Background: Lesions within the sella can be clinically silent and be identified incidentally on imaging, or present with symptoms related to endocrine abnormalities due to dysfunction of the hypothalamic-pituitary axis (HPA) or as a result of compression of nearby structures. Sudden onset of symptoms may occur with pituitary apoplexy, a condition where the lesion hemorrhage or outgrows its blood supply resulting in infarction; both circumstances can result in rapid enlargement of the lesion in question. Apoplexy is most commonly found with pituitary adenomas, although rarely, it has been reported in association with other lesions of the pituitary, including Rathke cleft cysts (RCC).
Case Description: A 38-year-old woman with a known pituitary lesion, stable for eight years, presented with sudden onset of headache, nausea, vomiting and changes in vision. Imaging at presentation revealed the presence of a suprasellar lesion containing hemorrhagic products and exerting significant mass effect on nearby structures, most consistent with pituitary apoplexy. The patient underwent emergent transsphenoidal resection of the lesion with gross total resection and pathology supported a diagnosis of apoplexy. The patient presented an additional two times with similar symptoms with imaging demonstrating recurrent hemorrhage into the sella necessitating urgent re-exploration. After the third exploration, pathology revealed an underlying RCC.
Conclusions: Pituitary apoplexy results in the sudden onset of symptoms, in a lesion that might have previously been considered to be stable. Although commonly found in pituitary adenomas, RCC can rarely present with apoplexy and the two lesions can only be differentiated by pathology. RCC can masquerade as an adenoma, especially when necrosis and haemorrhagic products are abundant in the tissue sample. Therefore, when a patient presents with symptoms and imaging suggestive of recurrent apoplexy, a broad differential should be maintained. Collaborative efforts between the neurosurgeon and pathologist will ultimately reveal the correct diagnosis.
Rathke cleft cysts (RCC) are benign lesions that may be found in the sellar or suprasellar regions. They are defined pathologically by their lining of cuboidal, columnar or ciliated epithelium and are likely derived from embryonic vestiges of Rathke’s pouch [1]. Radiographically, RCC appear cystic and without calcification; the presence of the latter would favour craniopharyngioma over RCC. Although RCC are often discovered incidentally, they can present similarly to other lesions of the pituitary with symptoms due to mass effect of the lesion on nearby structures. Hormonal imbalance, headaches and visual disturbances have sometimes been described in cases of RCC and rarely, hemorrhage into the cyst that results in acute clinical deterioration has been reported [2]. In most cases, incidental discovery of RCC can be managed expectantly with periodic follow-up. However, when symptoms do present either gradually or acutely, patients require surgical intervention to prevent progression of symptoms and to preserve ophthalmologic function. Here, we describe a patient with a known suprasellar lesion who presented with recurrent apoplexy, ultimately discovered to have pathologic features consistent with RCC.
A 38-year-old female presented with new-onset severe retro-orbital headache, changes in vision, nausea, vomiting and hand weakness. The headache symptoms began three days before the development of blurry vision, peripheral vision loss, nausea, vomiting and bilateral hand weakness the day prior to presentation. Her past medical history was unremarkable except for a known suprasellar mass found incidentally eight years earlier, for which the patient did not receive follow-up. The patient denied galactorrhea and reported regular menses. MRI of the brain without contrast showed a sellar/suprasellar lesion that measured 2.1 × 1.3 × 2.2 cm with T2 hyperintense signal (Figure 1A). A second signal, T1 hyperintense/T2 hypointense measured 0.2 × 0.4 cm anteriorly and 0.7 × 1.5 × 0.9 cm posteriorly, with internal haemorrhagic products, and compression on the bilateral cavernous sinuses, infundibulum, and optic chiasm. Laboratory studies revealed low AM cortisol, concerning for central adrenal insufficiency. All other laboratory studies were within normal limits.
