Diabetes is at an increased risk for development of dementia and effective treatments for diabetes-induced dementia are required with urgency. Yokukansan (YKS), a traditional Japanese Kampo medicine, is composed of seven dried medicinal herbs. YKS is used clinically to treat several neurological conditions including dementia. In this study, we aimed to show the effects of yokukansan on learning and memory functions in diabetes-induced dementia and to clarify the molecular mechanisms inducing the effects in the hippocampus of yokukansan- and non-treated diabetic model mice. Morris water maze test showed that yokukansan administration significantly improved learning and memory functions of diabetic model mice. In the proteomic analysis, significant changes in expression of 10 (4 up- and 6 down-regulated) proteins and 8 (3 up- and 5 down-regulated) phosphoproteins were observed in the hippocampus of yokukansan-treated diabetic model mice. These identified proteins and phosphoproteins could be functionally classified as neuronal development, cellular cytoskeleton, energy metabolism, oxidoreductase, protein deubiquitination, proton transport, and chaperone. Of these, proteins and phosphoproteins classified as neuronal development, cellular cytoskeleton, oxidoreductase, and protein deubiquitination have been reported to be associated with learning and memory dysfunction in AD. Additionally, western blotting validated the changes in CAPZB, tubulin beta-5 chain, and LDHB. Taken together, these results showed that yokukansan improved learning and memory functions in mice with diabetes-induced dementia, underlying the changed expression of proteins and phosphoproteins in the hippocampus. We propose that yokukansan could be effective for care of diabetes-induced dementia.
dementia, diabetes, hippocampus, proteome, yokukansan
Diabetes is a disease of impaired glucose homeostasis characterized by hyperglycemia and insulin resistance [1]. As a condition affecting the central nervous system, chronic hyperglycemia causes cognitive impairment. Diabetes-induced dementia is clinically and pathophysiologically different from Alzheimer’s disease (AD). Diabetes-induced dementia is clinically characterized by more impaired attention but less impaired word recall, and slow progression of cognitive impairment [2]. Amyloid β and phosphorylated tau show normal levels in cerebrospinal fluid of patients with diabetes-induced dementia [3]. Interestingly, epidemiological evidence suggest that diabetes increases risk of dementia such as AD [4]. Some biological mechanisms have been postulated through which diabetes might increase the risk of AD: vascular mechanisms, toxic effects of hyperglycemia, insulin resistance of the brain, formation of advanced glycation end-products, and competition for insulin-degrading enzyme resulting in reduced degradation of amyloid β [5]. Thus, we became interested in these differences and performed proteomic analysis of the hippocampus and cortex of AD and diabetic model mice in comparison, to clarify differences in molecular mechanisms of AD versus diabetes-induced dementia [6-8]. Moreover, interest in the improvement of diabetes-induced dementia in relation to normalization of these altered proteins is intriguing.
Yokukansan (YKS), a traditional Japanese Kampo medicine, is composed of seven dried medicinal herbs: Atractylodes lanceae rhizome (rhizome of Atractylodes lancea De Candolle), Poria sclerotium (sclerotium of Poria cocos Wolf), Cnidium rhizome (rhizome of Cnidium officinale Makino), Uncaria hook (hook of Uncaria rhynchophilla Miquel), Japanese Angelica root (root of Angelica acutiloba Kitagawa), Bupleurum root (root of Bulpleurum falcatum Linné), and Glycyrrhiza (root and stolon of Glycyrrhiza uralensis Fisher). YKS is currently used clinically to treat the behavioral psychological symptoms associated with dementia (BPSD), which is frequently observed in neurological disorders such as AD [9]. YKS decreased expression of 5-HT2A receptor in the prefrontal cortex, resulting in improvement of BPSD in mice treated with a 5-HT2A/2C receptor agonist, 2,5-dimethoxy-4-iodoamphetamine [10]. Recently, YKS has been reported to ameliorate cognitive function in AD model rats [11] and spatial cognitive deficiency in Tg2576 mice [12]. YKS increased spontaneous acetylcholine release and suppressed hippocampal apoptosis in a rat model of repeated cerebral ischemia [13]. YKS has neuroprotective effect against amyloid β-induced cytotoxicity in cortical neurons prepared from rat [14]. Therefore, it is necessary to investigate whether YKS is effective for dementia, and further study is required to elucidate the effects of YKS on diabetes-induced dementia.
