Abstract
Clinical practice guidelines are increasingly being used to support high-quality patient care. The use of evidence-based approaches is important in effectively addressing the side effects of oral mucositis (OM) resulting from chemotherapy. The purpose of this study was to test the effectiveness of evidence-based OM guidelines in improving the quality of life (QOL) of head and neck cancer (HNC) patients. This research used a prospective, repeated measure, quasi-experimental design with a control group. A convenience sample of 60 HNC patients was recruited from one medical centre in metropolitan Taipei City. The experimental group received care in accordance with OM-related clinical practice guidelines, while the control group received routine care only. QOL was measured using the Chinese version of the European Organization for Research and Treatment of Cancer (EORTC) QLQ-HN35 at baseline and at two times after the intervention. Sixty patients completed the pre-test and first post-test (10th day) measure. Forty-five of the participants (18 experimental group and 27 control group) completed the second post-test (14th day) measures. Generalised estimating equations revealed statistically significant group-by-time interactions in the QOL. Using the control group as the reference group and the baseline as the reference time, the post-intervention impact of mucositis on QOL was significantly less in the experimental group than in the control group: on day 10 (β = -0.91, p <.001) (Likert 4-point)/(β= -2.73, p <.001) (dichotomy) and day 14 (β = -1.10, p <.001) (Likert 4-point)/(β= -3.57, p <.001) (dichotomy). The findings of the present study support the effectiveness of using OM guidelines to alleviate the impact of OM on the QOL of HNC patients.
Keywords
cancer, chemotherapy, oral mucositis, clinical practice guidelines, quality of life
Introduction
Head and neck cancer (HNC) refers to various malignancies of the upper aerodigestive tract and is one of the most commonly diagnosed cancers worldwide [1]. Although advancements in cancer treatment have improved survival rates for many cancers, including HNC [2,3], these therapies induce numerous side effects such as complications in the oral cavity. One of the most significant oral complications of cancer treatment is oral mucositis (OM) [4-6].
Oral mucositis refers to inflamed erosive or ulcerative injuries of the oral mucosa. The features of OM include widespread erythema, pseudomembranous deterioration, and candida ulceration [7]. Chemotherapy typically causes OM to peak in terms of intensity at 7-14 days. Afterward, OM gradually resolves in the absence of secondary infections or of the further administration of chemotherapeutic agents [8].
Patients typically experience the most severe symptoms during the peak period of OM. For example, increased levels of OM due to pain and the regional impairment of physical functions such as swallowing, drinking, speaking, and sleeping may affect daily living profoundly and, hence, increase hospital costs (Nonzee et al., 2008) and the impact on quality of life (QOL) [9-11].
In certain conditions, the agent intensity of chemotherapy diminishes due to the side effects of OM, subsequently impacting the efficacy of the chemotherapeutic agent [12]. Therefore, it is important to apply evidence-based approaches in order to deal with chemotherapy-related OM in order to enhance healthcare quality and therapeutic outcomes [13]. However, as expert opinion and consensus-based clinical guidelines most frequently guide therapeutic practice, the base of published evidence base that is currently available on the management of OM in chemotherapy patients with HNC is limited.
Clinical practice guidelines have been defined as “systematic developed statements to assist practitioner and patient decisions about appropriate health care for clinical circumstances.” [14]. Evidence-based clinical practice guidelines are available to guide clinicians in the selection of effective strategies to manage chemotherapy-caused OM. The purpose of the present study was to analyse the effect of the Clinical Practice Guideline on Oral Mucositis in Cancer Patients (CPGOMCP) on the QOL of patients with HNC. The primary goal was to reduce the impact of mucositis on the QOL of patients undergoing chemotherapy. The results of the first and second post-test of participants for the experimental (CPGOMCP intervention) group and the control (routine care) group were compared.
Methods
The aim of the present study was to evaluate the effect of a clinical guideline on the QOL of HNC patients. This research used a prospective, repeated measure, quasi-experimental design with a control group. The effective sample size was calculated using G-power 3.0 [15]. Based on a power of .80, an alpha of .05, and an effect size (ES) based on the research results of Soga et al. [16] which indicated a prevalence of post-oral-care-intervention ulcerative OM of 31.0% (9/29) in the experimental group and of 79.2% (19/24) in the control group, a minimum sample size of 16 participants in each group (32 in total) was determined. Allowing for a 20% rate of refusal to participate and a 20% attrition rate, the researchers recruited an initial 30 participants for each group.
Sample and Procedures
The study was conducted in one medical centre in metropolitan Taipei City. Patients were recruited using a convenience sequential sampling approach. Patients with HNC admitted to an oncology ward were asked to participate in this study between January 1 and August 31, 2014. Patients that met the following inclusion criteria were eligible for enrolment as participants: (1) diagnosis of HNC and intent to receive systematic chemotherapy treatment for this cancer; (2) oral mucosa were intact at time of enrolment; (3) able to self-perform oral care or to perform oral care with assistance; (4) older than 18 years of age; and (5) conscious and able to sign the consent form. The exclusion criteria included having received other approaches such as topical oral-hygiene agents for managing oral mucosa. A total of 60 patients agreed to participate and completed the pre-test measure. Of these patients, 30 were assigned to the experimental group and 30 were assigned to the control group.
