The well-accepted assumption of metallic iron (Fe0) acting as electron donor for environmental remediation has created an unstable domain of knowledge for the past 23 years. This assumption is discouraging some outstanding and prospective scientists from correctly interpreting their experimental results. Such a situation is a recipe for long-term decline. The critical situation cannot be solved with simplistic approaches. It is now imperative to develop an understanding to defend the difficulties of this assumption and re-orient Fe0 mediated remediation research as a whole.
peer review, research funding, systematic flaws, water treatment, zero-valent iron
The research on using metallic iron (Fe0) for environmental remediation and water treatment is no more in a golden age. This opportunity can be used to address systemic flaws that have certainly threatened its development, and that were difficult to address some 10 years ago [1-3]. A central flaw is the long-held assumption that Fe0 is a direct reducing agent for the chemical transformation of contaminants [4-9]. As a result, the large majority of published works is still considering Fe0 as an environmental reducing agent [7,8,10-12]. The demonstration that this assumption has been a popular fallacy is now ten years old [2]. In a hyper-competitive atmosphere for research funding, scientific progress is broken as some have received their grants funded possibly for the propagation of misconceptions. Others have seen their grants rejected because they are dancing outside the circle. Thus, promising scientific careers are continued to be misdirected.
In retrospect, Fe0 remediation research was born with a mistake as the reductive transformation paradigm has never been convincingly established [13-16], but the flaw has been 'difficult' to recognize in the midst of success stories [17-24]. During the past two decades, Fe0 remediation researchers have (re) demonstrated the potential of Fe0 and Fe0-based filtration to remediate many cases of pollution including wastewater [10-12] and safe drinking water [25-30]. More than a century, Fe0 materials have been used in household water filters [31,32] and at large scale for the provision of cities [33-35]. More than 200 Fe0-based reactive walls are installed worldwide and are performing well [10,36]. Some rules of thumb are now available to design efficient and sustainable Fe0 filters, based on these and other achievements [37,38].
Despite such progress, it is remarkable that no bright future is generally expected from Fe0 filters [39-42]. Some systems have been almost abandoned [25,43] while even the proven efficient SONO filters [26,28] have not yet reached the expected large-scale distribution. The balance between ‘expectations and achievements’ are left out from the pure scientific perspective [44,45]. As an example, the very first Fe0 wall containing only 22% Fe0 (w/w) [14] is commonly referenced as a success story [10,16]. However, the majority of available Fe0 walls contain larger Fe0 proportions [21,22,24]. The rationale for their reported sustainability does not seem to have been discussed yet. Based on these observations and evidences that alternative views have been constantly refuted [8,46-50] or belittled [51,52]. It is fair to argue that the Fe0 remediation research has progressed on an unsustainable path [53]. In the light of these findings, an attempt has been made in this communication to describe how this situation arose and proposes/reiterates some possible remedies. Critical analysis of the published literature revealed that the writing style has been given more attention and weightage than the scientific content.
The root cause of the widespread misconception is twofold: (i) the assumption that an observed chemical reduction of chlorinated solvents in a Fe0-based vessel is mediated by electrons from the metal body (reduction by Fe0, as a direct reduction) [54], and (ii) the consensus-based approach adopted in the initial stage of research on Fe0 for environmental remediation [14]. The research community must realize now that this misconception has created a non-precedent confusion [35,44,45,55-60]. Over the last decade, isolated researchers [8,23,61-68] have corrected the mistake but the large majority is still confounding 'reduction in the presence of Fe0 (including indirect reduction) and 'reduction by Fe0 (direct reduction-electrons from Fe0) [1-3,69,70].
The reductive transformation concepts
The idea that electrons from Fe0 mediate contaminant reductive transformations was adopted from a seminal work by Matheson and Tratnyek [4]. Although controversial views were published during 1994 and 1995 [13,71,72] Weber [6] validated and generalized the results of Matheson and Tratnyek [4]. Despite this pseudo-scientific demonstration supported by further reports [5,73,74], O'Hannessin and Gillham [75] acknowledged that the reductive transformation concept was a 'broad consensus'. Recently, Ebelle et al. [76] presented a detailed discussion of Matheson and Tratnyek [4]. It is clearly demonstrated that the conclusions of Matheson and Tratnyek [4] were not supported by own results. The question arises why could such a fallacy be introduced in the scientific literature? The evidence that the whole research community has ignored ancient works on using Fe0 for safe drinking water provision [31-35,77,78] is not addressed herein. Using metallic elements (including Al0, Fe0 and Zn0) for water treatment was also common place during the 1980s [79-83].
The propagation of the misconception
The Fe0 remediation technology was born in North America (Canada, USA) and 'migrated' to Australia, Europe and the rest of the word over the time [18,42,84-89]. The American colleagues were considered the absolute experts of the 'new born'. For example, the Fe0 remediation technology was introduced in Germany by a literature review [90] presenting the technology as an American innovation. The original literature review was improved two years later to account for novelties mostly based on not readily accessible technical documents [91]. The introduction of Fe0 walls in Italy and Belgium followed a similar path [42,84,85,87,89,92,93]. The mistakes transported in this trial have largely been identified and discussed [55,70,94-100] and will not be duplicated herein. It is sufficient to recall two aspects: (i) no initial critical review of the related scientific literature was performed and (ii) the iron mass balance was fully ignored. Interestingly, a vertical look through the list of authors reveals that many of them are members of editorial boards of authoritative journals. Authors and editors must endorse now the responsibility of the propagated misconception [101]. The next section illustrates the extent of confusion using the Fe0/Se system [102].
Lessons from the Fe0/Se system
The efficiency of Fe0 filings to remove Se from agricultural drainage water was demonstrated during the 1980s [80,82,103]. The operating mode of Fe0 filters for Se removal was elucidated by Anderson [82]. Accordingly, Fe0 serves as generator of contaminant collectors and SeVI, SeIV and Se0 are all adsorbed and/or occluded (co-precipitation) in the matrix of iron corrosion products (FeCPs). Fe0 filters were efficient but not sustainable because iron particles were soon cemented to each other and the initial porosity of the filters was filled by in-situ generated FeCPs (yielding permeability loss). In other words, Harza Fe0 filters were efficient but not sustainable.
With the advent of Fe0 reactive walls (permeable reactive barriers), the Fe0/Se system was 'rediscovered' and (mostly independently) intensively investigated over the past 17 years [15,40,102,103,104-113]. SeVI was tested as redox-sensitive species to be reductively precipitated by Fe0. This effort was supported by an impressive number of modern analytical tools, using both bulk and surface methods [15,109,110,114,115]. Related studies tested several types of Fe0 materials including: (i) iron filings (IF), (ii) sponge iron and (iii) steel wool (SW). Available results can be summarized as follows: (i) Fe0 reduction can significantly decrease selenium concentrations at laboratory scale, (ii) Fe0 filters have successfully treated Se in full-scale operations at some sites, (iii) At some sites neither IF (granular Fe0) nor SW (fibrous Fe0) was efficient to meet regulatory requirements, (iv) at other sites SW were successful were IF failed and (v) no clear trend between the residence time in the filters and the efficiency could be established.
