Background: This study aimed to analyze and evaluate the protective and therapeutic effects of citicoline (CDP-choline) on neonatal rats with necrotizing enterocolitis (NEC).
Methods: Forty 3-day-old Sprague-Dawley rats, of either gender, were randomly divided into four treatment groups of 10 rats each: control, NEC model, NEC+CDP-choline, and CDP-choline. After the experimental period, intestinal tissue was extracted, stained with hematoxylin and eosin (HE), and observed under a light microscope. Additionally, the samples were scored according to the method of Shiou et al. Immunofluorescence was used to detect beclin-1 and LC3A/B to allow a comparison of autophagy and apoptosis in each group. The inflammatory factors TNF-α, IL-1β, and IL-6 were detected by enzyme-linked immunosorbent assay (ELISA).
Results: HE staining revealed that the inflammatory response in the NEC+CDP-choline treatment group significantly improved compared with that in the NEC model group; furthermore, a pathological examination showed a significant difference in Shiou scores (P < 0.05). Beclin-1 and LC3A/B levels significantly decreased in intensity and the levels of inflammatory factors TNF-α, IL-1β, and IL-6 significantly decreased (P < 0.05).
Conclusions: CDP-choline exerted protective and therapeutic effects on neonatal rats with NEC.
autophagy and apoptosis, citicoline, inflammatory factors, necrotizing enterocolitis (NEC), Shiou score
Necrotizing enterocolitis (NEC) is a common gastrointestinal emergency for newborns, with a mortality rate of 20–30% [1]. However, there is still no clear and effective clinical therapeutic strategy for it owing to the inexact pathogenesis of the disease [2]. Recent studies have shown that NEC intestinal mucosal injury results from the release of inflammatory mediators mediated by oxygen free radicals. It was also reported that inflammatory responses and inflammatory factor release significantly increased the incidence of nervous impairment in children with NEC [3]. Phosphatidylcholine, the main lipid of the gastrointestinal mucosal barrier, exerts a protective effect on the mucosa as a hydrophobic intestinal surfactant and regulates the inflammatory signaling system. Moreover, studies have demonstrated that defect or damage of the phospholipid layer causes inflammation and increases susceptibility to NEC [4]. Thus, exogenous phosphatidylcholine may exert a therapeutic effect on NEC. This study planned to examine the protective effects against NEC-induced intestinal inflammation, necrosis, and apoptosis following the administration of citicoline (cytidine 5′-diphosphocholine, CDP-choline) in neonatal rats with NEC, which provided a basis for investigating the mechanism responsible for the development of NEC and a new search strategy to effectively prevent and treat NEC.
Experimental animals
Forty 3-day-old neonatal rats were divided into four groups. 1) The control group was conventionally fed after separation from mother rats without any intervention or stimulation [5]. 2) In the NEC model group, rats were fed with intestinal formula milk after separation from the mother rats and the method of hypoxia-hyperoxia exposure and cold stress were used to induce NEC. First, neonatal rats were placed in an environment with 100% CO2 for 10 min. Then, they were subjected to 4ºC for 5 min followed by 97% O2 for 5 min. This process was performed twice daily for three consecutive days and then saline was intraperitoneally injected. 3) In the NEC+CDP-choline treatment group, CDP-choline was intraperitoneally injected once daily at a dose of 300 mg/kg/day for three consecutive days after NEC was successfully induced. 4) Lastly, in the CDP-choline group, neonatal rats were conventionally fed after separation from the mother rats. CDP-choline was administered by intraperitoneal injection once daily at a dose of 300 mg/kg/day for three consecutive days. At day 7, the rats were sacrificed and gastrointestinal tissue from the end of duodenum to ileocecum was extracted. The institutional and national guide for the care and use of laboratory animals was followed.
Experimental methods
Pathological examination of intestinal tissue in rats by hematoxylin and eosin (HE) staining
Main reagents: (1) Xylene (Kelong Chemical Reagent Factory, Chengdu); (2) Eagle (Maikun Chemical, Shanghai, batch number: 20120831); (3) Hematoxylin (Sigma, batch number: 041M0014V).
Main instruments: (1) Rotary slicer (RM2235, LEICA company); (2) Bleaching machine for pathological tissue (Tec 2500, Hao Silin Instrument Equipment Co., Ltd, Changzhou); (3) Microscope (BX43, OLYMPUS); (4) Waterproof constant-temperature incubator (PYX-DHS500BS-II, Yuejin Medical Equipment Co., Ltd, Shanghai).