In order to prevent interaction among the interventional effects, the initial 30 qualified participants were assigned to the control group and the latter 30 were assigned to the experimental group. (Table 1) illustrates the process that was used in the present study.
Table 1. Two-group, quasi-experimental design.
| January - April, 2014 | April - August, 2014 |
| Admission | 10th day | 14th day | Admission | 10th day | 14th day |
Control group | O1 | O2 | O3 | | | |
Experimental group | | | | O1 X | O2 | O3 |
Note: O1- First interview; O2-Second interview; O3-Third interview; X-Implementation of CPGOMCP program.
The institutional review board of the medical centre approved the present study. The study consent form was signed prior to participant enrolment. Participants were informed that confidentiality was guaranteed, and that participation was voluntary. All participants were free to withdraw from the research at any time during the research process. Participants received a research survey packet after providing informed consent to participate. After a participant completed and returned his/her survey, a researcher reviewed the survey form to confirm completeness and asked the participant to complete any missing items. Details of the medical characteristics of the participants were collected from hospital medical records. The outcome measure of QOL was assessed at three-time points, including at pre-test and on the 10th and 14th days after the intervention (post-test). (Figure 1) presents the flow chart that was used for the present study.
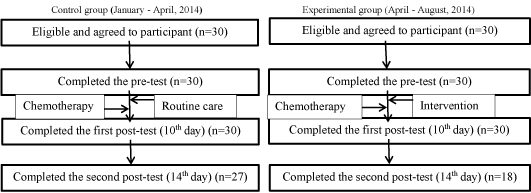
Figure 1. Study flow diagram.
Clinical Practice Guideline of Oral Mucositis in Cancer Patients (CPGOMCP)
This CPGOMCP was developed by Chou, Hsieh, Lee, Chiang, and Chi [17] based on evidence-based guidelines and on a systematic review of the evidence from various scholarly sources, including clinical practice guidelines, randomized controlled clinical trials, systematic reviews, and meta-analyses, that was published between 2001 and 2011. The CPGOMCP involves a variety of interventions. Major sections are summarized in the following:
- Evaluation and risk factor identification: including the OM assessment tool selected, inspection facilities for evaluating the health of the oral cavity, and holistic history assessment for potential OM such as the regimen of chemotherapy and the pre-treatment oral evaluation that was conducted by dentists.
- Education and management: including patient education promoting the prevention and management of OM (for example: using mouth rinse, preventive oral care, oral cryotherapy, and specific approaches / pharmacologic agents for conditional therapy) and the oral care protocol for managing grade 1 - 4 OM (for example: nutritional monitoring, blood cell monitoring, therapeutic oral care, and the management of symptoms such as pain).
- Continued care: including education for continued homecare; care for complications such as infections, bleeding, and dry mouth; and situations requiring hospitalized.
Measures
Sociodemographic Variables
The sociodemographic variables that were assessed included: age, gender, body mass index, education, religion, marital status, employment status, and relationship to the caregiver. The medical variables that were assessed included: concurrent radiotherapy, periodontosis, mouth rinsing, frequency of tooth brushing, the use of analgesics, tooth treatment in the past 6 months, and the presence of chronic disease.
Quality of life
Quality of life was measured using the Chinese version of European Organization for Research and Treatment of Cancer (EORTC) QLQ-HN35 [18]. The EORTC QLQ-HN35 is a self-report questionnaire that was designed specifically for HNC patients. This instrument consists of 35 items on health-related quality of life, with 7 subscales (pain, swallowing, senses, speech, social eating, social contact, and sexuality) and 11 single items (problems with teeth, problems opening the mouth, dry mouth, sticky saliva, cough, feeling ill, pain killers, nutritional supplements, feeding tube, weight loss, and weight gain). The timeframe addressed by the EORTC QLQ-HN35 module is “during the past week”. The subscale items are scored on a scale ranging from 1-4, with 1= “not at all”, 2= “a little”, 3= “quite a bit”, and 4= “very much”. The single items are scored on a yes (2) or no (1) response format. Higher scores for the instrument indicate relatively worse QOL.
Statistics
Data were analysed using SPSS for Windows 18.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics (mean, standard deviation (SD), frequency, and percentage) were used to characterize the total sample and QOL. The Fisher’s exact test and the independent-samples t test for homogeneity was used to identify any significant group differences in the demographic variables and in the pre-test measure of QOL. The effect of CPGOMCP on QOL was evaluated using generalized estimating equations (GEE) to analyse the repeated measured of the outcomes. Covariates (control variables) were selected from the demographic and medical variables in order to identify significant differences in the base data.
Results
A total of 60 participants (30 experimental group and 30 control group) completed the pre-test measures and the first posttest (10th day) measures. However, only 45 of the participants (18 experimental group and 27 control group) completed the second posttest (14th day) measures. The main reasons for dropping out of the experimental group was hospital discharge prior to the second posttest and refusal to continue participation.