The example of the Fe0/Se system illustrates the extent of confusion within the Fe0 research community, despite available powerful analytical tools [38,53]. This communication advocates that the confusion is primarily due to the false assumption that Fe0 is a reducing agent. It appears that considering Fe0 as generator of Se collectors solves reported discrepancies and enable the design of (efficient and) sustainable filters. In fact, what is the long-term kinetics of Fe0 corrosion? Which Fe0 materials have the (long-term) ability to produce enough FeCPs for the collection of Se from the polluted water of concern? Answering these questions starts with two points: (i) avoiding pure Fe0 systems (columns with a zone containing 100 % Fe0) like in the Harza Process and (ii) characterizing the intrinsic reactivity of individual materials [38]. If such a systematic approach is broadly adopted, the modified Harza Process will be sustainable. Moreover, because co-precipitation and size-exclusion are primarily non (or less) selective mechanisms, Fe0 filters do not only address species with high affinities to FeCPS [59]. This is the rationale for making Fe0 filters as universal devices for safe drinking water and sanitation [38,69].
The assessment of the peer review system questioned
As it is now established that systematic flaws were governing research on Fe0 for environmental remediation [35,44,45, 53,55,57-61], there have been some injustice within the research community. In particular, the demands for research money is much larger than the supply. This hyper-competition is supposed to retain just the best scientific workforce for the limited funds available. However, in a context where the expertise of the referees is questioned, it appears that the best scientific workforce is rather punished for its clairvoyance. The great majority of environmental research is conducted by PhD students and postdoctoral fellows. But as a rule, the grants are obtained by more experienced scientists, including professors. In another phrase for two decades (5 generations of PhD student or 10 generations of postdoctoral fellows), experienced scientists working on Fe0 for environmental remediation have been the 'blind guide of the blind'. Consequently, a growing number of PhDs is graduating within a knowledge system inhibiting their creativity. In this vicious circle, they are performing a contra-productive job without advantage for the taxpayers’ investment in their lengthy career of education. The current knowledge system on Fe0 remediation is a perpetual danger for the integrity of science.
Undermining the scientific knowledge-building process
Science is a collective activity aiming at building a reliable body of knowledge about the physical world. This goal can only be achieved if all scientists are honest and dedicated to the service of their respective nations. In this common project, there is no place for consensus and frauds. Admittedly, some consensus could be temporally met but just in the sake of more clarity. In other words, scientists who are committed to the task must take serious conscious steps to ensure that no falsehoods are introduced in the common knowledge database.
It is difficult to understand why scientists are undermining their own art. Hundreds of articles contain expressions like ‘to the best of the author’s knowledge’ in the introduction to rationalize the investigation of things that are known for more than one century [33-35,77,78]. Moreover, several authors have put various arguments forward to maintain that Fe0 is an environmental reducing agent despite controversial views [8,46-52]. This attitude runs the risk of derailing the project of establishing the science of Fe0 remediation. Scientific knowledge-building should remain the paramount goal, whether it is accompanied by scientific scorekeeping or not.
This section analytically describes the Fe0/H2O system while ignoring primarily, intentionally achievements in the framework of 'Fe0 remediation'. The reader is then confronted with the dilemma of the 'Fe0 remediation' research.
An analysis of the Fe0/H2O system
A piece of reactive Fe0 immersed in an aqueous solution undergoes spontaneous oxidative dissolution (aqueous iron corrosion) according to Eq. 1 (69,70):
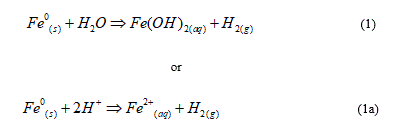
Equation 1 shows that Fe0 is a source of FeII and H species. Thus, Fe0 can be universally applied to generate FeII and H species (reducing agents) for any relevant application, including the generation of FeIII and FeIV species [31,34,45,56,116]. Eq. 1 recalls that Fe0 is oxidized by water (H2O) while Eq. 1a recalls that iron corrosion, and the corresponding H2 generation, is more quantitative under acidic conditions (abundance of H+). Another explanation of Eq. 1a is that the consumption of H+ yields to an increase of the pH value.
Under anoxic conditions, Fe(OH)2 is polymerized and precipitated as hydroxide which is mostly further transformed to magnetite (Fe3O4 or FeO.Fe2O3). A multi-layered oxide scale is formed on the Fe0 surface and acts as physical barrier for the transport of reactive species, from and to the Fe0 surface [1-3,117,119]. This is the rationale for the universally reported decreased kinetics of iron corrosion by the oxide scale [44,117,118]. It is essential to recall that under natural conditions (no external current), no direct Fe0 oxidation to FeIII is possible. Moreover, FeIII species are constantly present within anoxic Fe0/H2O systems and their formation, rationalized by several mechanisms, is not addressed herein [119,120].
Under oxic conditions (presence of oxidizing agents including O2), Eq. 1 is still the main reaction for aqueous iron corrosion [121]. Its reaction kinetics is accelerated by the consumption of FeII species (LeChatelier Principle) which are further oxidized to less soluble FeIII species (mostly hydroxides and oxides). In another phrase, species reductive transformation in a Fe0/H2O system results from a chemical reaction between FeII and H species (indirect reduction) and not from an electrochemical reaction (electrons from Fe0, direct reduction). Thus, reaction as per Eq. 2 is impossible under environmental conditions. RCl stands for a halogenated chlorinated hydrocarbon.
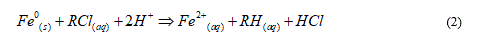
Three other important features of the Fe0/H2O system are: (i) the volumetric expansive nature of iron corrosion [122], (ii) the fact that iron hydroxides precipitate in the presence of foreign species (co-precipitation) [123,124], and (iii) the evidence that iron hydroxides and oxides are good adsorbents for a wide range of dissolved species [29,125,126]. These three features act in synergy for the processes of aqueous contaminant removal in the presence of Fe0. In particular, in Fe0-based packed beds (Fe0 filters), volumetric expansion implies that porosity and thus permeability loss occurs with increasing operational duration. The progress of iron corrosion (corrosion rate) depends mainly on the porosity and stability of the oxide scale on Fe0 [117,118]. Thus, the importance of solution chemistry on the evolution of the oxide scale should be systematically characterized [38].