In this study, we examined the effects of YKS on diabetic model mice showing dementia by means of Morris water maze (MWM), and then the expression changes of hippocampal proteins and phosphoproteins in diabetic model mice treated with YKS using two-dimensional gel electrophoresis (2-DE) followed by staining with SYPRO Ruby and Pro-Q Diamond and subsequent mass spectrometry to elucidate the molecular mechanisms underlying the effects of YKS on diabetes-induced dementia. Our results reveal that the identified proteins and phosphoproteins are involved in the improvement of learning memory function by YKS in the diabetic model.
Materials
Streptozotocin (STZ), urea, thiourea, SDS, 3-[(3-Cholamidopropyl) dimethylammonio] propanesulfonate (CHAPS), 2-mercaptoethanol, dithiothreitol (DTT), bromophenol blue, iodoacetamide, RNase A, and DNase I were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan). Dry powdered extract of YKS was supplied by TSUMURA & Co (Tokyo, Japan). Source information for all other assay reagents and materials is stated in the respective subsections of Materials and Methods below.
Animals
Seven-month-old male C57BL/6 mice were used in this study. C57BL/6 mice (Japan SLC, Inc. Shizuoka, Japan) were housed with a 12-h light/dark cycle and with ad libitum access to food and water. The frequency of animal monitoring was done once a week. Each time, the health, body condition, and wellbeing of the animals were assessed.
Generation of diabetic model mice treated with STZ
STZ is toxic to insulin-producing β cells in pancreatic islets, and STZ administration induces a constant state of hyperglycemia [15]. The diabetic model mouse showing cognitive impairments was established by intraperitoneal injection of STZ (50 mg/kg dissolved in 100 mM citrate buffer, pH 4.5) once a day for 5 consecutive days [6,16]. One week after injection, the mice were divided into 2 groups: a control group, fed standard pellet chow (CLEA Rodent Diet CE-2, CLEA Japan, Inc., Tokyo, Japan) for 9 weeks during isolation, and a treatment group, fed standard pellet chow containing 1% (w/w) YKS for 9 weeks during isolation. Subsequently, blood glucose levels were measured from tail vein blood using blood glucometer (Terumo Co. Tokyo, Japan). The mice with a glucose level above 400 mg/dL were chosen for the subsequent study [6,17].
Behavioral test
Learning and memory function was examined in both groups of mice using Morris water maze (MWM) test in accordance with previous report [18]. The maze consisted of a round black pool, 96 cm in diameter. The maze was filled with water having a temperature of about 23°C. Four identically spaced locations at the circumference of the pool divided the pool into four quadrants and were used as beginning points. An escape platform of 10 cm diameter was located 2 cm beneath the surface of the water at a fixed position in the center of one of the quadrants. All male mice were given daily sessions of 5 training trials for 5 consecutive days. Each mouse was allowed up to 60 seconds to find the latent platform. The time spent to detect the platform (escape latency) was recorded. One day after acquisition, a probe test was performed to evaluate memory level by removing platform. Locomotor activity of the mice was examined by an open-field test [19].
Protein extraction
Protein extraction was performed as previously described [6]. Both the YKS- and non-treated diabetic model mice were killed under anesthesia with pentobarbital sodium. The hippocampi were isolated from three mice in each group and mixed separately for the two groups. The mouse hippocampal samples were lysed by sonication in lysis buffer composed of 7 M urea, 2 M thiourea, 5% CHAPS, 2% immobilized pH gradient (IPG) buffer (GE Healthcare UK Ltd., Buckinghamshire, UK), 50 mM 2-mercaptoethanol, 2.5 μg/ml DNase I, and 2.5 μg/ml RNase A. Any insoluble material was removed by centrifugation at 15,000 × g for 30 min, 4°C.
Two-DE, gel staining and Image analysis
Two-DE is a combination of isoelectric focusing and SDS-polyacrylamide gel electrophoresis, which allows the separation of proteins by molecular charge and molecular weight. 2-DE was performed as previously described [6]. Samples (1000 μg of protein) were applied to IPG gel strips (pH 4-7; 7 cm; GE Healthcare). Focusing started at 50 V for 6 h, at 100 V for 6 h, and finally at 2000 V for 6 h. After focusing, the IPG strips were first equilibrated with rocking for 15 min in 50 mM Tris-HCl, pH 8.8, 6 M urea, 1% SDS, 30% (v/v) glycerol, and 1% (w/v) DTT followed by 15 min with the same buffer with 2.5% (w/v) iodoacetamide replacing DTT. The equilibrated IPG strips were transferred onto 15% SDS-Polyacrylamide gel electrophoresis (PAGE) gels at 5 mA/gel for 7 h.