The general demographic and medical characteristics for participants are summarized in (Table 2). The mean age was 52.9 years (SD = 10.8, range 25-74) and the mean body mass index was 22.3 kg/m2 (SD = 3.4, range 16.0-33.1). A majority of participants were men (n = 54, 90.0%), indicated a religious affiliation (n = 47, 78.3%), were married (n =53, 88.3%), and were not employed (n = 33, 55.0%). Most had not received concurrent radiotherapy (n = 43, 71.7%) or tooth treatment during the past 6 months (n = 54, 90.0%). Table 2 presents the demographic data of participants.
Table 2. Comparison of demographic characteristics, by group (n=60).
| Variable | Participant N (%) | Group | | t | p |
| Exp. N (%) | Cont. N (%) |
Gender | | | | - | .671 a |
Male | 54 (90.0) | 26 (86.7) | 28 (93.3) | | |
Female | 6 (10.0) | 4 (13.3) | 2 (6.7) | | |
Age (in years, mean ± SD) | 52.9 ±10.8 | 53.4 ±11.2 | 52.4 ±10.6 | -0.36 | .723 |
Body mass index (kg/m2, mean ± SD) | 22.3 ±3.4 | 22.2 ±2.8 | 22.4 ± 4.0 | 0.25 | .802 |
Education level | | | | - | .533 a |
Illiterate | 1 (1.7) | 0 (0.0) | 1 (3.3) | | |
Primary school | 17 (28.3) | 10 (33.3) | 7 (23.3) | | |
Junior high school | 15 (25.0) | 9 (30.0) | 6 (20.0) | | |
Senior high school | 13 (21.7) | 6 (20.0) | 7 (23.3) | | |
Post-secondary | 14 (23.3) | 5 (16.7) | 9 (30.0) | | |
Religion affiliation | | | | - | .457 a |
None | 13 (21.7) | 7 (23.3) | 6 (20.0) | | |
Buddhism | 12 (20.0) | 4 (13.3) | 8 (26.7) | | |
Taoism | 34 (56.7) | 19 (63.3) | 15 (50.0) | | |
Catholicism | 1 (1.7) | 0 (0.0) | 1 (3.3) | | |
Marital status | | | | - | .506 a |
Single | 4 (6.7) | 3 (10.0) | 1 (3.3) | | |
Married | 53 (88.3) | 25 (83.3) | 28 (93.3) | | |
Divorced | 3 (5.0) | 2 (6.7) | 1 (3.3) | | |
Caregiver | | | | - | .005 a** |
Spouse | 12 (20.0) | 3 (10.0) | 9 (30.0) | | |
Children | 3 (5.0) | 0 (0.0) | 3 (10.0) | | |
Foreign caregiver | 5 (8.3) | 5 (16.7) | 0 (0.0) | | |
Local caregiver | 1 (1.7) | 0 (0.0) | 1 (3.3) | | |
None | 39 (65.0) | 22 (73.3) | 17 (56.7) | | |
Employment | | | | - | .017a* |
Unemployed | 33 (55.0) | 11 (36.7) | 22 (73.3) | | |
Part time | 21 (35.0) | 15 (50.0) | 6 (20.0) | | |
Full time | 6 (10.0) | 4 (13.3) | 2 (6.7) | | |
Chronic disease | | | | | |
Diabetes | 10 (16.7) | 5 (16.7) | 5 (16.7) | - | 1.000 a |
Hypertension | 8 (13.3) | 4 (13.3) | 4 (13.3) | - | 1.000 a |
Hepatitis | 1 (1.7) | 0 (0.0) | 1 (3.3) | - | 1.000 a |
Kidney disease | 1 (1.7) | 1 (3.3) | 0 (0.0) | - | 1.000 a |
Other | 2 (3.3) | 0 (0.0) | 2 (6.7) | - | .492 a |
Periodontosis | | | | - | N.A. |
No | 60 (100.0) | 30 (100.0) | 30 (100.0) | | |
Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | | |
Had tooth treatment in the past 6 months | | | | - | .671 a |
No | 54 (90.0) | 28 (93.3) | 26 (86.7) | | |
Yes | 5 (8.3) | 2 (6.7) | 3 (10.0) | | |
Unknown | 1 (1.7) | 0 (0.0) | 1 (3.3) | | |
Prior incidence of oral mucositis | | | | - | .412 a |
No | 40 (66.7) | 18 (60.0) | 22 (73.3) | | |
Yes | 20 (33.3) | 12 (40.0) | 8 (26.7) | | |
Mouth rinsing | | | | - | .105 a |
None | 38 (63.3) | 15 (50.0) | 23 (76.7) | | |
Every day | 7 (11.7) | 5 (16.7) | 2 (6.7) | | |
Conditional rinsing | 15 (25.0) | 10 (33.3) | 5 (16.7) | | |
Frequency of tooth brushing | | | | - | .076 a |
None | 3 (5.0) | 2 (6.7) | 1 (3.3) | | |
1 / daily | 23 (38.3) | 8 (26.7) | 15 (50.0) | | |
2 / daily | 25 (41.7) | 12 (40.0) | 13 (43.3) | | |
3 / daily | 6 (10.0) | 5 (16.7) | 1 (3.3) | | |
4 / daily | 3 (5.0) | 3 (10.0) | 0 (0.0) | | |
Analgesics | | | | - | .353 a |
No | 55 (91.7) | 29 (96.7) | 26 (86.7) | | |
Yes | 5 (8.3) | 1 (3.3) | 4 (13.3) | | |
Concurrent radiotherapy | | | | - | 1.000 a |
No | 43 (71.7) | 22 (73.3) | 21 (70.0) | | |
Yes | 17 (28.3) | 8 (26.7) | 9 (30.0) | | |
a Fisher’s exact test; *p < .05; **p < .01.; Exp. Experimental group, Cont. Control group.