Separation processes in Fe0 Filters
Akageneite, amorphous iron hydroxides, goethite, green rusts, lepidocrocite, and magnetite are some common identified solid iron corrosion products from field and laboratory studies using Fe0 [21,22,127-131]. These minerals possess little or no permanent surface charge, but variable surface charge is generated through the adsorption of protons to surface hydroxyl groups [20]. For the near neutral pH range, it can be considered that iron oxides/hydroxides exhibit a positively charged surface [132]. This implies that Fe0 shielded with ion oxides cannot directly influence the transport of dissolved species to its surface. In other words, no matter the chemical reactivity of Fe0 towards a species (difference between the relative standard electrode potentials), the extent of its effective transformation depends on the species affinity to the oxide scale [59,132,133]: adsorption on the outer surface and diffusion through the film. This basic argument makes on the contaminant removal process makes unstable any discussion not properly considering the oxide scale as a physical barrier [1-3,35,44,45,55-60].
As an example, nitrate is considered a nonspecific ion that participate only in electrostatic ion exchange at the oxide surface, and do not undergo chemisorption through inner-sphere attachment [20,134]. This assumption is valid but must be completed by the size of NO3- and the diffusion potential across the oxide scale (porosity and tortuosity of the oxide scale). In other words, although the adsorption of some species (e.g. oxyanions) on iron oxides has been properly considered, diffusion behavior across the oxide scale has not been thoroughly examined. However, only such a holistic approach could enable the establishment of the science of Fe0 remediation.
Table 1 summarizes the key processes mediating contaminant removal in Fe0 filters. It is seen that a Fe0 filter acts as a media filter for the separation of particles, ideally only in-situ generated ones (iron oxides and hydroxides), but also as a chromatographic system for the separation of molecules. Iron oxides and hydroxides are generated in the presence of trace amounts of contaminants. Thus, contaminants are physically enmeshed in the matrix of these iron minerals (co-precipitation). Iron minerals can be regarded as contaminant collectors in Fe0 filters [31,32,135]. Lastly, because the precipitated iron minerals progressively fill the pore space within the filter, permeability loss occurs but contaminant removal by size-exclusion is increased [70,94-97].
Table 1. Separations processes involved in a Fe0-based filtration system.
Technique |
Separated |
Region A |
Region B |
Comments |
Adsorption |
Molecules |
Filter |
Filtrate |
Highly selective |
Co-precipitation |
Molecules |
Reactive zone |
Filtrate |
Non-specific |
Precipitation |
Ions |
Filter |
Filtrate |
Not relevant |
Reduction |
Molecules/ions |
Filter |
Filtrate |
Not relevant |
Screening |
Particles |
On the screen |
Past the screen |
Increased efficiency |
|
|
Diameter > screen |
Diameter < screen |
with time |
Size-exclusion |
Molecules |
Filter |
Filtrate |
Non-specific |
Finally, two mechanisms should be shortly addressed here (precipitation and reduction) for an improved understanding. Both are quantitative at relatively high contaminant concentrations. Water treatment with Fe0 filters typically implies the removal of contamination at mg/L-level from large volume of waters. Under these conditions, neither precipitation nor reduction could be quantitative [136]. Moreover, the trace amounts of contaminants are immersed in an ‘ocean’ of iron hydroxides and oxides (oxide scale) such that the opportunity of a quantitative chemical reaction is not given [1-3]. For these reason chemical precipitation and reduction in the aqueous phase are not relevant in the context of environmental remediation and safe drinking water provision. In other words, the suitability of Fe0 for environmental remediation arises from its capacity to generate iron minerals which acts as contaminant collectors [26,82,69,135,136]. This knowledge was used by Tseng et al. [137] to concentrate 60Co from sea water and by James et al. [138] to remove phosphate from wastewater. As discussed herein, contaminant reduction, when it occurs is mediated by iron corrosion products (e.g. FeII, H/H2, green rusts, magnetite) and is not a relevant removal mechanism for any contaminant [70,95].
Back to the Fe0 remediation research
Fe0-based filters are an effective technology for environmental remediation [8,11,12,14,75,82,88], safe drinking water production [25-30,139], and waste water treatment (11,21-23,140). Fe0 barriers is an established technology for groundwater remediation [10,24]. Reported contaminant removal mechanisms include adsorption, biologically mediated transformations, chemical reduction (degradation/precipitation) and precipitation [20-24,35,56,57,58,67]. It is still mostly considered that Fe0 filters are to be used (i) for the dechlorination of halogenated hydrocarbons via reduction reactions, and (ii) for the reduction of heavy metals, relevant inorganic oxyanions (including nitrate and perchlorate) and radionuclides. Hereby, reduction is mostly regarded as a stand-alone removal mechanism and possible paths for contaminant reduction in Fe0/systems include: (i) direct reduction at the Fe0 surface, possibly via pitting in the oxide surface, (ii) reduction by dissolved FeII species, (iii) reduction by hydrogen species (H/H2), and (iv) reduction by adsorbed FeII species (structural FeII) [21,141,142]. The removal of arsenic and selenium species have also been documented, but the importance of reduction was less emphasized [40, 108,134,143-147].
The analysis of the Fe0/H2O system (sections 3.1 and 3.2) has demonstrated that this popular state-of-the-art knowledge on the operating mode of Fe0 filters is non-acceptable. In particular, because Eq. 1 is universal, all corrosion products are present in every Fe0/H2O system. Accordingly, even where reduction is believed to play a key role, adsorbing agents are abundantly available [31,32,82,130,131,139] and have enmeshed some contaminant during their precipitation [1-3,56,59,61,62,67,70,95]. Moreover, screening (Table 1) is improved by porosity loss and contaminant removal by size-exclusion is not avoidable. Given that this knowledge is present in the peer-reviewed scientific literature since 2007 [2], it is difficult to understand why the reductive transformation concept is still prevailing [8]. Perhaps changes are more painful to some contemporary scientists than in the days of Carl Sagan (section 3.4). However, the objectivity is the main characteristic of science [148]. A view can be expressed agressively, freshly, harshly or politely in one hand while it should remain objective on the other.
Should scientists learn from politicians?
In 1987, Carl Sagan stated: “In science it often happens that scientists say, 'You know that's a good argument; my position is mistaken,' and then they would actually change their minds and you never hear that old view from them again. They really do it. It doesn't happen as often as it should, because scientists are human, and change is sometimes painful. But it happens every day”. The continuation of the citation reads as: "I cannot recall the last time something like that happened in politics or religion". Would researchers on Fe0 remediation agree with Carl Sagan?
