Diabetes is one of the largest global health emergencies of the 21st Century. Among its complications, diabetic kidney disease (DKD), and the number of cases progressing to end-stage renal disease (ESRD) from DKD is increasing continuously. To prevent DKD, beside anti-hypertensive agents including either inhibition of the ACE or angiotensin receptor blocker (ARB), the efficacy of physical activity/exercise has been remarked. There is no evidence that vigorous-intensity exercise increases the rate of progression of DKD, and there appears to be no need for specific exercise restrictions for people with DKD. The U.S. Department of Health and Human Services’ physical activity guidelines for Americans suggest that adults over age 18 years do 150 min/week of moderate-intensity or 75 min/week of vigorous-intensity aerobic physical activity, or an equivalent combination of the two. In addition, the guidelines suggest that adults do muscle-strengthening activities that involve all major muscle groups 2 or more days/week. Physical activity does carry some potential health risks for people with diabetes, including acute complications like cardiac events, hypoglycemia, and hyperglycemia. In low- and moderate intensity activity undertaken by adults with type 2 diabetes, the risk of exercise induced adverse events is low. Individuals with type 1 diabetes the only common exercise-induced adverse event is hypoglycemia. To prevent hypoglycemia during aerobic exercise, additional carbohydrate intake and/or reductions in insulin are typically required. In future, it is needed to reveal whole mechanisms of the physical activity for DKD, and to establish the exercise training recommendations for DKD.
Diabetes is one of the largest global health emergencies of the 21st Century. Because Diabetes and its complications are major causes of death in most countries. Furthermore, the incidence of type 2 diabetes mellitus has been increasing worldwide. At 2015, one in 11 adults has diabetes, but at 2040, one in 10 adults will have diabetes [1]. Among its complications, diabetic kidney disease (DKD), and the number of cases progressing to end-stage renal disease (ESRD) from DKD is increasing continuously, accounting for approximately 50% of cases in the developed world [2]. As a result, Diabetes is currently the most common cause of kidney disease among patients receiving renal replacement therapy. Of course, anti-hypertensive agents, inhibitors of the renin–angiotensin system (RAS) with either angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB), have been reported to prevent the development and progression of DKD [2-9]. But these agents do not prevent DKD. A substantial residual risk of progression of kidney disease remains. Besides, lifestyle modification is recommended as a primary treatment approach for type 2 diabetes [10,11] and several studies have shown that exercise can be beneficial for DKD in obese Zucker rats [12-14]. However, relatively little is known about the benefits and risks of renal rehabilitation in DKD. So present article discusses the effects and safety of renal rehabilitation in patients with DKD in light of current literature.
Before pursuing an exercise program, in patients already had complications or certain subjects, it should undergo a thorough history and physical examination. Patients with symptoms suggestive of coronary artery disease (CAD) should be evaluated appropriately, irrespective of diabetes status [15]. However, the best protocol for screening asymptomatic patients with diabetes for coronary artery disease remains unclear. The ADA consensus report “Screening for Coronary Artery Disease in Patients with Diabetes” [16] concluded that routine testing is not recommended. Providers should perform a careful history being aware of the atypical presentation of coronary artery disease in patients with diabetes and assess other cardiovascular risk factors. Providers should assess patients for conditions that might contraindicate certain types of exercise or predispose to injury, such as uncontrolled hypertension, autonomic neuropathy, peripheral neuropathy, a history of foot lesions, and untreated proliferative retinopathy. The patient’s age and previous physical activity level should be considered [17].
Physical activity can acutely increase urinary protein excretion. However, there is no evidence that vigorous-intensity exercise increases the rate of progression of DKD, and there appears to be no need for specific exercise restrictions for people with DKD [17,18]. The U.S. Department of Health and Human Services’ physical activity guidelines for Americans [19] suggest that adults over age 18 years do 150 min/week of moderate-intensity or 75 min/week of vigorous-intensity aerobic physical activity, or an equivalent combination of the two. In addition, the guidelines suggest that adults do muscle-strengthening activities that involve all major muscle groups 2 or more days/week. The guidelines suggest that adults over age 65 years or those with disabilities follow the adult guidelines if possible or, if this is not possible, be as physically active as they are able. Recent evidence supports that all individuals, including those with diabetes, should be encouraged to reduce the amount of time spent being sedentary (e.g., working at a computer, watching TV), particularly, by breaking up extended amounts of time (>90 min) spent sitting by briefly standing or walking (Table 1) [20,21].