Table 2 shows the baseline characteristics of the study population. The two groups were homogeneous with the exception that the experimental group had significantly more participants who were currently employed (p = 0.005) and who had no caregiver (p = 0.017) than the control group. Moreover, no statistically significant differences between the two groups were found for the EORTC QLQ-HN35, with the exception that the control group had significantly higher mean scores for speech problems (p=0.019) and sexuality problems (p=0.021) than the experimental group (Table 3).
Table 3. Comparison of pre-test subscale and total scale EORTC QLQ-HN35 scores, by group.
| Subscale/Total scale | Total sample
(n= 60) Mean (SD) | Group | t | p |
| Exp. (n = 30) Mean (SD) | Cont. (n = 30) Mean (SD) |
Pain | 1.50±0.65 | 1.38 ± 0.52 | 1.62 ± 0.74 | 1.46 | .151 |
Swallowing | 1.29±0.61 | 1.14 ± 0.42 | 1.43 ± 0.73 | 1.90 | .062 |
Senses | 1.28±0.57 | 1.18 ± 0.33 | 1.38 ± 0.73 | 1.37 | .176 |
Speech | 1.40±0.59 | 1.22 ± 0.38 | 1.58 ± 0.71 | 2.42 | .019* |
Social eating | 1.39±0.68 | 1.28 ± 0.51 | 1.51 ± 0.81 | 1.33 | .187 |
Social contact | 1.16±0.49 | 1.05 ± 0.18 | 1.28 ± 0.66 | 1.87 | .067 |
Sexuality | 1.53±0.79 | 1.30 ± 0.53 | 1.77 ± 0.94 | 2.37 | .021* |
Likert 4-point of EORTC† | 1.35±0.56 | 1.21 ± 0.34 | 1.49 ± 0.70 | 1.95 | .056 |
Dichotomy of EORTC‡ | 0.62±1.65 | 0.33 ± 0.88 | 0.90 ± 2.14 | 1.34 | .185 |
†Likert 4-point, 1: not at all, 4: very much (for 7 subscales); ‡ Dichotomy, 0: no, 1: yes (for 11 single items); *p < .05.; Exp. Experimental group, Cont. Control group.
The effects of CPGOMCP on quality of life
Table 4 shows the effects of the CPGOMCP intervention on QOL, as assessed using the GEE. These effects were controlled for bias in terms of the variables: having a caregiver and employment status. Significant interaction effects (group×time) were found for QOL. Compared with their control group peers, the experimental group reported a significantly lower impact of mucositis on patients’ EORTC QLQ-HN35 (Likert 4-point) on day 10 (B = -0.91, p <.001) and on day 14 (B = -1.10, p <.001); a significantly lower impact of EORTC QLQ-HN35 (dichotomy) on day 10 (B= -2.73, p <.001) and on day 14 (B= -3.57, p <.001); and a significantly lower impact of the seven dimensions of the EORTC QLQ-HN35 on day 10 (B= -0.70 to -1.05, p <.001) and on day 14 (B= -0.88 to -1.30, p <.001; Table 5). Figure 2 and Figure 3 reveal the changes in EORTC QLQ-HN35 over the three observation time points for the control and experimental groups.
Table 4. GEE analysis of the CPGOMCP effects on QOL.