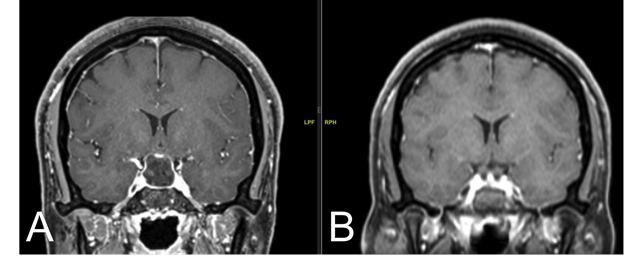
Figure 1. T2 MRI coronal images at initial presentation (A) and post-transsphenoidal resection (B)
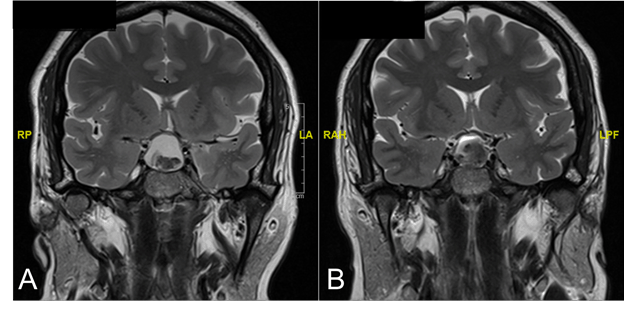
(A) Imaging at initial presentation showed a lesion within the sella which displayed hyperintense signaling on T2 MRI and a second, hypointense signal within the lesion suggesting internal hemorrhagic products. Displacement of the optic chiasm, infundibulum and bilateral cavernous sinuses can be seen. (B) Post-resection, the sella displayed post-surgical changes with hemorrhage and fluid-fluid levels.
Due to the acute onset of neurological symptoms combined with imaging findings, the patient underwent emergent endoscopic transsphenoidal resection of her pituitary lesion. Upon opening of the sellar floor dura, a significant amount of mucoid/haemorrhagic appearing fluid was released under pressure. Decompression also revealed necrotic material and solid tissue suspicious for tumour, all of which was collected for histopathology. Fluid cytology showed ciliated, columnar-shaped cells with goblet cells and histopathology revealed fragments of necrotic pituitary gland, acellular proteinaceous material and hemorrhage (Figure 2A, B). The patient’s vision improved, and headaches resolved following surgery. Imaging post-resection showed post-surgical changes with fluid-fluid levels and haemorrhagic products (Figure 1B).
Figure 2. Histopathology
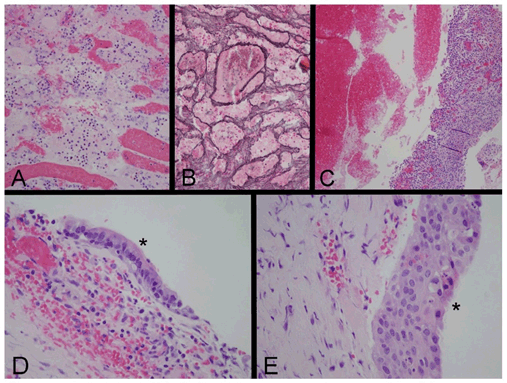
(A, B) Specimen from the first surgery. (A) Hematoxylin and eosin (H&E) section demonstrates small groups of necrotic cells that express synaptophysin by immunohistochemistry (not shown), establishing their identity as neuroendocrine in origin. Vascular congestion is also visible; acute hemorrhage present in the sample is not shown. (B) A reticulin stain demonstrates that the neuroendocrine cells have an acinar architectural pattern that is characteristic of non-neoplastic anterior pituitary gland. (C) This area of the specimen from the second surgery, stained with H&E, consists of fresh hemorrhage, at left, and a sample of granulation tissue, at right, that is composed of proliferating blood vessels, fibroblasts and chronic inflammation. A component of each of the specimens from the first two surgical procedures consisted of acellular proteinaceous material, consistent with cyst contents (not shown). (D, E) H&E sections of the specimen from the third surgical procedure reveal fragments of cyst wall, focally lined by ciliated columnar epithelial cells (D, *) that are the characteristic lining cells of a Rathke cleft cyst, and focally lined by nonkeratinized stratified squamous epithelium (E, *), interpreted as a zone of squamous metaplasia that can occur in a Rathke cleft cyst. Original magnifications: (A, B) 200x; (C) 100x; (D, E) 400x.