Table 1. Exercise training recommendations; types of exercise, intensity, duration, frequency, and progression [21].
| Aerobic |
Resistance |
Flexibility and Balance |
Type of exercise |
Prolonged, rhythmic activities using large muscle groups (e.g., walking, cycling, and swimming) May be done continuously or as HIIT
|
|
Stretching: static, dynamic, and other stretching; yoga Balance (for older adults): practice standing on one leg, exercises using balance equipment, lower-body and core resistance exercises, tai chi
|
Intensity |
|
|
|
Duration |
At least 150 min/week at moderate to vigorous intensity for most adults with diabetes For adults able to run steadily at 6 miles per h (9.7 km/h) for 25 min, 75 min/week of vigorous activity may provide similar cardioprotective and metabolic benefits
|
|
|
Frequency |
|
|
|
Progression |
A greater emphasis should be placed on vigorous intensity aerobic exercise if fitness is a primary goal of exercise and not contraindicated by complications Both HIIT and continuous exercise training are appropriate activities for most individuals with diabetes
|
Beginning training intensity should be moderate, involving 10-15 repetitions per set, with increases in weight or resistance undertaken with a lower number of repetitions (8-10) only after the target number of repetitions per set can consistently be exceeded Increase in resistance can be followed by a greater number of sets and finally by increased training frequency
|
|
For the effects of renal rehabilitation, whether an intensive lifestyle intervention (ILI) affects the development of nephropathy was reported. In this report, 5145 overweight or obese persons aged 45–76 years with type 2 diabetes were randomized to ILI designed to achieve and maintain weight loss through reduced caloric consumption and increased physical activity or to a diabetes support and education (DSE) group. The ILI aimed to achieve and maintain weight loss of at least 7% through reduced caloric intake and increased physical activity. 9 Strategies included a calorie goal of 1200 to 1800 kcal per day (with <30% of calories from fat and >15% from protein), meal-replacement products, and at least 175 minutes of moderate-intensity physical activity per week. DSE group sessions focused on diet, exercise, and social support. Results was that the incidence rate of very-high-risk chronic kidney disease (CKD) was 31% lower in ILI than DSE with hazard rates of 0.90 cases/100 person-years in DSE and 0.63 in ILI (difference=0.27 cases/100 person-years, hazard ratio and 95% confidence interval: HR=0.69, 0.55 to 0.87) (Figure 1). This effect was partly attributable to reductions in weight, HbA1c, and blood pressure [11]. As this report, the effectiveness of renal rehabilitation including exercise might be suggested.
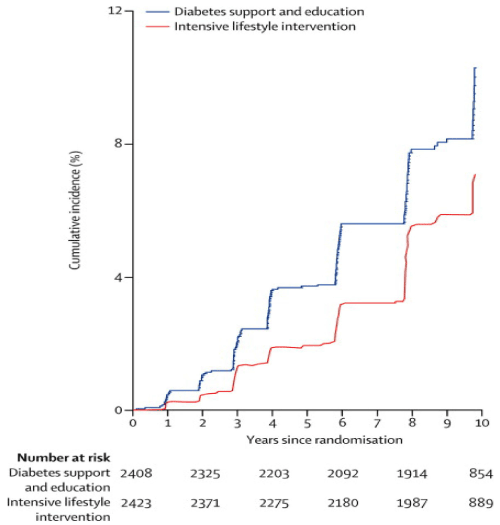
Figure 1. Cumulative incidence of very-high-risk CKD by treatment group through year 10. Too few observations were available beyond year 10 for reliable estimates. DSE is the Diabetes Support and Education group, and ILI is the Intensive Lifestyle Intervention group. The numbers of persons at risk at the beginning of the even-numbered years since randomization are shown. The hazard ratio (ILI vs. DSE) is 0.69, 95% confidence interval = 0.55 to 0.87, p=0.002.
