012) Biopsy of diffuse intrinsic pontine glioma? J Neurosurg Pediatr 10: 79-80.
- Burzynski SR (1986) Antineoplastons: Biochemical defense against cancer. Physiol Chem Phys 8: 275-279. [Crossref]
- Burzynski SR (1986) Synthetic antineoplastons and analogs. Drugs Future 11: 679-688.
- Burzynski SR, Weaver RA, Bestak M, Lewy RI, Janicki TJ, et al. (2003) Phase II study of Antineoplastons A10 and AS2-1 (ANP) in children with recurrent and progressive multicentric glioma. A preliminary report. Neuro Oncol 5: 358. [Crossref]
- Burzynski SR, Lewy RI, Weaver R, Janicki T, Jurida G, et al. (2004) Long-term survival and complete response of a patient with recurrent diffuse intrinsic brain stem glioblastoma multiforme. Integ Cancer Ther 3: 257-261. [Crossref]
- Burzynski SR, Weaver R, Lewy R, Janicki T, Jurida G, et al. (2004) Phase II study of antineoplaston A10 and AS2-1 in children with recurrent and progressive multicentric glioma. A Preliminary Report. Drugs R D 5: 315-326. [Crossref]
- Burzynski SR, Weaver R, Bestak M, Janicki T, Jurida G, et al. (2004) Phase II studies of antineoplastons A10 and AS2-1 (ANP) in children with atypical teratoid/rhabdoid tumors (AT/RT) of the central nervous system. A preliminary report. Neuro Oncol 6: 427.
- Burzynski SR, Weaver R, Bestak M, Janicki T, Szymkowski B, et al. (2004) Treatment of primitive neuroectodermal tumors (PNET) with antineoplastons A10 and AS2-1 (ANP). Preliminary results of phase II studies. Neuro Oncol 6: 428.
- Burzynski SR, Weaver RA, Janicki T, Szymkowski B, Jurida G, et al. (2005) Long-term survival of high-risk pediatric patients with primitive neuroectodermal tumors treated with Antineoplastons A10 and AS2-1. Integ Cancer Ther 4: 168-177. [Crossref]
- Burzynski SR (2006) Targeted Therapy for Brain Tumors. In: Brain Cancer Therapy and Surgical Interventions. Nova Science Publishers, Inc.
- Burzynski SR, Janicki, TJ, Weaver RA, Burzynski B (2006) Targeted therapy with Antineoplastons A10 and AS2-1 of high grade, recurrent, and progressive brainstem glioma. Integ Cancer Ther 5: 40-47. [Crossref]
- Burzynski SR (2006) Treatments for astrocytic in children: Current and emerging strategies. Ped Drugs 8: 167-168. [Crossref]
- Burzynski SR (2007) Recent clinical trials in diffuse intrinsic brainstem glioma. Cancer Ther 5: 379-390.
- Burzynski S, Janicki T, Burzynski G, Marszalek A (2013) Long-term survival (>13 years) in a child with recurrent diffuse pontine gliosarcoma: A case report. J Ped Hematol Oncol 36: e433-e439.
- Burzynski SR, Janicki TJ, Burzynski GS, Marszalek A (2014) A phase II study of antineoplastons A10 and AS2-1 in children with high-grade glioma. Final report (Protocol BT-06) and review of recent trials. J Cancer Ther 5: 565-577.
- Burzynski SR, Janicki TJ, Burzynski GS (2014) A phase II study of antineoplastons A10 and AS2-1 in adult patients with recurrent glioblastoma multiforme: Final report (Protocol BT-21). J Cancer Ther 5: 946-956.
- Burzynski SR, Burzynski GS, Janicki TJ (2014) Recurrent glioblastoma multiforme—A strategy for long- term survival. J Cancer Ther 5: 957-976.
- Burzynski SR, Janicki TJ, Burzynski GS, Marszalek A, Brookman S (2014) A phase II study of antineoplastons A10 and AS2-1 in children with recurrent, refractory or progressive primary brain tumors—Final report (Protocol BT-22). J Cancer Ther 5: 977-988.
- Burzynski SR, Janicki TJ, Burzynski GS, Brookman S (2014) Preliminary findings on the use of targeted therapy with pazopanib and other agents in combination with sodium phenylbutyrate in the treatment of glioblastoma multiforme. J Cancer Ther 5: 1423-1437.