Four months after the surgery, the patient returned with a one-month history of left-sided monocular temporal hemianopsia, blurry vision, and left-sided periorbital headache. MRI of the brain without contrast showed a 2 × 2.1 × 1.3 cm T1 and T2 hyperintense mass within the sellar and suprasellar cistern with compression of the optic chiasm (Figure 3A). The size of the lesion was comparable to the preoperative lesion. She underwent emergent endoscopic transsphenoidal hypophysectomy with evacuation of the sellar hematoma. During the operation, an efflux of dark red blood was released, and although tumour was not visualized, several samples of tissue were collected for pathological examination. Microscopic examination revealed acellular proteinaceous material, inflammation and proliferation of small blood vessels and fibroblasts, consistent with subacute reactive changes (Figure 2C). The patient’s vision was restored, and she improved clinically after surgery, with post-operative imaging showing reduced compression on the infundibulum and optic chiasm (Figure 3B).
Figure 3. T2 MRI coronal images four months after initial presentation (A) and post-re-exploration of the sella (B)
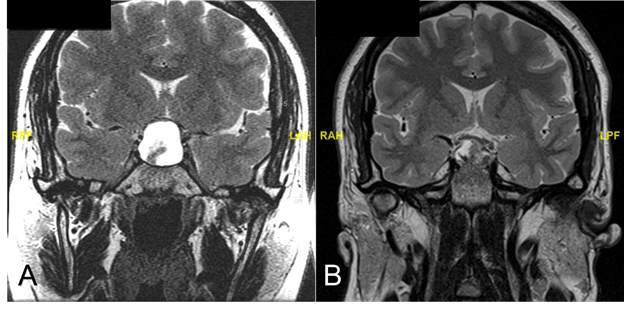
(A) Imaging four months after initial presentation displayed a lesion within the sella, similar to the one observed initially. The lesion was hyperintense on T2 MRI with a second, hypointense signal suggesting hemorrhage. Compression of the optic chiasm was observed. (B) Post-surgical changes were observed on imaging post-op, with a heterogeneous collection seen on T2 MRI, with decreased mass effect on the optic chiasm and infundibulum.
On follow-up one month after the second surgery, the patient complained of residual blurry vision in her left eye. Repeat MRI showed reaccumulation of T1 and T2 hyperintense signal in the sella, favouring proteinaceous cystic fluid rather than hemorrhage (Figure 4). The mass measured 2.1 × 2 × 1.2 cm with mass effect on the optic chiasm, causing repeated visual symptoms in the patient and the patient was taken back to the operating room for endoscopic transsphenoidal sinus surgery for the third time. Upon opening the floor of the sella, a large amount of mucoid-appearing material was released, and cystic wall components were collected. At this intervention, the sella was marsupialized and left open to allow drainage to the sphenoid sinus and prevent reaccumulation of fluid. Pathological examination identified the presence of a cyst wall epithelium, which appeared most consistent with a diagnosis of RCC (Figure 2D, E). Following this final intervention, the patient’s symptoms resolved, and she continued to improve in the post-operative period. Imaging post-operatively displayed expected post-surgical changes, with mixed hyperintense and isointense signal within the sella, and decompression of the optic chiasm (Figure 5A). Five months later, imaging did not show any evidence of cyst recurrence and patient remained neurologically stable (Figure 5B).