Physical activity does carry some potential health risks for people with diabetes, including acute complications like cardiac events, hypoglycemia, and hyperglycemia. In low- and moderate intensity activity undertaken by adults with type 2 diabetes, the risk of exercise induced adverse events is low. In individuals with type 1 diabetes (any age) the only common exercise-induced adverse event is hypoglycemia. Variable glycemic responses to physical activity [22] make uniform recommendations for management of food intake and insulin dosing difficult. To prevent hypoglycemia during prolonged (≧30 min), predominantly aerobic exercise, additional carbohydrate intake and/or reductions in insulin are typically required. For low- to moderate-intensity aerobic activities lasting 30~60 min undertaken when circulating insulin levels are low (i.e., fasting or basal conditions), 10~15 g of carbohydrate may prevent hypoglycemia [23]. For activities performed with relative hyperinsulinemia (after bolus insulin), 30~60 g of carbohydrate per hour of exercise may be needed [24], which is similar to carbohydrate requirements to optimize performance in athletes with [25] or without [26] type 1 diabetes. As recommended in Table 2, blood glucose concentrations should always be checked prior to exercise undertaken by individuals with type 1 diabetes. The target range for blood glucose prior to exercise should ideally be between 90 and 250 mg/dL (5.0 and 13.9 mmol/L). Carbohydrate intake required will vary with insulin regimens, timing of exercise, type of activity, and more [28], but it will also depend on starting blood glucose levels [21].
Table 2. Suggested carbohydrate intake or other actions based on blood glucose levels at the start of exercise [21].
Pre-exercise blood glucose |
Carbohydrate intake or other action |
˂90 mg/dL (˂5.0 mmol/L) |
Ingest 15-30 g of fast-acting carbohydrate prior to the start of exercise, depending on the size of the individual and intended activity; some activities that are brief in duration (˂30 min) or at a very high intensity (weight training, interval training, etc.) may not require any additional carbohydrate intake. For prolonged activities at a moderate intensity, consume additional carbohydrate, as needed (0.5-1.0 g/kg body mass per h of exercise), based on blood glucose testing results.
|
90-150 mg/dL (5.0-8.3 mmol/L) |
|
150-250 mg/dL (8.3-13.9 mmol/L) |
|
250-350 mg/dL (13.9-19.4 mmol/L) |
Test for ketones. Do not perform any exercise if moderate-to-large amounts of ketones are present Initiate mild-to-moderate intensity exercise. Intense exercise should be delayed until glucose levels are ˂250 mg/dL because intense exercise may exaggerate the hyperglycemia.
|
≥ 350 mg/dL (≥ 19.4 mmol/L) |
Test for ketones. Do not perform any exercise if moderate-to-large amounts of ketones are present. If ketones are negative (or trace), consider conservative insulin correction (e.g., 50% correction) before exercise, depending on active insulin status. Initiate mid-to-moderate exercise and avoid intense exercise until glucose levels decrease.
|
Adapted from Zaharieva and Riddell [27].
The exercise training recommendations for diabetes has done by several reports [15,17,21]. However, those for DKD has not yet established, and so as the limitation of the exercise therapy for DKD. Furthermore, the mechanisms of the physical activity for DKD have not fully elucidated [12-14]. Therefore, it is needed to reveal whole mechanisms of the physical activity for DKD, and to establish the exercise training recommendations for DKD, in near future.
The auhors declare that they have no conflict of interest.
- (2015) IDF DIABETES ATLAS, Seventh Edition.
- Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, et al. (2014) Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis 64: 510-533. [Crossref]
- Koya D, Araki S, Haneda M (2011) Therapeutic management of diabetic kidney disease. J Diabetes Investig 2: 248-254. [Crossref]
- Saha SA, Tuttle KR (2010) Influence of glycemic control on the development of diabetic cardiovascular and kidney disease. Cardiol Clin 28: 497–516. [Crossref]
- Ruggenenti P, Cravedi P, Remuzzi G (2010) The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol 6: 319-330. [Crossref]
- Hollenberg NK1 (2010) Direct renin inhibition and the kidney. Nat Rev Nephrol 6: 49-55. [Crossref]
- Kilpatrick ES, Rigby AS, Atkin SL (2009) The Diabetes Control and Complications Trial: the gift that keeps giving. Nat Rev Endocrinol 5: 537-545. [Crossref]
- de Zeeuw D, Agarwal R, Amdahl M (2009) Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 376: 1543–1551. [Crossref]
- Staels B, Maes M, Zambon A (2008) Fibrates and future PPARalpha agonists in the treatment of cardiovascular disease. Nat Clin Pract Cardiovasc Med 5: 542-553. [Crossref]
- The Look AHEAD Research Group, Knowler WC, Bahnson JL, Bantle JP, Bertoni AG, et al. (2014) Effect of a Long-Term Behavioral Weight Loss Intervention on Nephropathy in Overweight or Obese Adults with Type 2 Diabetes: the Look AHEAD Randomized Clinical Trial. Lancet Diabetes Endocrinol 2: 801–809.