It seems that nowadays, it is the scientist who must learn from politicians. In democratic systems, any politician confronted with any single behaviour contrary to the constitution (the fundamental law of politics) should resign. Accordingly, any scientist confronted with a behaviour contrary to Chemical Thermodynamics (a fundamental law of chemistry) should rectify his understanding. If such an approach was universally adopted, the systematic flaw addressed herein would have not (i) been introduced and (ii) maintained for two decades. The situation is worsen by the evidence that the Fe0 filtration technology was introduced by distorting the stream of corrosion science and overseeing many other aspects of General Chemistry. In particular, the favoured reductive transformation concept is even not valid in the concentration range of concern (mg/L) [38,53,56,57]. Thus a paradigm shift of the current understanding for Fe0 filtration technology is very much demanding before the future researchers are dragged deeper into confusion [95].
The Fe0 remediation research community cannot continue to ignore the warning signs of a knowledge system under great stress. There is even a risk for incipient decline for an already proven efficient technology. It is certain that the tax payer will continue its strong support for a cleaner environment. It is also certain that desirable larger budgets for this aim are possible and defensible. However, for a purposeful use of such budgets, structural flaws within the system should be addressed in a systematic way. There is evidence that the established scientific approaches/methods can correct its vulnerabilities. Some fundamental changes are required to bring the Fe0 research community back on the highway of iron corrosion science. These changes need to be made in a comprehensive fashion. An immediate paradigm shift is called for considering the worsening of current situation during the last two decades. Widespread engagement is necessary to bring about this paradigm shift, beginning with immediate debate, strong advocacy for change, and action by individual scientists, the funding agencies, academic institutions, and other entities that control and pay for the conduct of science [149].
The envisioned future world of Fe0 researchers is not more or less talented than the current. It does not need more or less financial support as well. It will just perform better investigations based on the science of aqueous iron corrosion [117,118]. In this perspective, even small laboratories will restore an environment in which talented trainees and scientists can achieve excellent results within some years [38,59,150]. The immediate goal of this communication has been to stimulate the debate on important issues that concern the future of a potentially universal frugal technology, eventually based on a century old knowledge [31,32,33,34,35,77,78]. This task cannot be left to a self-appointed subset of scientists [56] or to the leaders of funding agencies. Therefore, academic institutions, scientific societies, funding organizations, and other interested parties are encouraged to organize discussions (at regional, national and international levels) with a wide range of relevant constituencies [149]. Some discussions of this type have already begun in countries like Cameroon, Germany, India, Lebanon, Romania and Tanzania [8,55,150-159]. However, critical actions are needed on several fronts by many parties to re-orient research on Fe0 for water treatment. No less than the future credibility of natural science is at stake.
The manuscript was improved by the insightful comments of anonymous reviewers on previous versions. We acknowledge support by the German Research Foundation and the Open Access Publication Funds of the Göttingen University.
- Noubactep C (2006) Contaminant reduction at the surface of elemental iron: The end of a myth. Wissenschaftliche Mitteilungen Freiberg 31: 173-179.
- Noubactep C (2007) Processes of contaminant removal in “Fe0–H2O” systems revisited: The importance of co-precipitation. Open Environ Sci 1: 9-13.
- Noubactep C (2008) Processes of contaminant removal in “Fe0–H2O” systems revisited: The importance of co-precipitation. Open Environ Sci 1: 9-13.
- Matheson LJ, Tratnyek PG (1994) Reductive dehalogenation of chlorinated methanes by iron metal. Environ Sci Technol 28: 2045-2053. [Crossref]
- Roberts AL, Totten LA, Arnold WA, Burris DR, Campbell TJ (1996) Reductive elimination of chlorinated ethylenes by zero-valent metals. Environ Sci Technol 30: 2654-2659.
- Weber EJ (1996) Iron-mediated reductive transformations: investigation of reaction mechanism. Environ Sci Techno. 30: 716-719.
- Santisukkasaem U, Olawuyi F, Oye P, Das DB (2015) Artificial Neural Network (ANN) for evaluating permeability decline in permeable reactive barrier (PRB). Environ Process 2: 291-307.
- Kumar R, Sinha A (2017) Biphasic reduction model for predicting the impacts of dye-bath constituents on the reduction of tris-azo dye Direct Green-1 by zero valent iron (Fe0). J Environ Sci (China) 52: 160-169. [Crossref]
- Zhang X, Li J, Sun Y, Li L, Pan B, Zhang W, Guana X (2018) Aging of zerovalent iron in various coexisting solutes: Characteristics, reactivity toward selenite and rejuvenation by weak magnetic field. Sep Purif Technol 191: 94-100.
- Naidu R, Birke V, Eds. (2015) Permeable Reactive Barrier: Sustainable Groundwater Remediation. CRC Press 2014, eBook ISBN: 978-1-4822-2448-1, 333 pp.
- Guan X, Sun Y, Qin H, Li J, Lo IMC, et al. (2015) The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res 75: 224-248. [Crossref]
- Guo X, Yang Z, Dong H, Guan X, Ren Q, et al. (2016) Simple combination of oxidants with zero-valent-iron (ZVI) achieved very rapid and highly efficient removal of heavy metals from water. Water Res 88: 671-680. [Crossref]
- Warren KD, Arnold RG, Bishop TL, Lindholm LC, Betterton EA (1995) Kinetics and mechanism of reductive dehalogenation of carbon tetrachloride using zero-valence metals. J Hazard Mater 41 :217-227.
- Gillham RW, O’Hannesin SF (1994) Enhanced degradation of halogenated aliphatics by zero-valent iron. Ground Water 32: 958-967.
- Qiu SR, Lai H-F, Roberson MJ, Hunt ML, Amrhein C, et al. (2000) Removal of contaminants from aqueous solution by reaction with iron surfaces. Langmuir 16: 2230-2236.
- Lavine BK, Auslander G, Ritter J (2001) Polarographic studies of zero valent iron as a reductant for remediation of nitroaromatics in the environment. Microchem J 70: 69-83.
- Bigg T, Judd SJ (2000) Zero-valent iron for water treatment. Environ Technol 21: 661-670.
- Scherer MM, Richter S, Valentine RL, Alvarez PJ (2000) Chemistry and microbiology of permeable reactive barriers for in situ groundwater clean-up. Crit Rev Microbiol 26: 221-264. [Crossref]
- Jambor JL, Raudsepp M, Mountjoy K (2005) Mineralogy of permeable reactive barriers for the attenuation of subsurface contaminants. Can Miner 43: 2117-2140.
- Moore A, Young T (2005) Chloride interactions with iron surfaces: Implications for perchlorate and nitrate remediation using permeable reactive barriers. J Environ Eng 131: 924-933.
- Henderson AD, Demond AH (2007) Long-term performance of zero-valent iron permeable reactive barriers: a critical review. Environ Eng Sci 24: 401-423.