Figure 4. T2 MRI coronal images five months after initial presentation
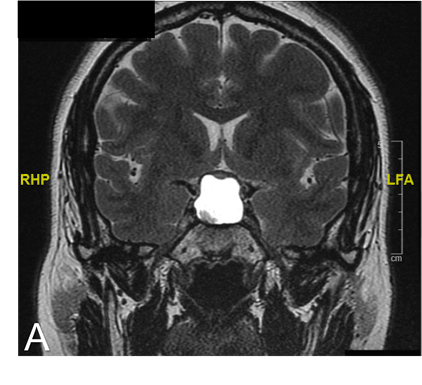
Imaging at five months after initial presentation showed T2 hyperintense signal in the sella which suggested accumulation of proteinaceous fluid within the previous tumor/resection site. Compression on the optic chiasm is again visualized.
Figure 5. T1 MRI coronal images post-re-exploration of sella for the third time
(A) Post-surgical changes were noted on the post-operative scan, with both hyperintense and isointense signaling within the sella. The optic chiasm appears to be decompressed post-operatively. (B) At follow-up five months later, complete resolution of the cyst is note
Management of pituitary lesions is determined by several factors. One important factor is the presence and severity of symptoms at presentation. For example, patients with visual field deficits due to compression of the lesion on the optic chiasm require intervention to restore and preserve ophthalmologic function. With identification of a non-compressive lesion, the decision for continued observation versus surgical intervention is most often defined by endocrinologic function or presumed diagnosis. In the case of a Rathke cleft cyst (RCC) or nonfunctional adenoma with an intact HPA and absence of chiasmic compression, observation with serial imaging is the most commonly accepted course.
In this instance, the patient had a known pituitary lesion identified in 2011 when she underwent neuroimaging for trauma but was subsequently lost to follow-up. As she was asymptomatic for years, she did not seek medical attention until 2019, when she began experiencing retro-orbital headaches, changes in vision, nausea and vomiting. Her symptoms were acute in onset, concerning for an acute neurologic process or hemorrhage. Indeed, CT revealed a lesion that likely hemorrhage with mass effect on the optic chiasm. Although previously asymptomatic, this acute onset of neurologic symptoms warranted immediate neurosurgical intervention.
Radiographs are limited in their diagnostic utility of pituitary lesions. Adenomas frequently appear as solid lesions with varying intensities on T1 and T2-weighted MRI; cystic adenomas are occasionally encountered as well. Often pituitary adenomas include evidence of necrosis and hemorrhage on MRI [3]. RCC appear as hyperintense on T1 and hypointense on T2-weighted images; the intensity can vary based on cystic contents. The key distinguishing features of RCC from an adenoma is its midline location without stalk deviation and its position between the anterior and posterior glands on sagittal images. Initially, the CT scan of our patient suggested the lesion was a macroadenoma with hemorrhage and subsequent MRI supported this diagnosis. The patient was taken to the operating room to remove the lesion, as she was symptomatic due to compression of the optic chiasm and delayed intervention risks compromised ophthalmic function. Lab work to evaluate pituitary function was significant only for mild elevation in prolactin most consistent with stalk effect in the setting of a non-secretory macroadenoma presenting with apoplexy.
Definitive diagnosis of a pituitary lesion requires histopathology. Normally, the pituitary gland contains distinct acini which produce the different hormones intrinsic to that region. Unsurprisingly, pituitary adenomas, which are inherently lesions of disordered growth, display loss of the normal acinar architectural pattern of the pituitary.[4] RCC by comparison, are defined by a cyst wall of simple cuboidal or pseudostratified columnar cells, or ciliated epithelial cells as well as cystic contents which usually stain with eosin. The histopathology of the surgical specimens from the patient presented here revealed different results with each intervention. Initially, pathology showed necrosis and hemorrhage, most consistent with pituitary apoplexy. After the second intervention, hemorrhage with acellular proteinaceous material and subacute granulation tissue with mild inflammation was seen. Finally, after the patient was taken back to the operating room for a third resection, pathology of the cyst wall revealed epithelium, including ciliated columnar and cuboidal cells and stratified squamous cells, with proteinaceous cyst contents, most consistent with a diagnosis of RCC.
