Background: Establishing prognostic factors is very important in treatment of cancer patients. In Head and Neck Cancer- (HNC) patients, diagnosis occurs usually in already advanced disease, therefore, there is a special need to early detect the disease and its recurrence.
Aim: We aimed to evaluate the survival (OS, DFS) and its prediction by a panel of serum Tumor Markers: CEA (Carcino Embryonic Antigen), SCC (Squamous Cell Carcinoma Antigen), TPS (Tissue Polypeptide Specific Antigen), CYFRA 21-1 (Cytokeratin 19 Fragment) and sIL-2R (soluble Interleukin 2 Receptor) in HNC patients, for establishing prognosis in those patients.
Patients and methods: We evaluated 293 blood samples from 194 HNC patients. Blood Tumor Markers levels were evaluated by conventional ELISA assays, at the first date of diagnosis and during follow-up. Comparison of marker levels between 76 healthy subjects and HNC patients, alive vs dead, response to therapy and survival – were performed.
Results: Significantly lower levels of all Tumor Markers were demonstrated in patients alive, as opposed to all those who died. HNC patients who died 0-24 m after entering the study, were found to be the most sensitive time parameter to evaluate, as compared to 0-12 m, 0-36m, 0-48m or 0-60 m. We compared the alive and dead patients according to gender and found more male patients than females. Serum levels of all Tumor Markers of our panel were higher before therapy and decreased significantly thereafter, in patients responding to various therapies. The Tumor Markers sIL-2R and Cyfra 21-1 levels, were best correlated to prediction of patient’s survival. Patients having low levels had the best clinical outcome and a longer survival – according to ROC analysis.
Conclusion: CEA, SCC, TPS, CYFRA 21-1 and sIL-2R are all useful Tumor Markers in the management of HNC patients. They assessed response to therapy, were prognostic for recurrence earlier than CT and predicted survival. From our panel of Tumor Markers, sIL-2R and Cyfra 21-1, proved to be the most sensitive predictors of advanced disease with poor prognosis vs those with a good and longer survival.
tumor markers, squamous cell carcinoma antigen, cytokeratin 19 fragment, HNC patients
Head and Neck Squamous Cell Carcinoma is the sixth most common cancer worldwide. HNC is also considered as the third most frequent group of neoplasms among males and the fourth most frequent among females. The main etiologies of HNC are smoking and alcohol consumption [1]. Furthermore, Human Papilloma Virus (HPV) infection is considered to have an important role in the occurrence and prognosis of HNC patients [2].
Head and Neck Cancer (HNC) represents an aggressive tumor type with unfavorable prognosis, with many cases of recurrent disease. The Squamous Cell Carcinoma (HNSCC) sub-group includes about 90% of all HNC cases. HNSCC represents a heterogeneous group of tumors - as to their different origin, etiology and pathobiology, clinical behavior and prognosis. The worldwide incidents, includes around a half to 2 million cases annually [1].
Unfortunately, worldwide mortality from HNSCC remains high, partially due to limits on therapy secondary to the significant morbidity associated with current treatments. Therefore, immunotherapeutic approaches to HNSCC treatment are attractive for their potential to reduce morbidity while improving survival [3].
Prognosis of HNC patients depends on stage of the disease, lymph nodes involvement and response to therapy. The main problem with HNC therapy is that about 70% of HNC patients present at diagnosis with advanced disease, already. This requires a multimodality therapy, including surgery, radiation, chemotherapy, Chemo-Radiotherapy (CRT) or other combinations. However, survival of HNC patients, did not improve during last years, in spite of recently development of those therapies [4,5].
Therefore, identification of novel molecules to be used as diagnostic, prognostic and predictive biomarkers, are required for the improvement of early detection, prognosis and therapeutic options for HNC.
Recently, bioinformatics analysis, revealed a number of genes [6], some of them already known and used previously (LDH and CEACAM1). LDH, although not specific to any cancer type, has been used for many years as a Tumor Marker in various malignancies [7].
In a recent study, a total of nine hub genes (SPP1, ITGA6, TMPRSS11D, MMP1, LAMC2, FAT1, ACTA1, SERPINE1 and CEACAM1) were identified to be significantly correlated to survival of HNC patients [6-9].