- Comba S, Di Molfetta A, Sethi R (2011) A Comparison between field applications of nano-, micro-, and millimetric zero-valent iron for the remediation of contaminated aquifers. Water Air Soil Pollut 215: 595-607.
- Gheju M (2011) Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut 222: 103-148.
- Wilkin RT, Acree SD, Ross RR, Puls RW, Lee TR, et al. (2014) Fifteen-year assessment of a permeable reactive barrier for treatment of chromate and trichloroethylene in groundwater. Sci Total Environ 468-469: 186-94. [Crossref]
- Ngai TKK, Murcott S, Shrestha RR, Dangol B, Maharjan M (2006) Development and dissemination of Kanchan™ Arsenic Filter in rural Nepal. Water Sci Technol Water Supply 6: 137-146.
- Hussam A, Munir AKM (2007) A simple and effective arsenic filter based on composite iron matrix: Development and deployment studies for groundwater of Bangladesh. J Environ Sci Health A Tox Hazard Subst Environ Eng 42: 1869-1878. [Crossref]
- Ngai TKK, Shrestha RR, Dangol B, Maharjan M, Murcott SE (2007) Design for sustainable development – Household drinking water filter for arsenic and pathogen treatment in Nepal. J Environ Sci Health A Tox Hazard Subst Environ Eng 42: 1879-1888. [Crossref]
- Neumann A, Kaegi R, Voegelin A, Hussam A, Munir AK, et al. (2013) Arsenic removal with composite iron matrix filters in Bangladesh: a field and laboratory study. Environ Sci Technol 47: 4544-4554. [Crossref]
- Casentini B, Falcione FT, Amalfitano S, Fazi S, Rossetti S (2016) Arsenic removal by discontinuous ZVI two steps system for drinking water production at household scale. Water Res 106: 135-145. [Crossref]
- Smith K, Li Z, Chen B, Liang H, Zhang X, et al. (2017) Comparison of sand-based water filters for point-of-use arsenic removal in China. Chemosphere 168: 155-162. [Crossref]
- Bischof G (1877) On putrescent organic matter in potable water. I. Proceedings of the Royal Society of London 26: 179-184.
- Bischof G (1878) On putrescent organic matter in potable water. II. Proceedings of the Royal Society of London 27: 258-261.
- Devonshire E (1890) The purification of water by means of metallic iron. Journal of the Franklin Institute 129: 449-461.
- Baker M (1934) Sketch of the history of water treatment. Journal American Water Works Association 26: 902-938.
- Mwakabona HT, Ndé-Tchoupé AI, Njau KN, Noubactep C, Wydra KD (2017) Metallic iron for safe drinking water provision: Considering a lost knowledge. Water Res 117: 127-142. [Crossref]
- Warner SD (2015) Two decades of application of permeable reactive barriers to groundwater remediation. in permeable reactive barrier sustainable groundwater remediation, R. Naidu and V. Birke (Editors), CRC Press, pp. 25–39, ISBN: 978-1-4822-2447-4.
- Btatkeu-K BD, Olvera-Vargas H, Tchatchueng JB, Noubactep C, Caré S (2014) Characterizing the impact of MnO2 on the efficiency of Fe(0)-based filtration systems. Chem Eng J 250: 416-422.
- Naseri E, Ndé-Tchoupé AI, Mwakabona HT, Nanseu-Njiki CP, Noubactep C, et al. (2017) Making Fe0-based filters a universal solution for safe drinking water provision. Sustainability 9: 1224.
- Mueller NC, Braun J, Bruns J, ÄŒernÃk M, Rissing P, et al. (2012) Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe. Environ Sci Pollut Res Int 19: 550-558. [Crossref]
- CH2M Hill Inc (2013) White paper report addendum selenium treatment. Technical addendum, CH2M Hill: Englewood, CO, USA, 29 March 2013, 68 pp.
- Singh A, Smith LS, Shrestha S, Maden N (2014) Efficacy of arsenic filtration by Kanchan arsenic filter in Nepal. J Water Health 12: 596-599. [Crossref]
- Madaffari MG (2015) New mixtures to be used in permeable reactive barrier for heavy-metals contaminated groundwater remediation: long-term removal efficiency and hydraulic behavior. PhD Thesis, Ecole Centrale Paris, 290 pp.
- Chiew H, Sampson ML, Huch S, Ken S, Bostick BC (2009) Effect of groundwater iron and phosphate on the efficacy of arsenic removal by iron-amended BioSand filters. Environ Sci Technol 43: 6295-6300. [Crossref]
- Noubactep C (2016a) Predicting the hydraulic conductivity of metallic iron filters: Modeling gone astray. Water 162.
- Noubactep C (2016b) Designing metallic iron packed-beds for water treatment: A critical review. CLEAN - Soil, Air, Water 44: 411-421.
- Ebert M, Birke V, Burmeier H, Dahmke A, Hein P, et al. (2007) Kommentar zu den Beiträgen "Das Ende eines Mythos" sowie "Zur Funktion reaktiver Wände" von Dr. Chicgoua Noubactep. Wasser, Luft und Boden (Terratech)7-8:TT 4–5.
- Elsner M, Cwiertny DM, Roberts AL, Lollar BS (2007) Response to Comment on "1,1,2,2-Tetrachloroethane Reactions with OH-, Cr(II), Granular Iron, and a Copper-Iron Bimetal: Insights from Product Formation and Associated Carbon Isotope Fractionation". Environ Sci Technol 41: 7949-7950.
- Kang S, Choi W (2009) Response to Comment on “Oxidative degradation of organic compounds using zero-valent iron in the presence of natural organic matter serving as an electron shuttle”. Environ Sci Technol 43: 3966-3967.
- Noubactep C (2010) Comments on "Degradation of 1,2,3-trichloropropane (TCP): hydrolysis, elimination, and reduction by iron and zinc". Environ Sci Technol 44: 3197. [Crossref]
- Chen L, Jin S, Fallgren PH, Swoboda-Colberg NG, Liu F, et al. (2012) Electrochemical depassivation of zero-valent iron for trichloroethene reduction. J Hazard Mater 239-240: 265-9. [Crossref]
- Ruhl AS, Ünal N, Jekel M (2012) Evaluation of two-component Fe(0) fixed bed filters with porous materials for reductive dechlorination. Chem Eng J 209: 401-406.
- Firdous R, Devlin JF (2014) Consideration of grain packing in granular iron treatability studies. J Contam Hydrol 164: 230-239. [Crossref]
- Makota S, Nde-Tchoupe AI, Mwakabona HT, Tepong-Tsindé R, Noubactep C, Nassi A, Njau KN (2017) Metallic iron for water treatment: Leaving the valley of confusion. Appl Water Sci.