RCC rarely become symptomatic and when they do, the symptoms are typically attributable to compression of adjacent structures. However, there have been isolated reports of RCC presenting as pituitary apoplexy, a pathological event more commonly associated with pituitary adenomas. In fact, clinical presentations of RCC apoplexy mimic that of pituitary apoplexy although often less severe. Sudden onset severe headache is almost always present in RCC apoplexy cases. Other symptoms include vision disturbances, nausea and vomiting, and less commonly meningismus, seizures, and altered mental status [5-12]. One group even devised the term “Rathke cleft cyst apoplexy” to describe this clinical phenomenon [2]. In many of these cases, the presentation, clinical course and radiographic studies favoured pituitary adenoma as the diagnosis, but pathology revealed an underlying RCC, as in our patient. In one report, two patients exhibited both RCC and pituitary adenoma, with a clinical presentation of pituitary apoplexy.[13] Other reports describe “non-haemorrhagic” RCC apoplexy, and they postulate that in these patients, leakage of cystic fluid causes similar apoplexy-like symptoms as in haemorrhagic apoplexy [6,14,15]. It is clear from these cases that although pituitary apoplexy is more commonly associated with pituitary adenoma, definitive diagnosis of a lesion cannot be made until surgical intervention allows gross examination of the lesion and provides a tissue sample for pathology.
The recurrence rate of RCC has been reported to be 3-30% over a follow-up duration between 2-5 years [1,16-19]. Recurrence of RCC is associated with squamous metaplasia, a feared consequence of RCC [20]. The frequency of required intervention in the patient described may be a concern for malignancy; fortunately, there was no evidence of this on pathology. The frequency with which this patient experienced symptoms, combined with her final diagnosis of RCC and risk for squamous metaplasia, may indicate a need for more frequent surveillance. To date, there are no identified triggers or precipitating events to RCC apoplexy and its recurrence. In the present case, our patient had a stable pituitary lesion which subsequently became unstable, three times within a six-month time period. One contributing factor may be the profound inflammatory process that ensues when cystic fluid and blood accumulate. Hemorrhage or cyst rupture may be the initial insult, which triggers a vicious cycle of inflammation, resulting clinically as recurrent apoplexy.
The patient presented here with a known pituitary lesion, stable for eight years with the sudden onset of headache, nausea, vomiting and visual symptoms. This presentation is most consistent with a pituitary adenoma, undergoing apoplexy. CT and MRI were consistent with this diagnosis, and even pathological studies supported this diagnosis after the first and second transsphenoidal resections. Finally, after the third exploration, pathology revealed an underlying Rathke cleft cyst. This case highlights the importance of maintaining a broad differential for pituitary lesions, to be wary of recurring apoplexy symptoms and to rely on pathology for definitive diagnosis.