After fixation with 50% methanol, containing 10% acetic acid, the gels were double stained, first with Pro-Q Diamond (Life Technologies, Carlsbad, CA, USA) and then with SYPRO-Ruby (Life Technologies) following the procedure described in [6]. Images were captured with Fluorophorestar 3000 image capture system (Anatech, Tokyo, Japan).
Image analysis and quantification of gel spots were performed using Prodigy SameSpots software (Nonlinear Dynamics, Newcastle upon Tyne, UK). Differentially expressed spots were identified by normalizing by the total intensity of all matched spots and comparing the difference in spot intensity between samples from YKS-treated and control mice using ANOVA.
Protein identification by MALDI-TOF-MS.
Spots of interest were excised, and in-gel digested with sequencing-grade modified trypsin (Promega, Madison, WI, USA) as described [6]. The digested peptide fragments were identified by MALDI TOF MS/MS (ABI PLUS 4800, Applied Biosystems, Foster City, CA, USA), and a Mascot MS/MS ion search through NCBInr databases was performed using the Mascot search engine (Matrix Science, Boston, MA, USA).
Western blotting
The isolated hippocampus samples were homogenized in buffer containing 20 mM Tris-HCl, pH 7.0, 6 M urea, 150 mM NaCl, 2 mM EDTA, and 1% Triton X-100. The homogenates were subjected to 8% SDS-PAGE and the gels were transferred onto an Immobilon-P polyvinylidene difluoride membrane (Millipore, Bedford, MA). Following 5% skimmed-milk blocking, the membranes were incubated with rabbit anti-CAPZB (diluted 1:1,000, Abcam, Cambridge, MA, USA), mouse anti- tubulin beta-5 chain (diluted 1:1,000, Abcam), rabbit anti-LDHB (diluted 1:1,000, Abcam), and rabbit anti-GAPDH (diluted 1:10,000, AbFrontier, Seoul, Korea) antibodies overnight at 4°C. The membranes were incubated with a secondary antibody (diluted 1:5000, GE Healthcare UK Ltd.) conjugated with horseradish peroxidase (HRP) for 1 h at room temperature. All values were corrected with reference to the value for GAPDH, used as an internal standard. The target proteins were detected using ECL Prime Western Blotting Detection Reagent (GE Healthcare UK Ltd.). Protein bands were scanned, and their optical density was analyzed with Multi Gauge version 2.2 software (Fuji Photofilm, Tokyo, Japan).
Statistical analysis
Respective densitometric quantifications are shown as mean ± S.E.M. All data were tested by Student’s t-test and repeated measures ANOVA followed by Bonferroni test to evaluate the differences between groups. P < 0.05 was considered statistically significant.
YKS improved learning and memory impairment of diabetic model mice
MWM test was performed to evaluate the learning and memory functions of diabetic model mice treated with YKS. Before MWM test, spontaneous locomotor activity of mice which was measured by the open-field test did not differ among all groups (data not shown). Compared with untreated diabetic model mice (CTL mice), YKS-treated diabetic model mice (YKS mice) showed tendency of decreased escape latency, indicating that administration of YKS induced improvement of learning and memory functions (Figure 1A). The time spent in the target quadrant was significantly increased (P < 0.05) in the YKS mice compared with the CTL mice (Figure 1B). These results indicate that YKS improved impaired learning and memory functions.