At present, more than 37 different human Cytokeratines (CK) genes have been identified, of which CK 8, 18, and 19 are the most abundant in various carcinomas [10-12].
We have also previously reported in a preliminary study, that Cyfra 21-1 and SCC may be used in the detection of recurrence in HNC, and their levels are correlated with stage of disease, lymph nodes and treatment response [13]. We have also studied and demonstrated IL-2R levels to be a significant predicting marker of response to therapy in HNC patients [14].
Some biomarkers have been reported to even distinguish primary HNSCC from metastatic Lung SCC, to determine the site of origin for HNSCC of unknown primary location [5,15].
However, in spite of many papers indicating the useful assessment of these markers in the clinical set-up of HNC, there is no introduction of using those markers in the routine workup in Oncology.
In the present study, we evaluated blood samples of:
- All HNC patients: 194
- All blood samples: 293
- All healthy controls: 76
- All sub-groups: our present cohort of HNC patients included the sub-groups of Larynx, Nasopharynx, Oral Cavity, Parotid and other salivary gland malignancies.
- We evaluated blood samples of HNC patients at diagnosis, before and after therapies (surgery, radiation, chemotherapy and combined modalities) during follow-up.
- We used ELISA Tumor Markers Kits from Diasorin, Italy.
- Correlations of Tumor Marker levels to gender, treatment response and to survival, were performed.
Statistical evaluation
A stepwise logistic regression analysis (SPSS: IBM SPSS Statistics for Windows, Version 25.0, 2017. Armonk, NY: IBM Corp.) was carried out to test TPS, IL-2R, SCC and Cyfra 21-1 as potential markers in predicting survival; CEA was also considered but dropped because of sparse data. IL-2R was the most effective marker (p=0.00354), followed by Cyfra 21-1 (p=0.0551); together they raise the success of the prediction from 54.1% to 69.4%, with an area under the ROC curve- AUC of 0.693 (p=0.000288). In addition to the statistics used for all patients, we analyzed also those who died 1-24 m (found as the best time point for analysis). In this logistic regression analysis, we used the patients, which revealed an AUC of 0.693.
- Alive, n = 60. Dead, n= 51 (0-24 m).
- Analysis, n= 111. Missing cases: 23. Total: 134.
In this study, we evaluated a cohort of 293 HNC patients from various sub-groups, as to their 5 Tumor Marker levels (CEA, SCC, TPS, Cyfra 21-1 and IL-2R) with correlation to clinical parameters (Figure 1 to Figure 7) (Table 1).
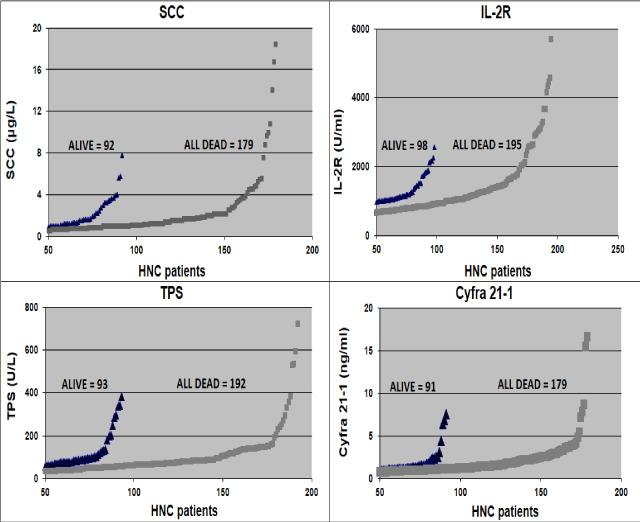
Figure 1. A cohort of patients still alive vs all who died, which performed the Tumor Markers tests. There is a good distinction between patients who died, most of them with high Tumor Markers levels, as compared to most of the low levels in patients still alive.