- Burzynski GS, Janicki TJ, Marszalek A (2014) Long-term survival (>20 years) of a child with brainstem glioma treated with antineoplastons A10 and AS2-1: a case report. Neuro Oncol 11: 16.
- Burzynski SR, Janicki TJ, Burzynski GS, Marszalek A (2014) The response and survival of children with recurrent diffuse intrinsic pontine glioma based on phase II study of antineoplastons A10 and AS2-1 in patients with brainstem glioma. Childs Nerv Syst 30: 2051-2061. [Crossref]
- Burzynski SR, Burzynski G, Janicki J, Marszalek A (2015) Complete response and Long-term survival (>20 years) of a child with tectal glioma: A case report. Pediatr Neurosurg 50: 99-103.
- Burzynski SR, Janicki TJ, Burzynski G (2015) A phase II study of Antineoplastons A10 and AS2-1 injections in adult patients with recurrent anaplastic astrocytoma - Final report (Protocol BT-15). Cancer Clin Oncol 442: 13-23.
- Burzynski SR, Janicki TJ, Burzynski GS, Marszalek A (2015) A Phase II Study of Antineoplastons A10 and AS2-1 in adult patients with Newly-Diagnosed Anaplastic Astrocytoma Final Report (Protocol BT- 08). Cancer Clin Oncol 4: 28-38.
- Burzynski SR, Burzynski GS, Marszalek A, Janicki J, Martinez-Canca J (2015) Long-term survival (over 20 years), complete response and normal childhood development in medulloblastoma treated with Antineoplastons A10 and AS2-1. J Neurol Stroke 2: 00054.
- Burzynski SR, Burzynski GS, Marszalek A, Janicki TJ, Martinez-Canca JF (2015) Long-term survival over 21 years and pathologically confirmed complete response in pediatric anaplastic astrocytoma: A case report. J Neurol Stroke 2: 00072.
- Burzynski SR, Burzynski GS, Brookman S (2015) A Case of Sustained Objective Response of Recurrent/Progressive Diffuse Intrinsic Pontine Glioma with Phenylbutyrate and Targeted Agents. J Cancer Ther 6: 40-44.
- Burzynski SR, Janicki T, Burzynski G (2015) A phase II study of Antineoplastons A10 and AS2-1 in adult patients with primary brain tumors. Final Report (Protocol BT-09). J Cancer Ther 6: 1063- 1074.
- Burzynski SR, Janicki TJ, Burzynski GS (2016) Primary CNS tumors and Leptomeningeal, Disseminated and/or Multicentric disease in children treated in Phase II studies with Antineoplastons A10 and AS2-1. Cancer Clin Oncol 5: 38-48.
- Burzynski SR, Janicki TJ, Burzynski GS (2016) A phase II study of antineoplastons A10 and AS2-1 in children with low-grade astrocytomas–Final report (Protocol BT-13). J Cancer Ther 7: 837-850.
- Burzynski SR, Janicki TJ, Burzynski GS (2017) Antineoplastons A10 and AS2-1 in the Treatment of children with optic pathway glioma: Final Report for protocol BT-23. Cancer Clin Oncol 6: 25-35.
- Burzynski SR, Janicki TJ, Burzynski GS, Marszalek A (2017) A phase II study of Antineoplastons A10 and AS2-1 in children with brain tumors. Final Report (Protocol BT-10). J Cancer Ther 8: 173- 187.
- Burzynski SR, Janicki T, Beenken S (2019) Treatment of Recurrent Glioblastoma Multiforme (rGBM) with Antineoplaston AS2-1 in Combination with Targeted Therapy. Cancer Clin Oncol 8.
- Burzynski SR, Janicki T, Burzynski GS, Beenken S (2021) Long-term survival (27.7 years) following IV Antineoplaston Therapy (ANP) in a 36-year-old-female with a progressive diffuse intrinsic pontine glioma (DIPG). Int J Radiol Imaging Technol 7: 073-078.
- Burzynski SR, Burzynski GS, Janicki T, Beenken S (2021) Long-term survival (23 years) in a 26-year-old male after Antineoplaston Therapy for a progressive, diffuse intrinsic pontine glioma: A case report. Int J Brain Disorder Treat.