Experimental steps: (1) Intestinal tissue was sliced. (2) Conventional HE staining was performed: the nucleus was blue, the cytoplasm was pink, and the blood cells were bright red.
Detection of autophagy and apoptosis by immunofluorescence
Main reagents: (1) Beclin-1 antibody (Abcam, product number ab55878, batch number GR26194-5); (2) LC3 antibody (Abcam, product number ab128025, batch number GR228439-7); (3) Donkey anti-rabbit secondary antibody (Life Technologies, product number A21206, batch number 1110071, excitation/absorption wavelengths 488 nm/525 nm).
Main instruments: (1) Rotary slicer (RM2235, LEICA company); (2) Bleaching machine for pathological tissue (Tec 2500, Hao Silin Instrument Equipment Co., Ltd, Changzhou); (3) Fluorescence microscope (BX43, OLYMPUS); (4) Waterproof constant-temperature incubator (type PYX-DHS500BS-II, Yuejin Medical Equipment Co., Ltd, Shanghai).
Experimental steps: (1) Intestinal tissue was conventionally sliced. (2) Beclin-1 and LC3A/B expression was detected by immunofluorescence in the following steps: 1) Xylene dewaxing and gradient alcohol rehydration were performed; 2) antigen repair was conducted; 3) the primary antibody was added, and the slices were incubated at 4 ºC overnight, washed with PBS for 3×3 min, and PBS was used as the negative control; 4) secondary antibody was added, the slices were incubated at 37ºC for 60 min, and washed with PBS for 3×5 min; 5) DAPI staining was performed at room temperature for 10 min; and 6) the slices were sealed with glycerol PBS and observed by fluorescence microscopy. (3) Analysis of the results: Green fluorescence indicated the presence of beclin-1 and LC3A / B; the nucleus was stained blue.
Detection of inflammatory factors using ELISA
Main reagents: (1) TNF-α ELISA reagent kit (CSB-E11987r); (2) IL-1β ELISA reagent kit (CSB-E08055r); (3) IL-6 ELISA reagent kit (CSB-E04640r).
Main instruments: (1) Microplate reader (BIO-RAD Model680); (2) Microplate Washer (Mindray MW-12A); (3) Constant-temperature incubator (DHG-9140A, Hongdu Electronics, Yangzhou); (4) High-speed centrifuge (Thermo ST16R).
Experimental steps: (1) For reagent preparation, all reagents and samples were equilibrated to room temperature; (2) preparation of the standard product; (3) sample loading and incubation; (4) addition of antibodies and incubation; (5) washing; (6) addition of HRP and incubation; (7) washing; (8) addition of TMB and incubation; (9) termination of the reaction and detection of the absorbance; and (10) result calculation: An average of two measurements of the OD values of the standard product and the sample minus the OD value of zero standard was used, standard curve and formula were established, and the concentrations of each sample were calculated by the detected OD value.
SPSS19.0 software was used for statistical analysis. Continuous data were expressed as the mean ± standard deviation(SD), with comparisons between groups conducted by one-way ANOVA with SNK post hoc test. The value of P < 0.05 was considered to be statistically significant.
Body weight gain
At 7 days of life, all pups in both the control group and the CDP-choline group survived but in these studies 2,3 animals died in the NEC model group and the NEC+CDP-choline treatment group prior to the end-point respectively, and these pups were excluded from all further analyses. Over the 7 days of study, The body weight gain of pups in the control group, NEC model group, NEC+CDP-choline treatment group and CDP-choline group was 5.102 ± 1.517, 0.053 ± 0.085,0.276 ± 0.171, 4.572 ± 1.927 respectively and the difference was statistically significant (F=38.543, P<0.01). The NEC+CDP-choline treatment group and the NEC model group gained significantly less weight from birth, when compared with the NEC model group (P < 0.05). However, there was no significant difference in weight gain between the NEC+CDP-choline treatment group and the NEC model group (P>0.05).