| | Likert 4-point of EORTC† | Dichotomy of EORTC‡ |
| Parameter | B | S.E. | Wald χ² | p | B | S.E. | Wald χ² | p |
Interaction (group×time) | | | | | ? | ? | | |
First posttest (10th day) | -0.91 | 0.18 | 24.99*** | <.001 | -2.73 | 0.62 | 19.22*** | <.001 |
Second posttest (14th day) | -1.10 | 0.16 | 46.05*** | <.001 | -3.57 | 0.55 | 42.27*** | <.001 |
Intercept | 1.45 | 0.18 | 63.66*** | <.001 | 0.51 | 0.62 | 0.68 | .409 |
Group | | | | | | | | |
Exp. vs. Cont. | -0.33 | 0.18 | 3.51 | .061 | -0.70 | 0.50 | 1.93 | .165 |
Time | | | | | | | | |
First posttest (10th day) vs. pre-test | 1.31 | 0.16 | 64.38*** | <.001 | 4.40 | 0.56 | 62.41*** | <.001 |
Second posttest (14th day) vs. pre-test | 1.39 | 0.14 | 98.56*** | <.001 | 5.13 | 0.43 | 139.94*** | <.001 |
Covariate | | | | | | | | |
Caregiver | | | | | | | | |
Children vs. Spouse | -0.04 | 0.33 | 0.01 | .905 | 0.65 | 0.66 | 0.97 | .324 |
Foreign caregiver vs. Spouse | -0.13 | 0.17 | 0.56 | .455 | -0.36 | 0.57 | 0.40 | .528 |
Local caregiver vs. Spouse | -0.59 | 0.15 | 14.87*** | <.001 | -1.69 | 0.51 | 11.05*** | <.001 |
None vs. Spouse | 0.06 | 0.16 | 0.16 | .692 | 0.56 | 0.48 | 1.35 | .246 |
Employment | | | | | | | | |
Part time vs. Unemployed | 0.18 | 0.18 | 1.03 | .311 | 0.38 | 0.45 | 0.72 | .398 |
Full time vs. Unemployed | -0.20 | 0.16 | 1.54 | .215 | -0.18 | 0.44 | 0.17 | .682 |
†Likert 4-point, 1: not at all, 4: very much (for 7 subscales); Likert 4-point of EORTC†: average score of total 7 subscales; ‡ Dichotomy, 0: no, 1: yes (for 11 single items); Dichotomy of EORTC‡: average score of total 11 single items; ***p < .001.
Table 5. GEE analysis of the effects of CPGOMCP on the subscales of QOL (table 1 of 3).
| | Pain | Swallowing | Senses |
| Parameter | B | S.E. | Wald χ² | p | B | S.E. | Wald χ² | p | B | S.E. | Wald χ² | p |
Interaction (group×time) | | | | | | | | | | | | |
First posttest (10th day) | -0.70 | 0.21 | 11.63*** | .001 | -0.97 | 0.22 | 19.00*** | <.001 | -0.77 | 0.20 | 14.14*** | <.001 |
Second posttest (14th day) | -1.03 | 0.18 | 31.86*** | <.001 | -1.30 | 0.21 | 38.10*** | <.001 | -0.88 | 0.18 | 23.25*** | <.001 |
Intercept | 1.53 | 0.19 | 63.26*** | <.001 | 1.39 | 0.20 | 46.74*** | <.001 | 1.37 | 0.18 | 58.72*** | <.001 |
Group | | | | | | | | | | | | |
Exp. vs. Cont. | -0.33 | 0.20 | 2.76 | .097 | -0.28 | 0.19 | 2.21 | .137 | -0.25 | 0.18 | 2.01 | .156 |
Time | | | | | | | | | | | | |
First posttest (10th day) vs. pre-test | 1.25 | 0.17 | 54.88*** | <.001 | 1.33 | 0.19 | 47.52*** | <.001 | 1.15 | 0.18 | 41.65*** | <.001 |
Second posttest (14th day) vs. pre-test | 1.34 | 0.14 | 89.09*** | <.001 | 1.50 | 0.19 | 62.31*** | <.001 | 1.27 | 0.14 | 76.54*** | <.001 |
Covariate | | | | | | | | | | | | |
Caregiver | | | | | | | | | | | | |
Children vs. Spouse | -0.12 | 0.24 | 0.25 | .615 | 0.22 | 0.41 | 0.30 | .586 | -0.29 | 0.36 | 0.66 | .417 |
Foreign caregiver vs. Spouse | -0.06 | 0.22 | 0.07 | .789 | -0.18 | 0.21 | 0.68 | .410 | -0.11 | 0.18 | 0.37 | .542 |
Local caregiver vs. Spouse | -0.48 | 0.18 | 7.51** | .006 | -0.58 | 0.18 | 11.00*** | .001 | -0.35 | 0.14 | 5.83* | .016 |
None vs. Spouse | 0.16 | 0.18 | 0.77 | .379 | 0.07 | 0.17 | 0.18 | .674 | 0.08 | 0.16 | 0.26 | .611 |
Employment | | | | | | | | | | | | |
Part time vs. Unemployed | 0.21 | 0.21 | 0.98 | .322 | 0.10 | 0.20 | 0.28 | .599 | 0.11 | 0.18 | 0.38 | .536 |
Full time vs. Unemployed | -0.30 | 0.19 | 2.48 | .115 | -0.28 | 0.23 | 1.46 | .227 | -0.24 | 0.20 | 1.38 | .240 |
*p < .05, **p < .01, ***p < .001.