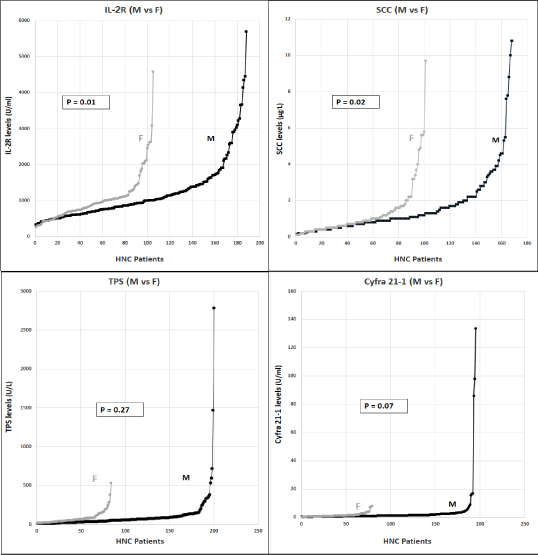
Figure 2a. We looked at the gender distribution in our HNC patients cohort. There is a significant higher number of males with HNC vs females, as can be seen here. Males had significantly higher levels of the Tumor Markers IL-2R (p=0.01) and SCC (p=0.02), while for TPS and Cyfra 21-1, differences in levels, were not significant (p=0.27, 0.07).
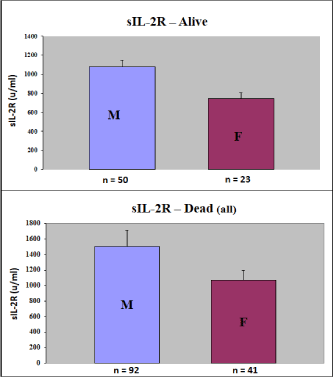
Figure 2b. We demonstrated a significant higher distribution of sIL-2R in male patients, as compared to females. This was correct for all individual markers, we showed it here only for sIL-2R, as an example of the Tumor Markers.
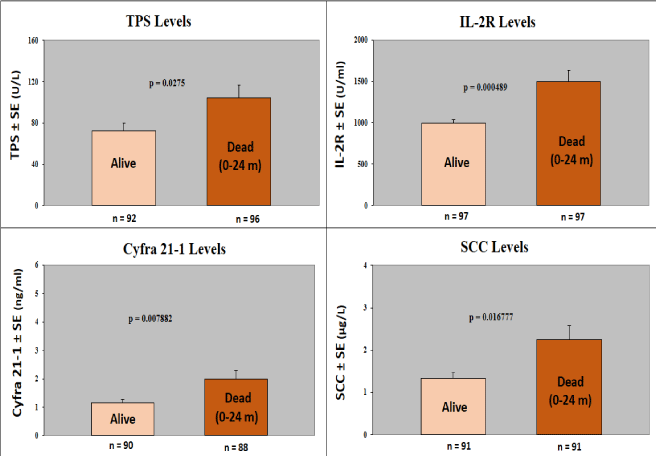
Figure 3. We compared levels of the Tumor Markers in alive patients with those who died 0-24 months after entering the study. This time of 2 years, was found as the most significant survival time evaluation (1y, 2y, 3y, 4y, 5y) after diagnosis of HNC. Levels of 4/5 Tumor Markers (IL-2R, TPS, SCC, Cyfra 21-1) distinguished significantly (p<0.05) between patients alive and those who died 0-24 months after treatments. The CEA Marker (performed only by some patients) did not distinguish significantly between these 2 groups, although there was a trend of higher levels in the patients who died (data not shown).
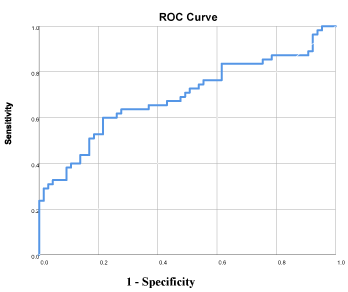
Figure 4. We performed ROC analysis on the group of patients who died 0-24 m vs 76 healthy controls, in order to understand which markers will predict survival best. Figure 4 shows clearly that sIL-2R and Cyfra 21-1 are the best predictors of survival, out of our panel of markers (AUC = 0.79). ROC: Area Under the Curve.