Pathological examination of intestinal tissue in rats by using HE staining
The HE staining of intestinal tissue in each group was examined under a light microscope. In the control group, the intestinal mucosal epithelium was complete and continuous, cells and glands were arranged regularly, and there was no inflammatory cell infiltration in mucosal and submucosal layers. In the NEC group, intestinal villus edema was visible and there was a large amount of inflammatory cell infiltration in the mucosal lamina propria and the submucosal layer (mainly neutrophils and eosinophils). Moreover, intestinal capillary congestion and even intestinal epithelial necrosis were visible. In the NEC+CDP-choline group, the intestinal mucosal epithelium was complete and continuous, and cells as well as glands, were regularly arranged. Furthermore, there was neither intestinal villus edema nor intestinal capillary congestion, but a small amount of inflammatory cell infiltration was visible in the lamina propria. There were no obvious inflammatory lesions in the normal+CDP-choline group (Figure 1).
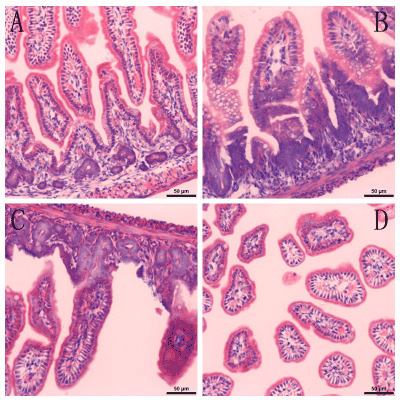
Figure 1. Examination of the HE staining of the intestinal tissue of rats by using light microscopy.
(A). The control group (×200). (B). NEC model group (×200). (C). NEC+CDP-choline treatment group (×200). (D). CDP-choline group (×200).
Intestinal pathology was scored according to the new double-blind standard by Shiou et al. [6], which uses the following scoring system: 0 points, intestinal mucosal villi integrity was retained and tissue structure was normal; 1 point, slight edema and separation in the submucosal layer and/or lamina propria; 2 points, moderate separation in the submucosal layer and/or lamina propria and edema in submucosal and/or muscular layers; 3 points, severe separation in the submucosal layer and/or lamina propria, edema in submucosal and/or muscular layers, and shedding of local villi; 4 points, disappearance of intestinal villi combined with intestinal necrosis. The induction of NEC was considered successful when a pathological score of ≥2 points was obtained.
The scores of the CDP-choline group, the NEC+CDP-choline treatment group, the NEC model group and the control group were 0.5 ± 0.707, 0.7 ± 0.823, 4.3 ± 0.823 and 0.4 ± 0.699 respectively. The scores of the NEC groups were significantly higher than those of the control groups (P < 0.05). There were no significant differences between the NEC+CDP-choline treatment, the normal control, and the CDP-choline groups (F = 0.42, P = 0.661).
Immunofluorescence detection of LC3II and beclin-1 and comparison of autophagy levels in rat intestinal tissue
The expression of fluorescence intensity for beclin-1 and LC3II in the NEC model group was significantly higher than that of the other three groups. However, there were no differences between the NEC+CDP-choline treatment group, the normal control group, and the CDP-choline group (Figures 2,3).
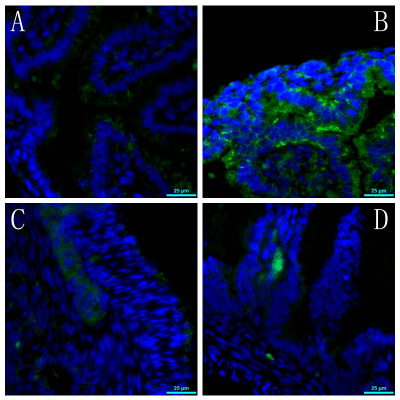
Figure 2. Fluorescence intensity staining of beclin-1 in the intestinal tissue of rats.
(A). The control group (×400). (B). NEC model group (×400). (C). NEC+CDP-choline treatment group (×400). (D). CDP-choline group (×400).