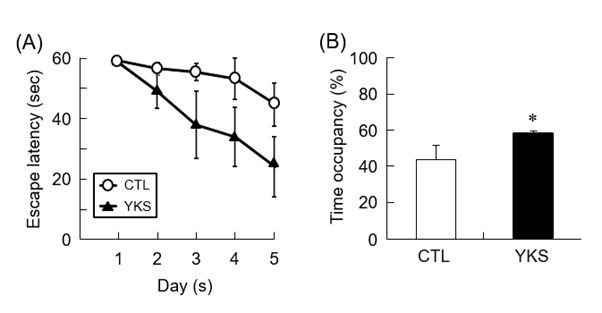
Figure 1. Learning and memory of YKS-treated diabetic model mice (YKS mice) and diabetic model mice (CTL mice). (A) Escape latency to find the hidden platform from the first to the fifth day. (B) The time spent in target quadrant within 60 seconds is shown. The results are means ± S.E.M. (n = 5). *p < 0.05 versus CTL mice
Identification of altered proteins and phosphoproteins in the hippocampus of YKS-treated diabetic model mice
The expression changes of proteins and phosphoproteins in the hippocampus of YKS mice and CTL mice were quantified and identified on 2-DE gels using Prodigy SameSpot software and MALDI-TOF MS/MS. Image analysis showed approximately 400 protein spots and 200 phosphoprotein spots on SYPRO Ruby-stained 2-DE gel (Figure 2A) and Pro-Q Diamond-stained 2-DE gel (Figure 2B), respectively. We detected 10 (4 up- and 6 down-regulated) proteins and 8 (3 up- and 5 down-regulated) phosphoproteins in the hippocampus (Table 1). These proteins and phosphoproteins were categorized into functional groups as shown in Table 1 using the PANTHER database. The 4 proteins with increased expression levels were identified as dihydropyrimidinase-related protein 2; F-actin-capping protein subunit beta isoform c; alpha-tubulin, partial; and enolase 1B, retrotransposed, and the 6 proteins with decreased expression levels were identified as glia maturation factor, beta, isoform CRA_a, partial; put. beta-actin (aa 27-375); tubulin beta-5 chain; ATP synthase, H+ transporting, mitochondrial F0 complex, subunit d; L-lactate dehydrogenase B chain isoform 1; and ubiquitin carboxy-terminal hydrolase L1, isoform CRA_a (Table 1). The 3 phosphoproteins with increased expression level were identified as gamma-enolase isoform 1; tubulin alpha-1C chain; and vacuolar adenosine triphosphatase subunit A, and the 5 phosphoproteins with decreased expression level were identified as pyridoxal kinase; gamma-enolase isoform 1; dihydropyrimidinase-related protein 2; keratin, type II cytoskeletal 6A; and heat shock protein 70 cognate (Table 1).
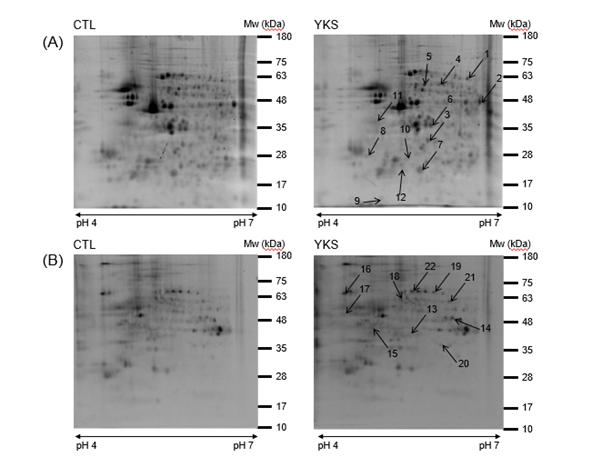
Figure 2. Representative 2-DE gels of hippocampal proteins (A) and phosphoproteins (B) in YKS-treated diabetic model mice (YKS mice) and diabetic model mice (CTL mice). About 1,000 μg of hippocampal proteins were loaded on an IPG strip (pH 4–7) and separated by two-dimensional electrophoresis (15%). Isoelectric points (PIs) and experimental masses are indicated on the X- and Y-axes. The proteins with changed expression levels are identified by numbers that correspond to spot numbers in Table 1. Experiments were performed three times independently. Representative gel images are shown
Table 1. Identified proteins and phosphoproteins in the hippocampus and its functional categories
Proteins and phosphoproteins of the mouse hippocampus were separated by 2-DE followed by in-gel digestion with trypsin and identified by MALDI-TOF MS/MS. The spots representing the identified proteins are indicated in Figure 2 and are designated by their gene ID accession numbers (GI acc. no.) used in the NCBI non-redundant (NCBI nr) protein database. Scores that relate to the probability assignment, molecular weight, pI, and sequence coverage (SC) are given. The score and sequence coverage were obtained by Mascot database searching (http://www.matrixscience.com). The spot densities were compared with diabetic model mice (CTL mice) values. P value was obtained by ANOVA, P < 0.05.