- Reynolds GW, Hoff JT, Gillham RW (1990) Sampling bias caused by materials used to monitor halocarbons in groundwater. Environ Sci Technol 24: 135-142.
- Noubactep C (2014) Flaws in the design of Fe(0)-based filtration systems? Chemosphere 117: 104-107. [Crossref]
- Ghauch A (2015) Iron-based metallic systems: An excellent choice for sustainable water treatment. Freiberg Online Geosci. 38, 80 pp.
- Noubactep C (2015) Metallic iron for environmental remediation: A review of reviews. Water Res 85: 114-123. [Crossref]
- Noubactep C (2016c) Research on metallic iron for environmental remediation: Stopping growing sloppy science. Chemosphere 153: 528-530. [Crossref]
- Gatcha-Bandjun N, Noubactep C, Loura Mbenguela B (2017) Mitigation of contamination in effluents by metallic iron: The role of iron corrosion products. Environ Technol Innov 8: 71-83.
- Gheju M, Balcu I, Enache A, Flueras A (2017) A kinetic approach on hexavalent chromium removal with metallic iron. J Environ Manage 203: 937-941. [Crossref]
- Ghauch A, Abou Assi H, Bdeir S (2010) Aqueous removal of diclofenac by plated elemental iron: bimetallic systems. J Hazard Mater 182: 64-74. [Crossref]
- Ghauch A, Abou Assi H, Baydoun H, Tuqan AM, Bejjani A (2011) Fe0-based trimetallic systems for the removal of aqueous diclofenac: Mechanism and kinetics. Chem Eng J 172: 1033-1044.
- Gheju M, Balcu I (2011) Removal of chromium from Cr(VI) polluted wastewaters by reduction with scrap iron and subsequent precipitation of resulted cations. J Hazard Mater 196: 131-138. [Crossref]
- Giles DE, Mohapatra M, Issa TB, Anand S, Singh P (2011) Iron and aluminium based adsorption strategies for removing arsenic from water. J Environ Manage 92: 3011-3022. [Crossref]
- Bilardi S, Calabrò PS, Caré S, Moraci N, Noubactep C (2013a) Improving the sustainability of granular iron/pumice systems for water treatment. J Environ Manage 121: 133-141. [Crossref]
- Biliardi S, Calabrò PS, Caré S, Moraci N, Noubactep C (2013b) Effect of pumice and sand on the sustainability of granular iron beds for the removal of CuII, NiII, and ZnII. Clean-Soil Air Water 41: 835-843.
- Gheju M, Balcu I, Vancea C (2016) An investigation of Cr(VI) removal with metallic iron in the co-presence of sand and/or MnO2. J Environ Manage 170: 145-151. [Crossref]
- Oladoja NA (2016) Appropriate technology for domestic wastewater management in under-resourced regions of the world. Appl Water Sci 7: 3391-3406.
- Noubactep C (2009) An analysis of the evolution of reactive species in Fe0/H2O systems. J Hazard Mater 168: 1626-1631. [Crossref]
- Noubactep C (2010a) The fundamental mechanism of aqueous contaminant removal by metallic iron. Water SA 36: 663-670.
- Lipczynska-Kochany E, Harms S, Milburn R, Sprah G, Nadarajah N (1994) Degradation of carbon tetrachloride in the presence of iron and sulphur containing compounds. Chemosphere 29: 1477-1489.
- Schreier CG, Reinhard M (1994) Transformation of chlorinated organic compounds by iron and manganese powders in buffered water and in landfill leachate. Chemosphere 29: 1743-1753.
- Arnold WA, Roberts AL (2000a) Inter- and intraspecies competitive effects in reactions of chlorinated ethylenes with zero-valent iron in column reactors. Environ Eng Sci 17: 291-302.
- Arnold WA, Roberts AL (2000b) Pathways and kinetics of chlorinated ethylene and chlorinated acetylene reaction with Fe(0) particles. Environ Sci Technol 34: 1794-1805.
- O´Hannesin SF, Gillham RW (1998) Long-term performance of an in situ "iron wall" for remediation of VOCs. Ground Water 36: 164-170.
- Ebelle TC, Makota S, Tepong-Tsindé R, Nassi A, Noubactep C (2017) Metallic iron and the dialogue of the deaf. Fres Environ Bull (Accepted 24.07.2016; F-2015-512).
- Tucker WG (1892) THE PURIFICATION OF WATER BY CHEMICAL TREATMENT. Science 20: 34-38. [Crossref]
- Van Craenenbroeck W (1998) Easton & Anderson and the water supply of Antwerp (Belgium). Industrial Archaeology Review 20: 105-116.
- Gould JP (1982) The kinetics of hexavalent chromium reduction by metallic iron. Water Res 16: 871-877.
- Harza Engineering Co (1986) Selenium removal study, Report to Panoche Drainage District. Harza Engineering Co., Firebaugh, California, USA.
- Murphy AP (1988) Removal of selenate from water by chemical reduction. Ind Eng Chem Res 27: 187-191.
- Anderson MA (1989) Fundamental aspects of selenium removal by Harza process. Rep San Joaquin Valley Drainage Program, US Dep Interior, Sacramento.
- Murphy AP (1991) Chemical removal of nitrate from water. Nature 350: 223-225.
- RTDF (1998) Remediation Technology Development Forum. Permeable Reactive Barrier Technologies for Contaminant Remediation. Office of Research and Development. EPA/600/R 98-125.
- ITRC (1999) International Technology Regulatory Council. Regulatory guidance for permeable reactive barriers designed to remediate chlorinated solvents. Prepared by the Interstate Technology Regulatory Council Permeable Reactive Barriers Team, Washington, D.C.
- Naftz DL, Morrison SJ, Davis JA, Fuller CC, Eds. (2002) Handbook of Groundwater Remediation Using Permeable Reactive Barriers, Applications to Radionuclides, Trace Metals, and Nutrients. Academic Press, San Diego, California.
- ITRC (2005) International Technology Regulatory Council. Permeable reactive barriers: Lessons learned/new directions. Prepared by the Interstate Technology Regulatory Council Permeable Reactive Barriers Team, Washington, D.C.
- Gillham RW (2010) Development of the granular iron permeable reactive barrier technology (good science or good fortune). In "Advances in environmental geotechnics : proceedings of the International Symposium on Geoenvironmental Engineering in Hangzhou, China, September 8-10, 2009"; Y. Chen, X. Tang, L. Zhan (Eds); Springer Berlin/London, pp. 5-15.
- ITRC (2011) International Technology Regulatory Council. Permeable reactive barrier: Technology update. Prepared by the Interstate Technology Regulatory Council Permeable Reactive Barriers Team, Washington, D.C.