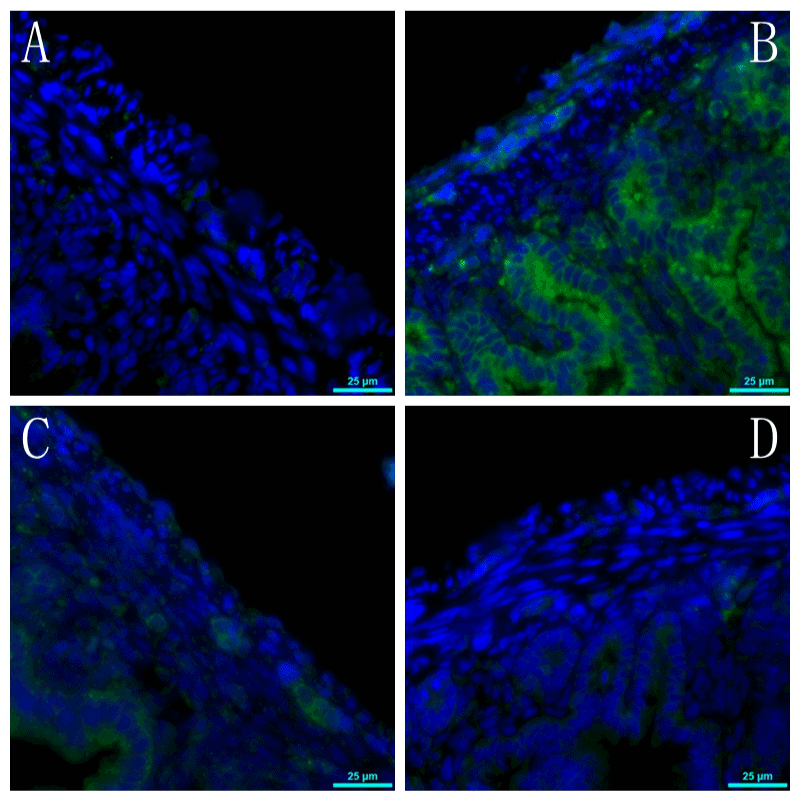
Figure 3. Fluorescence intensity staining of LC3II in the intestinal tissue of rats.
(A). The control group (×400). (B). NEC model group (×400). (C). NEC+CDP-choline treatment group (×400). (D). CDP-choline group (×400).
Comparison of the contents of inflammatory factors
The levels of the inflammatory factors TNF-α, IL-1β, and IL-6 showed a significant difference between the four groups (P < 0.05). The content of TNF-α, IL-1β, and IL-6 in the NEC model group was significantly higher than the other groups (P < 0.05), but there were no significant differences between the NEC+CDP-choline treatment group, the normal control group, and the CDP-choline group (P > 0.05) (Tables 1, Figure 4).
Table 1. ELISA detection of INF-α, IL-1β and IL-6 in intestinal tissue.
Cytokine |
The CDP-choline
group (n=10) |
The NEC+CDP-choline
treatment group (n=10) |
The NEC model
group(n=10) |
The control
group (n=10) |
F/P value |
The content of INF-α |
10.041 ± 4.721 |
8.265 ± 5.585 |
42.722 ± 18.461* |
8.731 ± 5.699 |
26.679/<0.001 |
The content of IL-1β |
43.012 ± 30.951 |
43.534 ± 40.862 |
312.250 ± 204.261* |
54.485 ± 34.405 |
15.475/<0.001 |
2021 Copyright OAT. All rights reserv
The content of IL-6 |
0.528 ± 0.215 |
0.664 ± 0.335 |
2.291 ± 0.898* |
0.867 ± 0.672 |
18.726/<0.001 |
Note: * P< 0.05, compared with the other group.
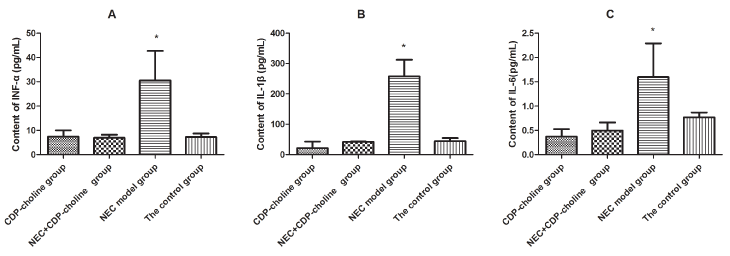
Figure 4. ELISA detection of INF-α, IL-1β and IL-6 in intestinal tissue of rats.
(A). The content of INF-α. (B). The content of IL-1β. (C). The content of IL-6. Each bar represents mean ± SD, n = 10 rats per group. *P < 0.05 versus the other groups.
