Chronic kidney disease (CKD) is a worldwide public health problem. In patients with CKD, exercise endurance is lowered and this phenomenon becomes more distinct as the renal dysfunction advances. Levels of physical activity and exercise tolerance among CKD patients with hemodialysis are low. Increased physical activity in this population has been associated with improved ability and capacity to perform activities in everyday life, occupational tasks, health-related quality of life and survival. Therefore, regular exercise is recommended to this population. Renal rehabilitation (RR) is coordinated, multifaceted interventions designed to optimize a renal patient’s physical, psychological, and social functioning, in addition to stabilizing, slowing, or even reversing the progression of renal deterioration, thereby reducing morbidity and mortality. We have established the Japanese Association of Renal Rehabilitation in 2011 to evaluate and promote RR. In Japan, April 2016, the Ministry of Health, Labor and Welfare that first decided to extend rehabilitation coverage to diabetic patients with pre-HD CKD patients at stage 4 by National Health Insurance Reimbursement in the world. RR is a feasible, effective and safe secondary prevention strategy following CKD, and offers a promising model for new field of rehabilitation.
chronic kidney disease, rehabilitation, exercise, renal protection, hemodialysis
Chronic kidney disease (CKD) is a worldwide public health problem. CKD patient is characterized by osteoporosis, muscle wasting, cardiovascular hypertrophy and vascular calcification and supposed to be a model of premature ageing [1].
In patients with CKD, exercise endurance, measured as maximal oxygen uptake (VO2 max), etc. is lowered and this phenomenon becomes more distinct as the renal dysfunction advances. Poor physical condition and skeletal muscle wasting are associated with CKD. This is due to the combined effects of uremic acidosis, protein-energy malnutrition and inflammatory cachexia, which lead to and are further aggravated by a sedentary lifestyle. Together, these factors result in a progressive downward spiral of deconditioning. The importance of renal rehabilitation is addressed in this review.
Physical inactivity is well recognized as a major health issue in today’s society. Regular exercise is important in maintaining health and preventing chronic disease, it is increasingly accepted as a valuable therapeutic intervention in many long-term conditions.
Patients with end-stage renal disease (ESRD) on maintenance hemodialysis have very high mortality, and yet higher mortality risk has been reported for sedentary hemodialysis patients [2]. As well as being a strong cardiovascular risk factor, physical inactivity is associated with increased risk of rapid kidney function decline in CKD [3].
Results from an international study of hemodialysis patients indicate that regular exercise is associated with better outcomes in this population and that patients at facilities offering exercise programs have higher odds of exercising. In DOPPS study, overall, 47.4% of participants were categorized as regular exercisers. The odds of regular exercise was 38% higher for patients from facilities offering exercise programs [4].
In DOPPS study, regular exercisers had higher health-related quality of life, physical functioning and sleep quality scores; reported fewer limitations in physical activities; and were less bothered by bodily pain or lack of appetite. Regular exercise was also correlated with more positive patient affect and fewer depressive symptoms. In models extensively adjusted for demographics, comorbidities and socio-economic indicators, mortality risk was lower among regular exercisers and at facilities with more regular exercisers [4].
A systematic literature search was completed in August 2010 to identify randomized, controlled trials of exercise training studies in hemodialysis patients. A subsequent meta-analysis was conducted and the search repeated in December 2010 [5]. Exercise training produced 26% improvements of peak VO2. Equivocal results. Significant improvements in lean body mass, quadriceps muscle area, knee extension, hip abduction and flexion strength were also reported [5]. They did not find any deaths directly associated with exercise in 28,400 patient-hours and no differences in withdrawal rates between exercise and control participants. Therefore, Exercise training is safe and imparts large improvements in peak VO2, and heart rate variability in hemodialysis patients [5].
Moreover, a growing evidence base suggests that exercise training in patients with hemodialysis improves in VO2max, left ventricular function, cardiac sympathetic and parasympathetic disharmony, malnutrition-inflammation-atherosclerosis syndrome, anemia, sleep quality, anxiety, health-related quality of life, activities of daily living, shunt size, Kt/V and mortality [6,7].
Also, there are few reports about the effect of exercise on renal function in animal models of chronic renal failure. We have been published several papers in this field recently.
First, we assessed the renal effects of moderate chronic treadmill exercise in a remnant kidney model of spontaneously hypertensive rats (SHR) with 5/6 nephrectomy and also assessed the effects of exercise and antihypertensive therapy on renal function [8]. The rats were divided into four groups: (i) no exercise (Non-EX); (ii) moderate exercise with treadmill running (20 m/min, 0 grade incline for 60 min) (EX); (iii) EX with an angiotensin converting enzyme (ACE) inhibitor, enalapril (2 mg/kg per day, intraperitoneally); and (iv) EX with an angiotensin receptor antagonist, losartan (5 mg/kg per day, i.p.), for 4 weeks. Chronic EX significantly attenuated the increase in proteinuria and significantly protected against increases in the index of glomerular sclerosis (IGS). Both enalapril and losartan with EX significantly decreased blood pressure, and further decreased the IGS. In the stepwise multiple regression analysis, only antihypertensive drug remained in the model as a significant predictor of IGS. In contrast, exercise, antihypertensive drug and mean systolic blood pressure remained in the model as significant predictors of proteinuria. These results suggest that exercise does not worsen renal function and has renal-protective effects in this model of rats. Moreover, the antihypertensive therapy has additional renal-protective effects in this model of rats.