Western blot analysis for the altered proteins in the hippocampus of YKS-treated diabetic model mice
Western blot analysis was performed to validate the identity of F-actin-capping protein subunit beta isoform c, tubulin beta-5 chain, and L-lactate dehydrogenase B chain isoform 1 as differentially expressed hippocampal proteins. The protein level of F-actin-capping protein subunit beta isoform c tended to increase (P = 0.54) (Figure 3A). The protein levels of tubulin beta-5 chain, and L-lactate dehydrogenase B chain isoform 1 tended to decrease (P = 0.68 and 0.47) (Figures 3B and C).
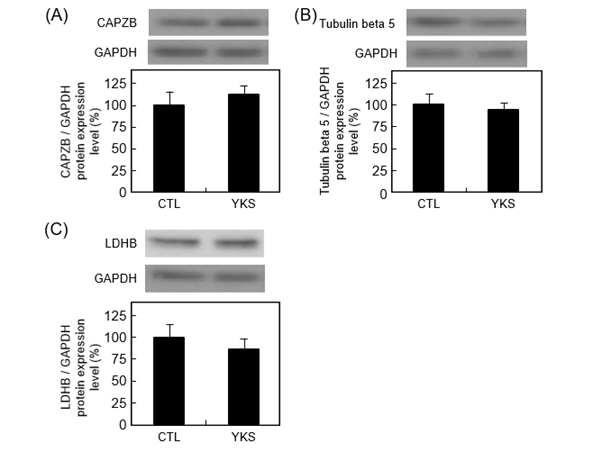
Figure 3. Representative images of western blotting conducted for evaluation of the expression of F-actin-capping protein subunit beta isoform c (CAPZB) (A), tubulin beta-5 chain (B), and L-lactate dehydrogenase B chain isoform 1 (LDHB) (C) in hippocampus from YKS-treated diabetic model mice (YKS mice) and diabetic model mice (CTL mice). The protein expression levels are normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression and indicated as the ratio relative to that in CTL mice. Data are presented as mean ± S.E.M. of four or five independent experiments. Representative bands are shown above each graph
We first showed that YKS improved learning and memory functions of STZ-induced diabetic model mice showing dementia using MWM test, and then identified the expression of 10 proteins and 8 phosphoproteins relevant to the molecular mechanisms underlying these beneficial effects of YKS using 2-DE coupled with MS. These proteins and phosphoproteins might contribute to the improvement of impaired learning and memory functions of diabetes-induced dementia.
MWM test
YKS has been reported to improve cognitive dysfunction in rodent models of AD [11,12]. Similarly, our findings in MWM test showed that YKS improved impaired learning and memory functions in diabetic model mice. Combined with other findings, it is suggested that YKS is beneficial to both AD and diabetes-induced dementia.
Neuronal development
Dihydropyrimidinase-related protein 2 (DPYSL2), also known as collapsin response mediator protein 2, binds to and regulates the assembly of microtubules, resulting in axonal growth and neuronal polarity [20]. Overexpression of DPYSL2 increases axon elongation in cultured hippocampal neurons [21]. Brain-specific DPYSL2 knockout mice exhibit deficits in spatial learning and memory in MWM [22]. The expression level of DPYSL2 protein is decreased in the hippocampus of AβPPswe/PS1dE9 mice [23]. Importantly, we also demonstrated that expression of DPYSL2 was increased in hippocampus during synaptic plasticity [24]. Thus, the expression of DPYSL2 increased by YKS treatment might affect synaptic plasticity in hippocampus, leading to improvement of diabetes-induced dementia.
Phosphorylation of DPYSL2 negatively regulates microtubule growth and stability [25]. DPYSL2 is phosphorylated at Ser522 by Cdk5 and subsequently at Ser518, Thr514, and Thr509 by GSK-3β in brain tissue of AD patients and some mouse models [26]. Phosphorylated DPYSL2 is associated with neurofibrillary tangles and abnormal neurites in AD brains [27]. In our previous study, expression of phosphorylated DPYSL2 was increased in hippocampus of STZ-induced diabetic model mice [6]. Our findings showed expression of phosphorylated DPYSL2 was decreased in hippocampus of YKS mice. Thus, the decreased expression of phosphorylated DPYSL2 in hippocampus by YKS treatment might contribute to improvement of impaired synaptic plasticity in diabetes-induced dementia.