- Shin JL, Asa SL, Woodhouse LJ, Smyth HS, Ezzat S (1999) Cystic lesions of the pituitary: clinicopathological features distinguishing craniopharyngioma, Rathke's cleft cyst, and arachnoid cyst. J Clin Endocrinol Metab 84: 3972-82. [Crossref]
- Chaiban JT, Abdelmannan D, Cohen M, Selman WR, Arafah BM (2011) Rathke cleft cyst apoplexy: a newly characterized distinct clinical entity. J Neurosurg 114: 318-24. [Crossref]
- Vasilev V, Rostomyan L, Daly AF, Potorac L, Zacharieva S, et al. (2016) MANAGEMENT OF ENDOCRINE DISEASE: Pituitary 'incidentaloma': neuroradiological assessment and differential diagnosis. Eur J Endocrinol 175: R171-84. [Crossref]
- Kleinschmidt-DeMasters BK (2016) Histological features of pituitary adenomas and sellar region masses. Curr Opin Endocrinol Diabetes Obes 23: 476-484. [Crossref]
- Kim E (2012) A Rathke's Cleft Cyst Presenting with Apoplexy. J Korean Neurosurg Soc 52: 404-6. [Crossref]
- Binning MJ, Liu JK, Gannon J, Osborn AG, Couldwell WT (2008) Hemorrhagic and nonhemorrhagic Rathke cleft cysts mimicking pituitary apoplexy. J Neurosurg 108: 3-8. [Crossref]
- Nishioka H, Ito H, Miki T, Hashimoto T, Nojima H, et al. (1999) Rathke's cleft cyst with pituitary apoplexy: case report. Neuroradiology 41: 832-4. [Crossref]
- Schooner L, Wedemeyer MA, Bonney PA, Lin M, Hurth K, et al. (2020) Hemorrhagic Presentation of Rathke Cleft Cysts: A Surgical Case Series. Oper Neurosurg (Hagerstown) 18: 470-479. [Crossref]
- Rosales MY, Smith TW, Safran M (2004) Hemorrhagic Rathke's cleft cyst presenting as diplopia. Endocr Pract 10: 129-34. [Crossref]
- Santos JM, Hannay M, Olar A, Eskandari R (2019) Rathke's Cleft Cyst Apoplexy in Two Teenage Sisters. Pediatr Neurosurg 54: 428-435. [Crossref]
- Ohnishi Y-I, Fujimoto Y, Iwatsuki K, Yoshimine T (2015) A Case of Apoplexy of Rathke's Cleft Cyst Followed by Cerebral Infarction. Case Rep Neurol Med 2015: 645370. [Crossref]
- Komatsu F, Tsugu H, Komatsu M, Sakamoto S, Oshiro S, et al. (2010) Clinicopathological characteristics in patients presenting with acute onset of symptoms caused by Rathke's cleft cysts. Acta Neurochir (Wien) 152: 1673-8. [Crossref]
- Gessler F, Coon VC, Chin SS, Couldwell WT, et al. (2011) Coexisting rathke cleft cyst and pituitary adenoma presenting with pituitary apoplexy: report of two cases. Skull Base Rep 1: 99-104. [Crossref]
- Yokota H, Ida Y, Wajima D, Nishimura F, Nakase H (2017) Rathke Cleft Cyst with Evidence of Rupture into Subarachnoid Space. World Neurosurg 97: 752.e1-752.e3. [Crossref]
- Neidert MC, Woernle CM, Leske H, Möller-Goede D, Pangalu A, et al. (2013) Ruptured Rathke cleft cyst mimicking pituitary apoplexy. J Neurol Surg A Cent Eur Neurosurg 74: e229-32. [Crossref]
- Lillehei KO, Widdel L, Astete CAA, Wierman ME, Kleinschmidt-DeMasters BK, et al. (2011) Transsphenoidal resection of 82 Rathke cleft cysts: limited value of alcohol cauterization in reducing recurrence rates. J Neurosurg 114: 310-7. [Crossref]
- Ross DA, Norman D, Wilson CB (1992) Radiologic characteristics and results of surgical management of Rathke's cysts in 43 patients. Neurosurgery 30: 173-8. [Crossref]
- Mukherjee JJ, Islam N, Kaltsas G, Lowe DG, Charlesworth M, et al. (1997) Clinical, radiological and pathological features of patients with Rathke's cleft cysts: tumors that may recur. J Clin Endocrinol Metab 82: 2357-62. [Crossref]
- Aho CJ, Liu C, Zelman V, Couldwell WT, Weiss MH (2005) Surgical outcomes in 118 patients with Rathke cleft cysts. J Neurosurg 102: 189-93. [Crossref]
- Chotai S, Liu Y, Pan J, Qi S (2015) Characteristics of Rathke's cleft cyst based on cyst location with a primary focus on recurrence after resectionc. J Neurosurg 122: 1380. [Crossref]