Second, we assessed the renal and peripheral effects of moderate to intense chronic exercise as well as the effects of the combination of chronic exercise and enalapril (ENA) in 5/6-nephrectomized Wistar-Kyoto rats [9]. The rats were divided into six groups according to the following treatment: 1) no exercise; 2) ENA (2 mg/kg/day, subcutaneously); 3) moderate exercise with treadmill running (20 m/min for 60 min/day, 5 days/week); 4) intense exercise with treadmill running (28 m/min for 60 min/day, 5 days/week); 5) EXm+ENA; and 6) sham operation. The rats were then treated for 12 weeks. Both chronic exercise and ENA blocked the development of hypertension, blunted increases in proteinuria, reduced serum creatinine and blood urea nitrogen, and improved IGS and the relative interstitial volume of the renal cortex (RIV). Moreover, IGS and RIV in the EXm+ENA group were the lowest among all other nephrectomized groups. Furthermore, EXm+ENA enhanced capillarization as well as the proportion of type-I fiber in the soleus muscle. These results suggest that EX and ENA have reno-protective effects. The findings also suggest that EXm+ENA provided greater reno-protective effects than those of ENA alone, and that EXm+ENA had some additional peripheral effects without any complications in this rat model.
Thirdly, we assessed the renal and peripheral effects of exercise in a rat model of Type 2 diabetic nephropathy (Goto–Kakizaki rats) and the benefits of combined exercise and ATIIA losartan [10].
The rats were divided into four groups: (i) no exercise (control); (ii) losartan; (iii) exercise with treadmill running; (iv) exercise plus losartan. All of groups were treated for 12 weeks. IGS and RIV of the renal cortex were significantly improved in the losartan, exercise, EX+LOS, and EX+LOS is the lowest. These results suggest that both exercise and losartan have reno-protective effects, and the combination of exercise and losartan provided greater reno-protective effects than losartan alone, and may affect macrophage infiltration, mesangial activation, and podocyte loss in this model of diabetic nephropathy. It is also suggested that exercise has a specific reno-protective effect that is not related to SBP reduction, and can enhance endurance without renal complications.
Finally, we evaluated the effects of exercise on the early stage of diabetic nephropathy with Zucker diabetic fatty (ZDF) rats. Exercise significantly reduced albumin excretion and normalized creatinine clearance in ZDF rats [11].
These results suggest that exercise training has renal protective effects in various animal models of pre-dialysis CKD.
Baria et al. reported first RCT that exercise 3 times/week, 30min/day, for 12wks at anaerobic threshold level improves renal function in obese CKD patients [12]. Greenwood et al. reported that Aerobic exercise (ergometer, 40min/day 3times/week 12months) improved the decline in rate of changes in eGFR in CKD stage 3,4 patients [13]. Chen et al. from Taipei reported the association of walking with overall mortality and renal replacement therapy (RRT) such as HD or renal transplant in patients with CKD stages 3–5 [14]. Therefore, our results with the animal models are at last supported by CKD patients.
We have established the Japanese Society of Renal Rehabilitation in 2011 to evaluate and promote renal rehabilitation (RR). We published the first book titled “Renal Rehabilitation” as RR in the world (Figure 1) [15]. We define RR as, “RR is coordinated, multifaceted interventions designed to optimize a renal patient’s physical, psychological, and social functioning, in addition to stabilizing, slowing, or even reversing the progression of renal deterioration, thereby reducing morbidity and mortality. RR includes five major components: such as exercise training, diet & fluid management, medication & medical surveillance, education, psychological & vocational counseling” [7,15]. The first step to successful RR is ensuring that the clinical prerequisites of anemia control, adequate dialysis, exercise, a well-functioning vascular access, and proper nutrition are in place.

Figure 1. The First Book on “Renal Rehabilitation” (Edited by Kohzuki M, Ishiyaku Publishers, Inc., Tokyo) [7]
Nature Review in Nephrology introduced Japanese Society of Renal rehabilitation as “In 2011, the Japanese Society of Renal rehabilitation was established to evaluate and promote renal rehabilitation.”, and Japanese National Health Insurance System for RR [16]. In Japan, April 2016, the Ministry of Health, Labor and Welfare extended rehabilitation coverage to diabetic patients with pre-HD CKD patients at stage 4 by National Health Insurance Reimbursement for the first time in the world.