Table 5. GEE analysis of the effects of CPGOMCP on the subscales of QOL (table 2 of 3)
| | Speech | Social eating | Social contact |
| Parameter | B | S.E. | Wald χ² | p | B | S.E. | Wald χ² | p | B | S.E. | Wald χ² | p | |
Interaction (group×time) | | | | | | | | | ? | ? | | | |
First posttest (10th day) | -1.01 | 0.21 | 23.49*** | <.001 | -1.05 | 0.23 | 20.68*** | <.001 | -0.97 | 0.19 | 25.72*** | <.001 | |
Second posttest (14th day) | -1.28 | 0.20 | 42.42*** | <.001 | -1.24 | 0.21 | 34.71*** | <.001 | -0.90 | 0.15 | 35.19*** | <.001 | |
Intercept | 1.58 | 0.19 | 66.51*** | <.001 | 1.36 | 0.21 | 42.65*** | <.001 | 1.34 | 0.17 | 62.67*** | <.001 | |
Group | | | | | | | | | | | | | |
Exp. vs. Cont. | -0.40 | 0.19 | 4.34* | .037 | -0.33 | 0.20 | 2.83 | .093 | -0.31 | 0.16 | 3.67 | .055 | |
Time | | | | | | | | | | | | | |
First posttest (10th day) vs. pre-test | 1.20 | 0.19 | 41.48*** | <.001 | 1.73 | 0.20 | 75.92*** | <.001 | 1.09 | 0.18 | 35.17*** | <.001 | |
Second posttest (14th day) vs. pre-test | 1.38 | 0.16 | 72.03*** | <.001 | 1.78 | 0.16 | 117.59*** | <.001 | 0.98 | 0.14 | 45.71*** | <.001 | |
Covariate | | | | | | | | | | | | | |
Caregiver | | | | | | | | | | | | | |
Children vs. Spouse | -0.03 | 0.43 | 0.00 | .947 | 0.02 | 0.46 | 0.00 | .961 | -0.11 | 0.37 | 0.09 | .762 | |
Foreign caregiver vs. Spouse | -0.07 | 0.22 | 0.10 | .756 | -0.19 | 0.17 | 1.27 | .259 | -0.09 | 0.16 | 0.31 | .581 | |
Local caregiver vs. Spouse | -0.55 | 0.17 | 9.94** | .002 | -0.53 | 0.16 | 10.73** | .001 | -0.76 | 0.14 | 31.05*** | <.001 | |
None vs. Spouse | 0.00 | 0.18 | 0.00 | .994 | 0.19 | 0.17 | 1.33 | .249 | -0.12 | 0.15 | 0.64 | .424 | |
Employment | | | | | | | | | | | | | |
Part time vs. Unemployed | 0.17 | 0.22 | 0.62 | .431 | 0.25 | 0.16 | 2.39 | .122 | 0.29 | 0.18 | 2.64 | .104 | |
Full time vs. Unemployed | -0.18 | 0.19 | 0.83 | .362 | 0.05 | 0.17 | 0.08 | .782 | -0.14 | 0.15 | 0.96 | .327 | |
*p < .05, **p < .01, ***p < .001.
Table 5. GEE analysis of the effects of CPGOMCP on the subscales of QOL (table 3 of 3)
| | Sexuality |
| Parameter | B | S.E. | Wald χ² | p |
Interaction (group×time) | | | | |
First posttest (10th day) | -0.82 | 0.23 | 12.85*** | <.001 |
Second posttest (14th day) | -0.97 | 0.19 | 24.61*** | <.001 |
Intercept | 1.79 | 0.23 | 58.31*** | <.001 |
Group | | | | |
Exp. vs. Cont. | -0.42 | 0.23 | 3.38 | .066 |
Time | | | | |
First posttest (10th day) vs. pre-test | 1.43 | 0.19 | 58.02*** | <.001 |
Second posttest (14th day) vs. pre-test | 1.70 | 0.16 | 107.04*** | <.001 |
Covariate | | | | |
Caregiver | | | | |
Children vs. Spouse | -0.11 | 0.22 | 0.25 | .614 |
Foreign caregiver vs. Spouse | -0.28 | 0.27 | 1.06 | .303 |
Local caregiver vs. Spouse | -0.83 | 0.18 | 20.53*** | <.001 |
None vs. Spouse | 0.11 | 0.22 | 0.26 | .610 |
Employment | | | | |
Part time vs. Unemployed | -0.08 | 0.22 | 0.14 | .713 |
Full time vs. Unemployed | -0.44 | 0.29 | 2.27 | .132 |
***p < .001.
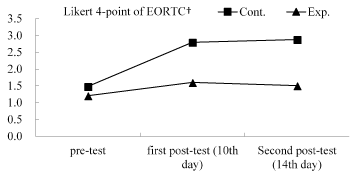
Figure 2. Average score changes in the EORTC (Likert 4-point) over time; Exp. = Experimental group; Cont. = Control group.
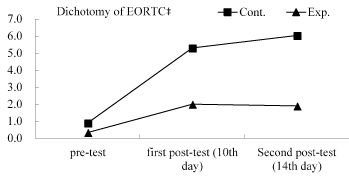
Figure 3. Total score changes in the EORTC (dichotomy) over time; Exp. = Experimental group; Cont. = Control group
Discussion
The present study investigated the effect on the quality of life of HNC patients of following the CPGOMCP guidelines. The results revealed that following these guidelines reduced by a statistically significant degree the decline in participant QOL, as compared with the control group. Furthermore, the use of the CPGOMCP guidelines was associated with reductions in the impact of the HNC chemotherapy treatment on all of the seven dimensions of the QOL, including pain, swallowing, senses, speech, social eating, social contact, and sexuality.