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, et al. (2012) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35: 1364–79. [Crossref]
- Ito D, Cao P, Kakihana T, Sato E, Suda C, et al. (2015) Chronic Running Exercise Alleviates Early Progression of Nephropathy withUpregulation of Nitric Oxide Synthases and Suppression of Glycation in Zucker Diabetic Rats. PLoS ONE 10: e0138037. [Crossref]
- Muhammad AB, Lokhandwala MF, Banday AA (2011) Exercise reduces oxidative stress but does not alleviate hyperinsulinemia or renal dopamine D1 receptor dysfunction in obese rats. Am J Physiol Renal Physiol 300: F98–104. [Crossref]
- Boor P, Celec P, Behuliak M, Grancic P, Kebis A, et al. (2009) Regular moderate exercise reduces advanced glycation and ameliorates early diabetic nephropathy in obese Zucker rats. Metabolism 58: 1669–77. [Crossref]
- Marwick TH, Hordern MD, Miller T, Chyun DA, Bertoni AG, et al. (2009) Exercise Training for Type 2 Diabetes Mellitus Impact on Cardiovascular Risk A Scientific Statement From the American Heart Association: Circulation 119: 3244-3262.
- Bax JJ, Young LH, Frye RL, Bonow RO, Steinberg HO, et al. (2007) Screening for coronary artery disease in patients with diabetes. Diabetes Care 30: 2729-36. [Crossref]
- American Diabetes Association (2016) Foundations of Care and Comprehensive Medical Evaluation. Diabetes Care 39: S23–S35. [Crossref]
- Colberg SR (2013) Exercise and Diabetes: A Clinician’s Guide to Prescribing Physical Activity. Alexandria, VA (Ed.), American Diabetes Association.
2021 Copyright OAT. All rights reserv
- U.S. Department of Health and Human Services (2008) physical activity guidelines for Americans [Internet], 2008.
- Katzmarzyk PT, Church TS, Craig CL, Bouchard C (2009) Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc 41: 998-1005. [Crossref]
- Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, et al. (2016) Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 39: 2065-2079. [Crossref]
- Biankin SA, Jenkins AB, Campbell LV, Choi KL, Forrest QG, et al. (2003) Target-seeking behavior of plasma glucose with exercise in type 1 diabetes. Diabetes Care 26: 297-301. [Crossref]
- Riddell MC, Milliken J (2011) Preventing exercise induced hypoglycemia in type 1 diabetes using real-time continuous glucose monitoring and a new carbohydrate intake algorithm: an observational field study. Diabetes Technol Ther 13: 819–825. [Crossref]
- Francescato MP, Stel G, Stenner E, Geat M (2015) Prolonged exercise in type 1 diabetes: performance of a customizable algorithm to estimate the carbohydrate supplements to minimize glycemic imbalances. PLoS One 10: e0125220. [Crossref]
- Adolfsson P, Mattsson S, Jendle J (2015) Evaluation of glucose control when a new strategy of increased carbohydrate supply is implemented during prolonged physical exercise in type 1 diabetes. Eur J Appl Physiol 115: 2599–2607. [Crossref]
- Baker LB, Rollo I, Stein KW, Jeukendrup AE (2015) Acute effects of carbohydrate supplementation on intermittent sports performance. Nutrients 7: 5733–5763. [Crossref]
- Zaharieva DP, Riddell MC (2015) Prevention of exercise-associated dysglycemia: a case study-based approach. Diabetes Spectr 28: 55-62. [Crossref]
- Colberg SR, Laan R, Dassau E, Kerr D (2015) Physical activity and type 1 diabetes: time for a rewire? J Diabetes Sci Technol 9: 609-618. [Crossref]