Cellular cytoskeleton
Actin is present in monomeric (G-actin) and filamentous (F-actin) forms in living cells. In neurons, regulation of spine morphology and synapse formation is based on reorganization of the actin cytoskeleton [28]. F-actin-capping protein subunit beta isoform c, also known as CAPZB, binds to the growing ends of actin filaments, leading to inhibition of the polymerization and depolymerization of actin [29]. Microtubules are dynamic polymers consisting of α/β tubulin dimers and are present throughout neuronal dendrites and axons. Microtubule dynamics in brain play crucial roles in the essential processes of learning and memory [30]. Morphological and molecular changes in both presynaptic and postsynaptic sites are widely regarded as substrates for learning and memory [31]. Thus, changes in the expression of actin and tubulin might affect synaptic plasticity, resulting in improvement of diabetes-induced dementia.
Oxidoreductase
L-lactate dehydrogenase (LDH) is an enzyme that catalyzes the interconversion of pyruvate and lactate [32]. The LDHA and LDHB gene products are widely expressed, and both are found in the central nervous system [33]. LDHA is associated with pyruvate-to-lactate conversion, and LDHB with lactate-to-pyruvate conversion [32]. The expression level of LDHB protein is decreased in the hippocampus of AD patients [34]. Lactate is decreased in hippocampus and cortex of amyloid β25-35-treated rats [35]. Combined with our previous findings that the expression level of LDHB was decreased in the hippocampus of diabetic model mice showing dementia [6], we expected YKS to increase the expression level of LDHB in this study. Regrettably, YKS failed to increase the expression level of LDHB in the hippocampus of diabetic model mice. These findings suggest that LDHB does not contribute to the improvement of diabetes-induced dementia by YKS.
Protein deubiquitination
Ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) is a deubiquitylating enzyme and is abundantly expressed in neuronal cells [36]. Dysfunction of UCH-L1 has been reported in the pathophysiology of neurodegenerative disorders, including AD [37] and Parkinson’s disease [38]. LDN-57444, a reversible competitive inhibitor of UCH-L1, leads to marked accumulation of mature APP in Swedish mutant APP cell line 20E2 cells transfected with pz-UCH-L1 [39]. Expression level of UCH-L1 is decreased in sporadic AD brains [40], whereas overexpression of UCH-L1 improves learning and memory deficits in AD model mice [41]. Our previous report showed that expression level of UCH-L1 was decreased in diabetes-induced dementia [6]. Thus, expression of UCH-L1, which was anticipated to be increased by YKS treatment, was indeed decreased, suggesting no effect of YKS on UCH-L1 in mice with diabetes-induced dementia.
YKS administration significantly improved the learning and memory dysfunction in diabetic model mice. Ten proteins and 8 phosphoproteins had significantly altered levels in the hippocampus of YKS-treated diabetic model mice. YKS might contribute to the amelioration of diabetes-induced dementia by affecting the impairment of synaptic plasticity in the hippocampus through these proteins and phosphorylated proteins. Further studies are needed to understand the molecular mechanism of dementia improvement by YKS.
Conceptualization: K.M, K.K, M.T and S.M; Methodology: K.M, E.K, K.K, M.T, K.M and S.M; Validation: K.M, K.K and K.M; Formal Analysis: K.M; Investigation: K.M, E.K, K.M, and K.K; Resources: M.T; Data Curation: K.M; Writing—Original Draft Preparation: K.M; Writing—Review and Editing: K.M and S.M; Visualization: K.M; Supervision: S.M; Project Administration: S.M; Funding Acquisition: S.M;
All experimental procedures were performed in accordance with the National Institutes of Health Guidelines on the Care and Use of Animals and confirmed by the Himeji Dokkyo University Animal Experiment Committee. All efforts were made to minimize animal use and suffering.
MS/MS data are available via jPOST with identifier JPST001252.
The authors are grateful to T. Nakamura (Himeji Dokkyo University) for generously supplying YKS.
No competing interests to declare. All authors have read and agreed to the published version of the manuscript.