In this study, we used the comprehensive stress method of enteral formula feeding, hypoxia or hyperoxia exposure, and cold stimulation to establish a neonatal rat model of NEC. We found that CDP-choline intervention significantly improved the Shiou score of intestinal tissue; reduced levels of autophagy, apoptosis, and inflammatory factors (INF-α, IL-1β, and IL-6); and exerted intestinal protective effects.
NEC is the most common neonatal gastrointestinal emergency and has a high mortality rate. Studies have shown that NEC intestinal mucosal injury is a result of the release of inflammatory mediators, such as oxygen free radical-mediated platelet activating factor (PFA), leukotriene, and tumor necrosis factor-α (TNF-α). The release of inflammatory mediators is the last common pathway or key link for the occurrence of NEC (4). We also found that the content of inflammatory factors (TNF-α, IL-1β, and IL-6) in the NEC model group was significantly higher than those in the normal control group and CDP-choline group, but there was no statistical significance between the CDP-choline treatment group and other groups, which suggested that TNF-α, IL-1β, and IL-6 were involved in NEC intestinal cell injury and subsequent cell death process. However, CDP-choline had little effect on the expression of INF-α, IL-1β, and IL-6. Therefore, it is still unconfirmed whether CDP-choline can inhibit the levels of inflammatory markers and oxidative stress to effectively protect against NEC-induced ileal degeneration.
The mucous layer in the gastrointestinal tract is a key factor in the protection of the gastrointestinal tract; phosphatidylcholine (lecithin) is the main lipid of the gastrointestinal mucosal barrier, which, as a hydrophobic intestinal surfactant, has protective effect on mucosa and can regulate the inflammatory signaling system. The occurrence of NEC was related to the defect or damage of this system; exogenous lecithin administration has shown a good effect on ulcerative colitis [4,5]. CDP-choline, an activated form of choline that plays an important role in lecithin synthesis, reduces the production of free fatty acids, increases triglyceride levels, and is commonly used as a metabolic activators of brain cells in clinical practice. Moreover, it has important significance, especially for delayed neurotoxicity, prevention of the activation of apoptosis regulatory factor Caspase-3, and reduction of cellular apoptosis [7,8]. In our study, we found that the Shiou score of the intestinal pathology in NEC rats was significantly greater than those in other groups, which was indicative of significant injury and necrosis. However, inflammation was improved significantly after CDP-choline intervention, which suggested that CDP-choline had a certain protective effect on NEC inflammation. Cetinkaya et al. [9] recently reported that the phospholipids of the tissue membrane were reduced by intestinal damage in NEC rats, but CDP-choline significantly increased the levels of total phospholipid and phosphatidylcholine.
Apoptosis may be the primary method of death for initial intestinal mucosal cells. It has been shown that intestinal mucosal permeability was an early event in the onset of NEC in premature infants. Moreover, we found that apoptosis and autophagy occurred during this process [10]. Our investigation also found that the expression of beclin-1 and LC3II in the NEC model group was stronger than those in the normal group and the CDP-choline group, whereas there were no differences between the NEC+CDP-choline treatment group and the other groups; hence, it was suggested that CDP-choline reduced autophagy and apoptosis and manifested a protective effect on the intestine. Cell autophagy and apoptosis, as interrelated cell processes, are currently considered to have the same processes of induction, regulation, and signal transduction. The activation of autophagy in the ileal intestinal epithelium in clinical cases of NEC and experimental rat models has been shown [11]. The autophagosome is formed by the mediation of the ATG12-Atg5-Atg16 complex and the microtubule-associated protein light chain 3 (LC3I)-phospholipid conjugate (LC3II) [12]; hence, LC3II is considered to be a sign of autophagy. Similarly, beclin-1 is a component of type III PI3-kinase complex that participates in the nucleation of autophagy bubbles, which is another autophagy marker [13]. In contrast, apoptosis may be the primary method of death of the initial intestinal mucosa cells, which may be achieved through TNF-α and other factors that induce mitochondrial dysfunction and activate the mitochondrial apoptosis cascade. It has been verified in an experimental NEC model that cell autophagy and apoptosis were activated, cell autophagy occurred before the occurrence of apoptosis, and the occurrence of intestinal necrosis was after cellular apoptosis [10]. A recent study has found that CDP-choline in NEC neonatal rats can significantly reduce cellular apoptosis and the level of activated caspase-3, which supported the results of this study.