- Dahmke A, Schlicker O, Wüst W (1995) Literaturstudie “Reaktive Wände- pH-Redox-reaktive Wände”. LfU-Berichte, Texte und Berichte zur Altlastenbearbeitung. 51S.
- Dahmke A, Schlicker O, Wüst W (1997) Aktualisierung der Literaturstudie “Reaktive Wände” pH-Redox-reaktive Wände, Texte und Berichte zur Altlastenbearbeitung LfU Baden-Württemberg ISSN 0944-3304.
- Roehl KE, Meggyes T, Simon FG, Stewart DI, Eds. (2005) Long-term Performance of Permeable Reactive Barriers. Elsevier, Amsterdam, The Netherlands.
- Zolla V, Rolle M, Sethi R, Di Molfetta A (2006) Performance evaluation of permeable reactive barrier using zero-valent iron at a chlorinated solvents’ site. XV International Symposium on Mine Planning Equipment Selection (MPES 2006), 20-22 September 2006, Torino-Italy.
- Noubactep C (2010b) Metallic iron for safe drinking water worldwide. Chem Eng J 165: 740-749.
- Noubactep C (2011a) Aqueous contaminant removal by metallic iron: Is the paradigm shifting? Water SA 37: 419-426.
- Noubactep C (2011b) Metallic iron for safe drinking water production. Freiberg Online Geosci 27-38.
- Noubactep C (2012) Investigating the processes of contaminant removal in Fe(0)/H2O systems. Korean J Chem Eng 29: 1050-1056.
- Noubactep C (2013a) Relevant reducing agents in remediation Fe(0)/H2O systems. Clean-Soil Air Water 41: 493-502.
- Noubactep C (2013b) Metallic iron for water treatment: A critical review. Clean-Soil Air Water 41: 702-710.
- Noubactep C (2014) Flaws in the design of Fe(0)-based filtration systems? Chemosphere 117: 104-107. [Crossref]
- Clement TP (2015) Who are coauthors and what should be their responsibilities? Environ Sci Technol 49: 3265-3266. [Crossref]
- Frankenberger Jr WT, Amrhein C, Fan TWM, Flaschi D, Glater J, et al. (2004) Advanced treatment technologies in the remediation of seleniferous drainage waters and sediments. Irrigat Drain Syst 18: 19-41.
- Lee EW (1994) Drainage water treatment and disposal, In K.K. Tanji & B. Yaron, eds. Management of water use in agriculture. New York, The United States of America, Springer-Verlag.
- Twidwell L, McCloskey J, Joyce H, Dahlgren E, Hadden A (2005) Removal of selenium oxyanions from mine waters utilizing elemental iron and galvanically coupled metals. In: Cea, Young (Ed.), Innovations in Natural Resoure Processing - Proceedings of the Jan. D. Miller Symposium, SME.
- Zhang Y1, Amrhein C, Frankenberger WT Jr (2005) Effect of arsenate and molybdate on removal of selenate from an aqueous solution by zero-valent iron. Sci Total Environ 350: 1-11. [Crossref]
- Zhang Y, Amrhein C, Chang A, Frankenberger WT (2008) Effect of zero-valent iron and a redox mediator on removal of selenium in agricultural drainage water. Sci Tot Environ 407: 89-96. [Crossref]
- Golder Associates (2009) Literature review of treatment technologies to remove selenium from mining-influenced water. Technical report; Golder Associates: Lakewood, CO, USA, July 2009, 40 pp.
- CH2M Hill Inc. (2013) White paper report addendum selenium treatment. Technical addendum, CH2M Hill: Englewood, CO, USA, 29 March 2013, 68 pp.
- Yoon IH, Kim KW, Bang S, Kim MG (2011) Reduction and adsorption mechanisms of selenate by zero-valent iron and related iron corrosion. Appl Catal B Environ 104: 185-192.
- Ahmed F, Natarajan P, Gulliver JS, Weiss PT, Nieber JL (2014) Assessing and improving pollution prevention by Swales, Report 2014-30.
- EPA (2014) Reference guide to treatment technologies for mining-influenced water. U.S. Environmental Protection Agency Office of Superfund Remediation and Technology Innovation. EPA 542-R-14-001.
- Tang C, Huang YH, Zeng H, Zhang Z (2014) Reductive removal of selenate by zero-valent iron: The roles of aqueous Fe2+ and corrosion products, and selenate removal mechanisms. Water Res 67: 166-174
- Khamkhash A, Srivastava V, Ghosh T, Akdogan G, Ganguli R, et al. (2017) Mining-related selenium contamination in Alaska, and the state of current knowledge. Minerals 7: 46.
- McGuire MM, Carlson DL, Vikesland PJ, Kohn T, Grenier AC, et al. (2003) Applications of surface analysis in the environmental sciences: dehalogenation of chlorocarbons with zero-valent iron and iron-containing mineral surfaces. Anal Chim Acta 496: 301-313.
- Santos S, Ungureanu G, Boaventura R, Botelho C (2015) Selenium contaminated waters: An overview of analytical methods, treatment options and recent advances in sorption methods. Sci Tot Environ 521-522: 246-260. [Crossref]
- Keenan C, Seldak DL (2008) Factors affecting the yield of oxidants from the reaction of nanoparticulate zero-valent iron and oxygen. Environ Sci Technol 42: 1262-1267. [Crossref]
- Nesic S (2007) Key issues related to modelling of internal corrosion of oil and gas pipelines -A review: Corros Sci 49: 4308-4338
- Lazzari L (2008) General aspects of corrosion, Chapter 9.1, Vol.V, Encyclopedia of Hydrocarbons, Istituto Enciclopedia Italiana, Rome, Italy.
- Odziemkowski MS, Simpraga RP (2004) Distribution of oxides on iron materials used for remediation of organic groundwater contaminants-Implications for hydrogen evolution reactions. Can J Chem 82: 1495-1506.
- Odziemkowski MS (2009) Spectroscopic studies and reactions of corrosion products at surfaces and electrodes. Spectrosc Prop Inorg Organomet Compd 40: 385-450.
- Stratmann M, Müller J (1994) The mechanism of the oxygen reduction on rust-covered metal substrates. Corros Sci 36: 327-359.
- Pilling NB, Bedworth RE (1923) The oxidation of metals at high temperatures. J Inst Metals 29: 529-591.
- Crawford RJ, Harding IH, Mainwaring DE (1993a) Adsorption and coprecipitation of single heavy metal ions onto the hydrated oxides of iron and chromium. Langmuir 9: 3050-3056.
- Crawford RJ, Harding IH, Mainwaring DE (1993b) Adsorption and coprecipitation of multiple heavy metal ions onto the hydrated oxides of iron and chromium. Langmuir 9: 3057-3062.