- American Diabetes Association (2011) Diagnosis and classification of diabetes mellitus. Diabetes Care 34: 62-69. [Crossref]
- Hanyu H, Hirose D, Fukasawa R, Hatanaka H, Namioka N, et al. (2015) Guidelines for the clinical diagnosis of diabetes mellitus-related dementia. J Am Geriatr Soc 63: 1721-1723.
- Kanetaka H, Fukasawa R, Shimizu S, Takenoshita N, Hanyu H (2017) Cerebrospinal fluid analysis in individuals with diabetes-related dementia. eNeurologicalSci 8: 9-10.
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P (2006) Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5: 64-74.
- Gudala K, Bansal D, Schifano F, Bhansali A (2013) Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J Diabetes Investig 4: 640-650.
- Matsuura K, Otani M, Takano M, Kadoyama K, Matsuyama S (2018) Proteomic analysis of hippocampus and cortex in streptozotocin-induced diabetic model mice showing dementia. J Diabetes Res 8953015. [Crossref]
- Takano M, Yamashita T, Nagano K, Otani M, Maekura K, et al. (2013) Proteomic analysis of the hippocampus in Alzheimer's disease model mice by using two-dimensional fluorescence difference in gel electrophoresis. Neurosci Lett 534: 85-89.
- Takano M, Maekura K, Otani M, Sano K, Nakamura-Hirota T, e al. (2012) Proteomic analysis of the brain tissues from a transgenic mouse model of amyloid beta oligomers. Neurochem Int 61: 347-355.
- Mizukami K, Asada T, Kinoshita T, Tanaka K, Sonohara K, et al. (2009) A randomized cross-over study of a traditional Japanese medicine (kampo), yokukansan, in the treatment of the behavioural and psychological symptoms of dementia. Int J Neuropsychopharmacol 12: 191-199.
- Egashira N, Iwasaki K, Ishibashi A, Hayakawa K, Okuno R (2008) Repeated administration of Yokukansan inhibits DOI-induced head-twitch response and decreases expression of 5-hydroxytryptamine (5-HT)2A receptors in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry 32: 1516-1520.
- Uchida N, Takasaki K, Sakata Y, Nogami A, Oishi H, et al. (2013) Cholinergic involvement and synaptic dynamin 1 expression in Yokukansan-mediated improvement of spatial memory in a rat model of early Alzheimer's disease. Phytother Res 27: 966-972.
- Tabuchi M, Yamaguchi T, Iizuka S, Imamura S, Ikarashi Y, et al. (2009) Ameliorative effects of yokukansan, a traditional Japanese medicine, on learning and non-cognitive disturbances in the Tg2576 mouse model of Alzheimer's disease. J Ethnopharmacol 122: 157-162.
- Nogami-Hara A, Nagao M, Takasaki K, Egashira N, Fujikawa R, et al. (2018) The Japanese Angelica acutiloba root and yokukansan increase hippocampal acetylcholine level, prevent apoptosis and improve memory in a rat model of repeated cerebral ischemia. J Ethnopharmacol 214: 190-196.
- Tateno M, Ukai W, Ono T, Saito S, Hashimoto E, et al. (2008) Neuroprotective effects of Yi-Gan San against beta amyloid-induced cytotoxicity on rat cortical neurons. Prog Neuropsychopharmacol Biol Psychiatry 32: 1704-1707.
- Szkudelski T (2001) The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiological Research 50: 537-546.
- Ramos-Rodriguez JJ, Ortiz O, Jimenez-Palomares M, Kay KR, Berrocoso E, et al. (2013) Differential central pathology and cognitive impairment in pre-diabetic and diabetic mice. Psychoneuroendocrinology 38: 2462-2475.
- Fox HS (1992) Androgen treatment prevents diabetes in nonobese diabetic mice. J Exp Med 175: 1409-1412.
- Umeda T, Tomiyama T, Kitajima E, Idomoto T, Nomura S, et al. (2012) Hypercholesterolemia accelerates intraneuronal accumulation of Abeta oligomers resulting in memory impairment in Alzheimer's disease model mice. Life Sci 91: 1169-1176.
- Iso H, Simoda S, Matsuyama T (2010) Environmental change during postnatal development alters behaviour, cognitions and neurogenesis of mice. Behav Brain Res 179: 90-98. [Crossref]
- Arimura N, Ménager C, Kawano Y, Yoshimura T, Kawabata S (2005) Phosphorylation by Rho kinase regulates CRMP-2 activity in growth cones. Mol Cell Bio. 25: 9973-9984.