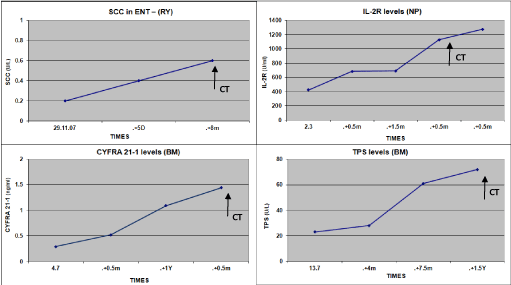
Figure 5. Kinetics of Tumor Markers in HNC patients responding to therapy, which decreased significantly. (1-12 m before indicated by CT).
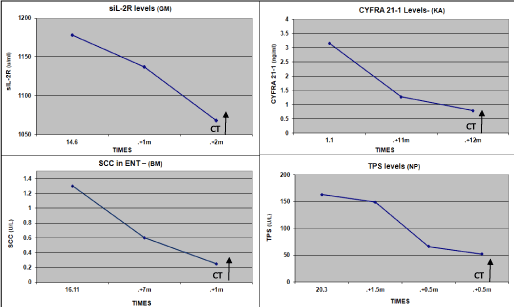
Figure 6. Kinetics of Tumor Markers in HNC patients non-responding to therapy, which increased significantly in all markers. (3 to 18m before indicated by CT).
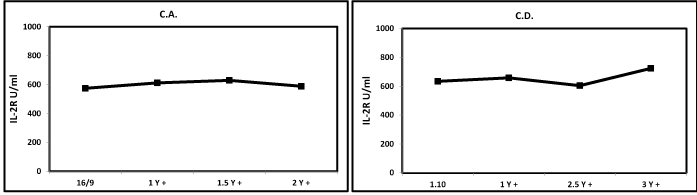
Figure 7. Kinetics of sIL-2R in a stable disease HNC patient.This is important information for the clinicians provided by tumor markers, without using CT or MRI.
Table 1. Test result.
During recent years, many efforts have been utilized toward identifying new Biomarkers in HNC patients, using different methods, from in vitro HNC cell-lines to Proteomics [16,17].
CEA and SCC (15) were the most reported Tumor Markers in HNC, correlated mostly to tumor mass, as reported for example, in a meta-analysis study of 493 patients [15,18]. Additional markers such as MMPs were shown with high pre-operative serum levels, but without follow up over years [19]. LDH, although old and non-specific marker, has been shown to be significant in HNSCC for many years. There was a significant correlation between serum LDH and grades of HNSCC showing high levels of expression in moderately differentiated SCC. This alteration among different tumor grades can be an indicator for the treatment and prognosis of HNC [7]. Many studies showed Tumor Markers to be important in therapy response assessment, or even predict survival in HNC patients: CEA, SCC [9,13,15,18]. Immune Cancer cells as CTC (22) and CSC (23) have been related with the capacity of metastasis, relapse and resistance to multiple drugs during chemotherapy. One study was conducted using articles from five databases (PubMed, Scopus, Web of Science, Lilacs and Scielo). CSC correlated with clinical characteristics of the tumor, such as staging, tumor size and lymph node metastasis and was used for prognosis in HNC patients (23).
Our results are very similar to many other studies showing prognostic significance of SCC [9,13,18]. Cyfra 21-1 and CEA (12, 13), or Cytokeratins - TPS [16,17]. There is a very good correlation between our preliminary results and the updated results here: The most significant markers we found in our previous [13] and present studies were:
SCC, [9,13,15,18-20], Cyfra 21-1 [13,21], and sIL-2R [14]. However, surprisingly for us, Cyfra 21-1 was not found as a tumor marker significantly correlated to metastases formation of HNC patients. This study [21] included n=55 patients, and the follow-up was about 3 years. This is the only paper, [21] which disagrees with our and other's studies finding that Cyfra 21-1 is a sensitive and prognostic marker in HNC [12,13]. Immune parameters [9] including CD44, were found in high levels in oral rinses and tissues from oral and oropharyngeal cancer patients [20] and higher levels of IL-6 expression and serum levels- were associated with active disease and metastases and poorer survival [8]. We have previously shown the immune-biomarker serum sIL-2R as a very sensitive, correlated to activity of disease vs response to treatments in HNC patients [14]. In the present study, according to the ROC analysis, we demonstrated again the high sensitivity of this immune Biomarker. A few papers suggested a higher frequency of males in HNC patients [15]. Since it interested us if we will find similar results in our cohort of HNC patients, we examined the gender distribution indeed we determined a higher male proportion of males in our cohort of patients, as well. These results maybe correlated to a higher degree of smoking and alcohol consuming in men vs females [15]. Despite advances in diagnostics, treatment, and close follow-up by serum Tumor Markers, the 5-year progression-free survival (PFS) or survival rates for recurrent/metastatic (R/M) disease - have not significantly changed over the past years [3-5,24]. Recent treatments include NIVOLUMAB –B and PEMBROLIZUMAB –C, in addition to formerly used Methotrexate, Docetaxel and Cetuximab [25]. In a randomized, open-label, phase 3 study at 97 medical centers in 20 countries, they demonstrated a prolongation of overall survival (OS) and favorable safety of Pembrolizumab in patients with recurrent or metastatic HNSCC. Pembrolizumab was therefore suggested as a monotherapy or as a part of combination therapy, in earlier stages of disease [26]. Nivolumab, is also a relatively new drug used in SCC [27]. Nivolumab appeared to improve efficacy of treatment versus IC, regardless of prior Cetuximab use, supporting its use in patients with R/M SCC HNC, with or without prior Cetuximab exposure.