In summary, CDP-choline can inhibit the intestinal inflammatory cascade by reducing the expression of inflammatory mediators such as TNF-α, IL1-β, and IL-6. Moreover, it can inhibit autophagy and apoptosis of the intestinal mucosal epithelial cells, which exerts a protective effect against the intestinal injury of neonatal rats with NEC.
Shuxia Liang, Jie Xu and Mingxing Ding conceived and coordinated the study, designed, performed and analyzed the experiments, wrote the paper. Xiaobing Li, Panjian Lai, Jinzhi Mei and Linqian Zhang carried out the data collection, data analysis. Yuanshu Fang and Fangming Guo performed and analyzed the experiments. Yunguang Bao and Kaixuan Wang revised the paper. All authors reviewed the results and approved the final version of the manuscript.
This study was supported by Experimental Animals Science and Technology Project of Zhejiang Province in China (2014C37024, 2017C37116) and Jinhua Science and Technology Research Project (2015-3-010).
This study was supported by Experimental Animals Science and Technology Project of Zhejiang Province in China (2014C37024, 2017C37116) and Jinhua Science and Technology Research Project (2015-3-010).
The authors declare that they have no conflict of interest.
The institutional and national guide for the care and use of laboratory animals was followed.
- Neu J, Walker WA (2011) Necrotizing enterocolitis. N Engl J Med 364: 255-264. [Crossref]
- Athalye-Jape G, More K, Patole S (2013) Progress in the field of necrotising enterocolitis--year 2012. J Matern Fetal Neonatal Med 26: 625-632. [Crossref]
- Lodha A, Asztalos E, Moore AM (2010) Cytokine levels in neonatal necrotizing enterocolitis and long-term growth and neurodevelopment. Acta Paediatr 99: 338-343. [Crossref]
- Stremmel W, Hanemann A, Ehehalt R, Karner M, Braun A (2010) Phosphatidylcholine (lecithin) and the mucus layer: Evidence of therapeutic efficacy in ulcerative colitis? Dig Dis 28: 490-496. [Crossref]
- Sheng Q, Lv Z, Cai W, Song H, Qian L, Wang X (2013) Protective effects of hydrogen-rich saline on necrotizing enterocolitis in neonatal rats. J Pediatr Surg 48: 1697-1706. [Crossref]
- Shiou SR, Yu Y, Chen S, Ciancio MJ, Petrof EO, Sun J, et al. (2011) Erythropoietin protects intestinal epithelial barrier function and lowers the incidence of experimental neonatal necrotizing enterocolitis. J Biol Chem 286: 12123-12132. [Crossref]
- Gutierrez-Fernandez M, Rodriguez-Frutos B, Fuentes B, Vallejo-Cremades MT, Alvarez-Grech J, et al. (2012) CDP-choline treatment induces brain plasticity markers expression in experimental animal stroke. Neurochem Int 60: 310-317. [Crossref]
- Zafonte RD, Bagiella E, Ansel BM, Novack TA, Friedewald WT, et al. (2012) Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA 308: 1993-2000. [Crossref]
- Cetinkaya M, Cansev M, Cekmez F, Tayman C, Canpolat FE, Kafa IM, et al. (2013) CDP-choline reduces severity of intestinal injury in a neonatal rat model of necrotizing enterocolitis. J Surg Res 183: 119-128. [Crossref]
- Yu Y, Shiou SR, Guo Y, Lu L, Westerhoff M, et al. (2013) Erythropoietin protects epithelial cells from excessive autophagy and apoptosis in experimental neonatal necrotizing enterocolitis. PLoS One 8: e69620. [Crossref]
- Maynard AA, Dvorak K, Khailova L, Dobrenen H, Arganbright KM, Halpern MD, et al. (2010) Epidermal growth factor reduces autophagy in intestinal epithelium and in the rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 299: G614-622. [Crossref]
- Walczak M and Martens S (2013) Dissecting the role of the Atg12-Atg5-Atg16 complex during autophagosome formation. Autophagy 9: 424-425. [Crossref]
- Park JM, Huang S, Wu TT, Foster NR, Sinicrope FA (2013) Prognostic impact of Beclin 1, p62/sequestosome 1 and LC3 protein expression in colon carcinomas from patients receiving 5-fluorouracil as adjuvant chemotherapy. Cancer Biol Ther 14: 100-107. [Crossref]