- Inagaki N, Chihara K, Arimura N, Ménager C, Kawano Y (2001) CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci 4: 781-782.
- Zhang H, Kang E, Wang Y, Yang C, Yu H, et al. (2016) Brain-specific Crmp2 deletion leads to neuronal development deficits and behavioural impairments in mice. Nat Commun 7: 11773.
- Fu Y, Zhao D, Pan B, Wang J, Cui Y, et al. (2015) Proteomic analysis of protein expression throughout disease progression in a mouse model of alzheimer's disease. J Alzheimers Dis 47: 915-926.
- Kadoyama K, Matsuura K, Nakamura-Hirota T, Takano M, Otani M (2015) Changes in the expression of collapsin response mediator protein-2 during synaptic plasticity in the mouse hippocampus. J Neurosci Res 93: 1684-1692.
- Sumi T, Imasaki T, Aoki M, Sakai N, Nitta E (2018) Structural Insights into the Altering Function of CRMP2 by Phosphorylation. Cell Struct Funct 43: 15-23.
- Cole AR, Noble W, van Aalten L, Plattner F, Meimaridou R (2007) Collapsin response mediator protein-2 hyperphosphorylation is an early event in Alzheimer's disease progression. J Neurochem 103: 1132-1144.
- Gu Y, Hamajima N, Ihara Y (2000) Neurofibrillary tangle-associated collapsin response mediator protein-2 (CRMP-2) is highly phosphorylated on Thr-509, Ser-518, and Ser-522. Biochemistry 39: 4267-4275.
- Sekino Y, Kojima N, Shirao T (2007) Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int 51: 92-104.
- Avenarius MR, Krey JF, Dumont RA, Morgan CP, Benson CB, et al. (2017) Heterodimeric capping protein is required for stereocilia length and width regulation. J Cell Biol 216: 3861-3881.
- Dent EW (2017) Of microtubules and memory: implications for microtubule dynamics in dendrites and spines. Mol Biol Cell 28: 1-8.
- Okabe S, Miwa A, Okado H (2001) Spine formation and correlated assembly of presynaptic and postsynaptic molecules. J Neurosci 21: 6105-6114.
- Ross JM, Öberg J, Brené S, Coppotelli G, Terzioglu M, et al. (2010) High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. Proc Natl Acad Sci U S A 107: 20087-20092.
- Laughton JD, Charnay Y, Belloir B, Pellerin L, Magistretti PJ (2000) Differential messenger RNA distribution of lactate dehydrogenase LDH-1 and LDH-5 isoforms in the rat brain. Neuroscience 96: 619-625.
- Zahid S, Oellerich M, Asif AR, Ahmed N (2014) Differential expression of proteins in brain regions of Alzheimer's disease patients. Neurochem Res 39: 208-215.
- Lu W, Huang J, Sun S, Huang S, Gan S (2015) Changes in lactate content and monocarboxylate transporter 2 expression in Abeta(2)(5)(-)(3)(5)-treated rat model of Alzheimer's disease. Neurol Sci 36: 871-876.
- Larsen CN, Krantz BA, Wilkinson KD (1998) Substrate specificity of deubiquitinating enzymes: ubiquitin C-terminal hydrolases. Biochemistry 37: 3358-3368.
- Tramutola A, Di Domenico F, Barone E, Perluigi M, Butterfield DA (2016) It Is All about (U)biquitin: Role of Altered Ubiquitin-Proteasome System and UCHL1 in Alzheimer Disease. Oxid Med Cell Longev 2016: 2756068.
- Leroy E, Boyer R, Auburger G, Leube B, Ulm G (1998) The ubiquitin pathway in Parkinson's disease. Nature 395: 451-452.
- Zhang M, Cai F, Zhang S, Zhang S, Song W (2014) Overexpression of ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) delays Alzheimer's progression in vivo. Sci Rep 4: 7298. [Crossref]
- Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M (2004) Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson's and Alzheimer's diseases. J Biol Chem 279: 13256-13264.
- Gong B, Cao Z, Zheng P, Vitolo OV, Liu S (2006) Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell 126: 775-788.