QOL is a crucial variable for evaluating the outcomes of management and the extended survival of cancer patients [19,20], as the objective of current cancer treatments is not to cure but rather to manage the symptoms and delay the progression of this disease. QOL is thus a critical indicator of symptom relief and of the rehabilitation needs of patients with cancer.
Patients undergoing chemotherapy for head and neck cancers may experience xerostomia, which increases the probability of OM [21] and the risks of oral infection and speaking and swallowing difficulties and, ultimately, significantly and negatively impacts the QOL of these patients [9-11]. The CPGOMCP guidelines provide a holistic tool for assessing the likely risks to oral cavity health. Participants in the experimental group underwent a dental evaluation prior to chemotherapy by a dental team that was experienced in oral oncology in order to identify and address the risk factors of oral mucositis such as spare plaque and dental caries. Thus, all of the prospect native sources of infection were excluded, and all suspected incidences of periodontal disease were cured [22].
Beginning assertive oral care prior to chemotherapy is crucial in order to reduce the severity of dry mouth and oral complications. Maintaining appropriate nutritional status, practicing effective oral hygiene, and identifying oral lesions in a timely manner are significant pre-treatment preparations. The CPGOMCP guidelines use an education and management program to prevent and manage oral mucositis. In the present study, the experimental group received pre-treatment oral care instructions, while the control group did not receive these instructions. The instructions included recommendations on using a mouth solution to prevent dry mouth, actively implementing preventive oral care, using oral cryotherapy for specific chemotherapy agents such as 5-fluorouracil (5-FU) bolus [23], and using a local agent such as aloe vera, polymixin/tobramycin/amphotericin (PTA), or Benzydamine HCL after concurrent radiotherapy in order to prevent oral mucositis [24,25].
Oral mucositis increases the risk of treatment-related morbidities including neutropenic sepsis and the need for nutritional support [4]. The current guidelines involve an oral care protocol for managing grade 1 - 4 OM that monitors platelets and neutrophil as well as nutrition. The guidelines further implement approaches to raise the moisture content of foods in order to facilitate swallowing and improve nutritional support.
The pain that is caused by mucositis is frequently a major problem for patients, often leading to difficulties with eating, drinking, and swallowing, which affects nutritional intake and leads to a worsening in QOL [26,27]. Therefore, pain management is an important issue for OM patients and includes topical coating agents for superficial lesions and opioids for the systematic relief of pain [28]. Indeed, OM-related pain management is a core objective of the CPGOMCP guidelines.
The results of the present study indicate that these guidelines hold strong potential for improving the QOL of HNC patients. However, several limitations should be highlighted. The participants were not randomly selected or randomly assigned. The experimental and control groups showed significant differences in terms of employment status and of relationship to caregiver as well as in terms of pre-test scores for the QOL dimensions of speech and sexuality. To address these pre-test differences between the groups, we structured our analysis to control for these potentially confounding variables. Moreover, we did not blind the nursing staff. Thus, the potential for the Hawthorne effect cannot be excluded. As they were aware of the study, the nursing staff that participated in the present study may have intentionally altered their nursing care behaviours, resulting in an unintended intervention effect. Further, the small sample size that was used in the present study may have diminished the power of the findings. A larger sample size is recommended for future studies. Finally, as attrition adversely influences both the individual participants that were lost to attrition and the intervention in total, the participants who withdrew from the present study may have also augmented the intervention effect.
Conclusion
The findings of the present study support that the CPGOMCP guidelines decrease the impact of chemotherapy-related oral mucositis on the quality of life of patients. Therefore, our results support the use of evidence-based clinical practice guidelines on HNC patients undergoing chemotherapy. As this study was a first and exploratory test, future trials are required to confirm the efficacy of the CPGOMCP guidelines.
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- Grégoire V, Lefebvre J-L, Licitra I, Felip F (2010) On behalf of the EHNS-ESMO-ESTRO Guidelines Working Group. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 21: v184-186. [Crossref]
- American Cancer Society (2013) Breast cancer facts and figures 2011-12. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-030975.pdf.