- Schwertmann U (1991) Solubility and dissolution of iron oxides. Plant and Soil 130: 1-25.
- Brown Jr GE, Henrich VE, Casey WH, Clark DL, Eggleston C, et al. (1999) Metal oxide surfaces and their interactions with aqueous solutions and microbial organisms. Chem Rev 99: 77-174. [Crossref]
- Mackenzie PD1, Horney DP, Sivavec TM (1999) Mineral precipitation and porosity losses in granular iron columns. J Hazard Mater 68: 1-17. [Crossref]
- Roh Y, Lee SY, Elless MP (2000) Characterization of corrosion products in the permeable reactive barriers. Environ Geol 40: 184-194.
- Furukawa Y1, Kim JW, Watkins J, Wilkin RT (2002) Formation of ferrihydrite and associated iron corrosion products in permeable reactive barriers of zero-valent iron. Environ Sci Technol 36: 5469-5475. [Crossref]
- Bartzas G, Komnitsas K (2010) Solid phase studies and geochemical modelling of low-cost permeable reactive barriers. J Hazard Mater 183: 301-308. [Crossref]
- Li L, Benson CH (2010) Evaluation of five strategies to limit the impact of fouling in permeable reactive barriers. J Hazard Mater 181: 170-180. [Crossref]
- Phukan M (2015) Characterizing the Fe(0)/sand system by the extent of dye discoloration. Freiberg Online Geosci 40: 70.
- Miyajima K (2012) Optimizing the design of metallic iron filters for water treatment. Freiberg Online Geosci 32:107.
- Su C, Puls RW (2001) Arsenate and arsenite removal by zerovalent iron: Effects of phosphate, silicate, carbonate, borate, sulfate, chromate, molybdate, and nitrate, relative to chloride. Environ Sci Technol 35: 4562-4568. [Crossref]
- Yao KM, Habibian MT, O’melia CR (1971) Water and waste water filtration: concepts and applications. Environ Sci Technol 5: 1105-1112.
- Howe KJ, Hand DW, Crittenden JC, Trussell RR, Tchobanoglous G (2012) Principles of Water Treatment. John Wiley & Sons, Inc., Hoboken, New Jersey, 674.
- Tseng CL, Yang MH, Lin CC (1984) Rapid determination of cobalt-60 in sea water with steel wool adsorption. J Radioanal Nucl Chem Lett 85: 253-260.
- James BR, Rabenhorst MC, Frigon GA (1992) Phosphorus sorption by peat and sand amended with iron oxides or steel wool. Water Environ Res 64: 699-705.
- Anderson W (1885) The purification of water by means of iron on the large scale. Minutes of the Proceedings of the Institution of Civil Engineers 81: 279-284.
- Sun Y, Chen SS, Tsang DCW, Graham NJD, Ok YS, et al. (2017) Zero-valent iron for the abatement of arsenate and selenate from flowback water of hydraulic fracturing. Chemosphere 167: 163-170. [Crossref]
- Klausen J, Troeber SP, Haderlein SB, Schwarzenbach RP (1995) Reduction of Substituted Nitrobenzenes by Fe(II) in Aqueous Mineral Suspensions. Environ Sci Technol 29: 2396-2404. [Crossref]
- Westerhoff P (2003) Reduction of nitrate, bromate, and chlorate by zero valent iron (Fe(0)). J Environ Eng 129: 10-16.
- Lackovic JA, Nikolaidis NP, Dobbs GM (2000) Inorganic arsenic removal by zero-valent iron. Environ Eng Sci 17: 29-39.
- Farrell J, Wang J, O'Day P, Conklin M (2001) Electrochemical and spectroscopic study of arsenate removal from water using zero-valent iron media. Environ Sci Technol 35: 2026-2032. [Crossref]
- Twidwell LG, McCloskey J (2011) A literature guide for removing arsenic from aqueous solution and long-term product storage. JOM 63: 94-100.
- Yang Z, Shan C, Zhang W, Jiang Z, Guan X, et al. (2016) Temporospatial evolution and removal mechanisms of As(V) and Se(VI) in ZVI column with H2O2 as corrosion accelerator. Water Res 106: 461-469. [Crossref]
- Sun Y, Chen SS, Tsang DCW, Graham NJD, Ok YS, et al. (2017) Zero-valent iron for the abatement of arsenate and selenate from flowback water of hydraulic fracturing. Chemosphere 167: 163-170. [Crossref]
- Lipton BH (2005) The biology of belief: Unleashing the power of consciousness, matter and miracles, mountain of love. Santa Rosa, CA, Mountain of Love/Elite Books, 224.
- Alberts B1, Kirschner MW, Tilghman S, Varmus H (2014) Rescuing US biomedical research from its systemic flaws. Proc Natl Acad Sci U S A 111: 5773-5777. [Crossref]
- Btatkeu-K BD1, Tchatchueng JB2, Noubactep C3, Caré S4 (2016) Designing metallic iron based water filters: Light from methylene blue discoloration. J Environ Manage 166: 567-573. [Crossref]
- Noubactep C1, Schöner A (2009) Fe0-based alloys for environmental remediation: thinking outside the box. J Hazard Mater 165: 1210-1214. [Crossref]
- Togue-Kamga F, Btatkeu BD, Noubactep C, Woafo P (2012) Metallic iron for environmental remediation: Back to textbooks. Fresenius Environ Bull 21: 1992-1997.
- Gatcha-Bandjun N, Noubactep C (2013) Metallic iron for environmental remediation: Missing the 'valley of death'. Fresenius Environ Bull 22: 2632-2639.
- Kobbe-Dama N, Noubactep C, Tchatchueng JB (2013) Metallic iron for water treatment: Prevailing paradigm hinders progress. Fresenius Environ Bull 22: 2953-2957.
- Noubactep C (2013c) Metallic iron for environmental remediation: the long walk to evidence. Corros Rev 31: 51-59.
- Gatcha-Bandjun N, Noubactep C, Loura Mbenguela B (2014) Water treatment with Fe0/H2O systems: Learning from internal electrolysis. Fresenius Environ Bull 23: 2663-2669.
- Ndé-Tchoupé AI, Crane RA, Mwakabona HT, Noubactep C, Njau K (2015) Technologies for decentralized fluoride removal: Testing metallic iron based filters. Water 7: 6750-6774.
- Tepong-Tsindé R, Crane R, Noubactep C, Nassi A, Ruppert H (2015) Testing metallic iron filtration systems for decentralized water treatment at pilot scale. Water 7: 868-897.
- Noubactep C (2016d) No scientific debate in the zero-valent iron literature. Clean-Soil Air Water 44: 330-332.