As early detection and treatment of cancer are the most effective means to reduce morbidity and mortality, tumor markers information will enable clinicians to a better understanding of the disease process, response to treatments and early therapeutic decision-making.
Our findings of the present study including a large number of patients and comparison to other studies- should refer to Multi-Centric or Meta-analysis studies including also large numbers of patients and long follow up- to establish the significance for routine clinical practice.
- In summary, the most important, out of our panel of Tumor Markers studied, are: Cyfra 21-1 and sIL-2R, providing good prognosis and therapy response assessment.
- Therefore, we strongly recommend including them in the routine set-up of HNC patients in the Clinical Oncology.
- Siddiqui F and Gwede CK (2012) Head and Neck Cancer in the elderly population. Semin Radiat Oncol 22: 321-333. [Crossref]
- Alsbeih G, Al-Harbi N, Judia SB, Al-Qahtani W, Khoja H, et al. (2019) Prevalence of Human Papillomavirus (HPV) Infection and the Association with Survival in Saudi Patients with Head and Neck Squamous Cell Carcinoma. Cancers (Basel) 11: 820. [Crossref]
- Nemunaitis J, O'Brien J (200) Biological approaches to treatment of Head and Neck Cancer. BioDrugs 13: 359-372. [Crossref]
- Ghosh G, Gupta G, Malviya A, Saroj D (2018) Comparison three-dimensional conformal radiotherapy versus intensity modulated radiation therapy in local control of Head and Neck Cancer. J Cancer Res Ther 14: 1412-1417. [Crossref]
- Dias FL (2013) Assessment of Treatment Response after Chemoradiation of Head and Neck Cancer. Curr Oncol Rep 15: 119-127. [Crossref]
- Zhao L, Chi W, Cao H, Cui W, Meng W, et al. (2019) Screening and clinical significance of Tumor Markers in Head and Neck Squamous Cell Carcinoma through bioinformatics analysis. Mol Med Rep 19: 143-154.
- Bhattacharjee A, Giri S, Roy M, Chakraborty A (2018) Correlation of serum Lactate Dehydrogenase (LDH) and Alkaline Phosphatase (AP) in different histological grades of Head and Neck Squamous Cell Carcinoma and premalignant lesions. J Cancer Res Ther 14: 934-940. [Crossref]
- Meyer F, Samson E, Douville P, Duchesne T, Liu G, et al. (2010) Serum prognostic markers in Head and Neck cancer. Clin Cancer Res 16: 1008-1015. [Crossref]
- Economopoulou P, de Bree R, Kotsantis I, Psyrri A (2019) Diagnostic Tumor Markers in Head and Neck Squamous Cell Carcinoma (HNSCC) in the Clinical Setting. Front Oncol 9: 827. [Crossref]
- Barak V, Goike H, Panaretakis KW and Einarsson R (2004) Clinical utility of Cytokeratins as Tumor Markers. Clin Biochem 37: 529-540. [Crossref]
- Einarsson R, Barak V (2000) TPS: A Cytokeratin serum Tumor Marker for effective therapy control of cancer patients with focus on Breast Cancer. J Clinical Ligand Assay 22: 348-352.