As super-aged society has come, the number of persons with multi-morbidity and multiple disabilities (MMD) and their needs of rehabilitation have increased rapidly more than we had expected [17]. Medical science basically aims to "Adding Years to Life" by increasing life expectancy. Rehabilitation generally aims to "Adding Life to Years" by helping patients with impairment achieve, and use, their full physical, mental and social potential. However, recent growing evidence suggests that rehabilitation for patients with visceral impairment such as cardiac, renal and pulmonary impairment can not only improve exercise performance and quality of life, but also increases survival (Figure 2) [18]. Therefore, modern comprehensive rehabilitation for patients with visceral impairment does not simply aim to "Adding Life to Years" but “Adding Life to Years and Years to Life” which is a new rehabilitation concept [18].
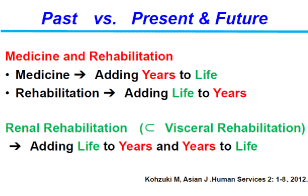
Figure 2. Renal Rehabilitation aim to "Adding Life to Years" but “Adding Life to Years and Years to Life” which is a new rehabilitation concept [18]
In RR, we should improve not only quality of life but also biological lifespan in patients with CKD. RR is a feasible, effective and safe secondary prevention strategy following CKD, and offers a promising model for new field of rehabilitation. Moreover, urgent efforts should be made urgently to increase the implementation rate of the RR.
- Kooman JP, Kotanko P, Schols AM, Shiels PG, Stenvinkel P (2014) Chronic kidney disease and premature ageing. Nat Rev Nephrol 10: 732-742. [Crossref]
- O’Hare AM, Tawney K, Bacchetti P, Johansen KL (2003) Decreased survival among sedentary patients undergoing dialysis: Results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis 41: 447–454. [Crossref]
- Johansen KL (2007) Exercise in the end-stage renal disease population. J Am Soc Nephrol 18: 1845-1854. [Crossref]
- Tentori F, Slder SJ, Thumma J (2010) Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): correlates and associated outcomes. Nephrol Dial Transplant 25: 3050–3062. [Crossref]
- Smart N, Steele M (2011) Exercise training in haemodialysis patients: a systematic review and meta-analysis. Nephrology (Carlton) 16: 626-632. [Crossref]
- Kohzuki M (2011) Exercise therapy for dialysis patients. Jap J Clin Dial 27: 1291-1298. (in Japanese with English abstract)
- Kohzuki M (2013) Renal rehabilitation: present and future perspectives. Hemodialysis, Suzuki H (Ed.), Intech, 743-751.
- Kohzuki M, Kamimoto M, Wu XM, Xu HL, Kawamura T, et al. (2001) Renal protective effects of chronic exercise and antihypertensive therapy in hypertensive rats with chronic renal failure. J Hypertens 19:1877-82. [Crossref]
2021 Copyright OAT. All rights reserv
- Kanazawa M, Kawamura T, Li L, Sasaki Y, Matsumoto K, et al. (2006) Combination of exercise and enalapril enhances renoprotective and peripheral effects in rats with renal ablation. Am J Hypertens 19: 80-6. [Crossref]
- Tufescu A, Kanazawa M, Ishida A, Lu H, Sasaki Y, et al. (2008) Combination of exercise and losartan enhances renoprotective and peripheral effects in spontaneously type 2 diabetes mellitus rats with nephropathy. J Hypertens 26: 312-21. [Crossref]
- Ito D, Ito O, Kohzuki M, Kiyomoto H. et al. Chronic running exercise alleviates early progression of nephropathy with upregulation of nitric oxide synthases and suppression of glycation in Zucker diabetic rats. PLoS ONE 10: e0138037. [Crossref]
- Baria F, Kamimura MA, Aoike DT, Ammirati A, Rocha ML, et al. (2014) Randomized controlled trial to evaluate the impact of aerobic exercise on visceral fat in overweight chronic kidney disease patients. Nephrol Dial Transplant 29: 857-864. [Crossref]
- Greenwood SA, Koufaki P, Mercer TH, MacLaughlin HL, Rush R, et al. (2015) Effect of exercise training on estimated GFR, vascular health, and cardiorespiratory fitness in patients with CKD: a pilot randomized controlled trial. Am J Kidney Dis 65: 425-34. [Crossref]
- Chen IR, Wang SM, Liang CC, Kuo HL, Chang CT, et al. (2014) Association of walking with survival and RRT among patients with CKD stages 3-5. Clin J Am Soc Nephrol 9: 1183-1189. [Crossref]
- Kohzuki M (2012) Definition of Renal Rehabilitation. In: “Renal Rehabilitation” Edited by Kohzuki M Ishiyaku Publishers, Inc., Tokyo.
- Zelle DM, Klaassen G, VanAdrichem E, Bakker SJ, Corpeleijn E, et al. (2017) Physical inactivity: a risk factor and target for intervention in renal care. Nat Rev Nephrol 13: 152-168. [Crossref]
- Kohzuki M (2014) Paradigm shift in rehabilitation medicine in the era of multimorbidity and multiple disabilities (MMD). Physical Medicine and Rehabilitation International 1: id1006.
- Kohzuki M, Sakata Y, Kawamura T, Ebihara S, Ito S (2012) A Paradigm Shift in Rehabilitation Medicine: From “Adding Life to Years” to “Adding Life to Years and Years to Life”. Asian J Human Services 2: 1-8.