- Rêgo DF, Pavan LM, Elias ST, De Luca Canto G, Guerra EN (2015) Effects of metformin on head and neck cancer : A systematic review. Oral Oncol 51: 416-422. [Crossref]
- Vera-Llonch M, Oster G, Ford CM, Lu J, Sonis S (2007) Oral mucositis and outcomes of allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies. Support Care Cancer 15: 491-496. [Crossref]
- Rambod M, Pasyar N, Ramzi M (2018) The effect of zinc sulfate on prevention, incidence, and severity of mucositis in leukemia patients undergoing chemotherapy. Eur J Oncol Nurs 33: 14-21. [Crossref]
- Onseng K, Johns NP, Khuayjarernpanishk T, Subongkot S, Priprem A, et al. (2017) Beneficial Effects of Adjuvant Melatonin in Minimizing Oral Mucositis Complications in Head and Neck Cancer Patients Receiving Concurrent Chemoradiation. J Altern Complement Med 23: 957-963. [Crossref]
- Bensadoun RJ, Magné N, Marcy PY, Demard F (2001) Chemotherapy- and radiotherapy-induced mucositis in head and neck cancer patients: new trends in pathophysiology, prevention, and treatment. Eur Arch Otorhinolaryngol 258: 481-487. [Crossref]
- Filicko J, Lazarus HM, Flomenberg N (2003) Mucosal injury in patients undergoing hematopoietic progenitor cell transplantation: new approaches to prophylaxis and treatment. Bone Marrow Transplant 31: 1-10. [Crossref]
- Chen SC, Lai YH, Huang BS, Lin CY, Fan KH, et al. (2015) Changes and predictors of radiation-induced oral mucositis in patients with oral cavity cancer during active treatment. Eur J Oncol Nurs 19: 214-219. [Crossref]
- Cheng KK, Lee V, Li CH, Yuen HL, Epstein JB (2012) Oral mucositis in pediatric and adolescent patients undergoing chemotherapy: the impact of symptoms on quality of life. Support Care Cancer 20: 2335-2342. [Crossref]
- Ganzer H, Touger-Decker R, Parrott JS, Murphy BA, Epstein JB, et al. (2013) Symptom burden in head and neck cancer: impact upon oral energy and protein intake. Support Care Cancer 21: 495-503. [Crossref]
- Naidu MU, Ramana GV, Rani PU, Mohan IK, Suman A, et al. (2004) Chemotherapy-induced and/or radiation therapy-induced oral mucositis-complicating the treatment of cancer. Neoplasia 6: 423-431. [Crossref]
- Shaneyfelt T (2012) In guidelines we cannot trust: comment on “Failure of clinical practice guidelines to meet institute of medicine standard”. Archives of internal medicine 172: 1633-1634.
- Field M, Lohr KN (1990) Clinical practice guidelines: Directions for a new program. Washington, DC: National Academy Press. [Crossref]
- Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175-191. [Crossref]
- Soga Y, Sugiura Y, Takahashi K, Nishimoto H, Maeda Y, et al. (2010) Progress of oral care and reduction of oral musositis—a pilot study in a hematopoietic stem cell transplantation ward. Support Care Cancer 19: 303-307. [Crossref]
- Chou HL, Hsieh CF, Lee CY, Chiang MG, Chi WC (2011) Clinical Practice Guideline on Oral Mucositis in Cancer Patients. J Oncol Nursing 11: 61-85.
- Chie WC, Hong RL, Lai CC, Ting LL, Hsu MM (2003) Quality of life in patients of nasopharyngeal carcinoma: validation of Taiwan Chinese version of the EORTC QLQ-C30 AND THE EORTC QLQ-H& N35. Qual Life Res 12: 93-98. [Crossref]
- Buchanan DR, O'Mara AM, Kelaghan JW, Minasian LM (2005) Quality-of-life assessment in the symptom management trials of the National Cancer Institute–Supported Community Clinical Oncology Program. J Clinical Oncol 23: 591-598.
- Sharma A, Walker LG, Monson JR (2013) Baseline quality of life factors predict long term survival after elective resection for colorectal cancer. Int J Surg Oncol 2013: 269510. [Crossref]
- Baydar M, Dikilitas M, Sevinc A, Aydogdu I (2005) Prevention of mucositis due to 5-fluorouracil treatment with oral cryotherapy. J Natl Med Assoc 97: 1161-1164. [Crossref]
- Woo SB, Matin K (1997) Off-site dental evaluation program for prospective bone marrow transplant recipients. J Am Dent Assoc 128: 189-93. [Crossref]
- Katranc? N, Ovayolu N, Ovayolu O, Sevinc A (2012) Evaluation of the effect of cryotherapy in preventing oral mucositis associated with chemotherapy – A randomized controlled trial. Eur J Oncol Nurs 16: 339-344. [Crossref]
- Sahebjamee M, Mansourian A, Hajimirzamohammad M, Zadeh MT, Bekhradi R, et al. (2015) Comparative Efficacy of Aloe vera and Benzydamine Mouthwashes on Radiation-induced Oral Mucositis: A Triple-blind, Randomised, Controlled Clinical Trial. Oral Health Prev Dent 13: 309-315. [Crossref]
- Worthington HV, Clarkson JE, Bryan G, Furness S, Glenny AM, et al. (2010) Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane database syst rev 13: CD000978. [Crossref]
- Elting LS, Keefe DM, Sonis ST, Garden AS, Spijkervet FK, et al. (2008) Patient-reported measurements of oral mucositis on head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer 113: 2704-2713. [Crossref]
- Nonzee NJ, Dandade NA, Patel U, Markossian T, Agulnik M, et al. (2008) Evaluating the supportive care costs of severe radio chemotherapy-induced mucositis and pharyngitis. Cancer 113: 1446-1452. [Crossref]
- Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, et al. (2007) Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109: 820-831. [Crossref]