- Kalfert D, Ludvik J, Kucera R, Topolcan O, Celakovsky P, et al. (2019) Pretreatment Serum Levels of Soluble Cytokeratin Fragments (Cyfra 21-1, TPS, MonoTotal) in Relation to Clinical and Patho-biological aspects of Head and Neck Squamous Cell Carcinomas. Anticancer Res 39: 5171-5177.
- Barak V, Meirovitz A, Rachmut K, Peretz T, Eliashar J, et al. (2015) The Diagnostic and prognostic value of Tumor Markers (SCC, TPS, CYFRA 21-1, CEA) in Head & Neck Cancer patients. Anticancer Res 35: 5519-5524.
- Gross M, Meirovitz A, Rachmut J, Kalichman I, Peretz T, et al. (2016) The diagnostic and prognostic value of sIL-2R as an immune Biomarker in Head and Neck Cancers Anticancer Res 36: 4347-4352.
- Travassos DC, Fernandes D, Massucato EMS, Navarro CM, Bufalino AJ (2018) Squamous Cell Carcinoma Antigen as a prognostic marker and its correlation with clinicopathological features in Head and Neck Squamous Cell Carcinoma: Systematic review and meta-analysis. Oral Pathol Med 47: 3-10. [Crossref]
- Rezende TM, de Souza Freire M, Franco OL (2010) Head and Neck Cancer: proteomic advances and biomarker achievements. Cancer 116: 4914-4925. [Crossref]
- Chen YT, Chong YM, Cheng CW, Ho CL, Tsai HW, et al. (2013) Identification of novel Tumor Markers for Oral Squamous Cell Carcinoma using glycoproteomic analysis. Clin Chim Acta 420: 45-53. [Crossref]
- Imai R, Takenaka Y, Yasui T, Nakahara S, Yamamoto Y, et al. (2015) Prognostic significance of serum SCC Antigen in patients with Head and Neck Cancer. Acta Otolaryngol 135: 295-301. [Crossref]
- Kalfert D, Ludvikova M, Topolcan O, Windrichova J, Malirova E, et al. (2014) Analysis of preoperative serum levels of MMP1, -2, and -9 in patients with site-specific Head and Neck Squamous Cell Cancer. Anticancer Res 34: 7431-741.
- Cohen ER, Reis IM, Gomez-Fernandez C, Smith D, Pereira L, et al. (2020) CD44 and associated markers in oral rinses and tissues from oral and Oropharyngeal Cancer patients. Oral Oncol 106: 104720. [Crossref]
- Rudhart SA, Schultz JD, Gehrt F, Pavel FL, Birk R, et al. (2019) CYFRA 21-1: a suitable Tumor Marker in patients with Head and Neck Cutaneous Squamous Cell Carcinoma? Eur Arch Otorhinolaryngol 276: 3467-3475. [Crossref]
- Wen Z, Li Z, Yong P, Liang D, Xie D, et al. (2019) Detection and clinical significance of circulating tumor cells (CTC) in patients with Nasopharyngeal Carcinoma. Oncol Lett 18: 2537-2547. [Crossref]
- Curtarelli RB, Gonçalves JM, Dos Santos LGP, Savi MG, Nör JE, et al. (2018) Expression of Cancer Stem Cell (CSC) Biomarkers in Human Head and Neck Carcinomas: a Systematic Review. Stem Cell Rev Rep 14: 769-784. [Crossref]
- Cline BJ, Simpson MC, Gropler M, Bukatko AR, Adjei Boakye E, et al. (2020) Change in Age at Diagnosis of Oropharyngeal Cancer in the United States, 1975-2016. Cancers (Basel) 12: 3191. [Crossref]
- Neal MEH, Haring CT, Mann JE, Brenner JC, Spector ME, Swiecicki PL (2019) Novel immunotherapeutic approaches in Head and Neck Cancer. J Cancer Metastasis Treat 5: 76.
- Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, et al. (2019) Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic Head-and-Neck Squamous Cell Carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 393: 156-167. [Crossref]
- Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas DA, et al. (2016) Nivolumab for recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 375: 1856-1867. [Crossref]








