Abstract
Objective: To systematically review the effectiveness and safety of antibiotic therapy in sepsis under the guidance of procalcitonin in ICU.
Data sources: PubMed, The Cochrane Library (through October 2016), EMbase, Web of Science, reference lists of retrieved publications. No limitations regarding the language of publications.
Study selection: We included only randomized controlled studies reporting on critically ill patients with sepsis managed with a PCT-guided algorithm vs. Routine practice.
Data extraction: Data on study characteristics, interventions, and outcomes were retrieved by two independent reviewers. Risk ratios, mean differences, and 95% confidence intervals were calculated by implementing both the Mantel-Haenszel fixed effect model and the Mantel-Haenszel random effect model.
Data synthesis: Thirteen randomized controlled studies involving 4728 ICU septic patients were included. Compared with the routine particle, PCT-guided algorithms decreased the total duration of antibiotic therapy (REM; MD=-1.60; 95%CI, -2.14 to -1.06; Z=5.79; P<0.00001), and the 28-day mortality had also been reduced ((FEM; MD=0.86; 95%CI, 0.76 to 0.97; Z=2.39; P=0.02). However, between the PCT-guided group and the routine practice group, the length of ICU and hospital stay, the ICU mortality, in hospital mortality and the rates of clinical cure had no significant difference.
Conclusions: The PCT-guided algorithms could decrease the total duration of antibiotic therapy and reduce the 28-day mortality of septic patients in ICU without improving the length of ICU and hospital stay, the ICU mortality, in hospital mortality and the rates of clinical cure. Further research is still necessary before the wide adoption of this strategy.
Key words
procalcitonin, sepsis, intensive care unit, meta-analysis
Introduction
Sepsis is defined as a systemic inflammatory response syndrome (SIRS) plus documented or suspected infection [1], associated with increasing hospitalization and mortality [2,3]. Antibiotic therapy is the key to cure and reduce the mortality of sepsis. Procalcitonin(PCT), a calcitonin precursor, is a promising biomarker to diagnose the potential sepsis [4]. Even though, Procalcitonin-guided algorithms of antibiotic therapy in intensive care unit is still not universally accepted [5]. Researchers have found that PCT-guided algorithm may reduce antibiotic exposure of sepsis in ICU, but cannot reduce the patients’ mortality [6,7]. It remains unclear if the Procalcitonin-guided algorithmic can improve health outcomes compared with routine practice in ICU. Therefore, we performed the Meta-analysis of the randomized controlled trials reporting on the outcomes of septic patients in ICU.
Materials and methods
Data sources and searches
We systematically searched MEDLINE, the Cochrane Library, EMbase and Web of Science (from inception up to October 31,2016) to identify relevant randomized controlled trials(RCTs) by using the search terms: procalcitonin AND sepsis AND intensive care unit AND (antibiotic therapy OR Anti-Bacterial Agents OR Anti-Infective) AND Random. The reference lists of the initially retrieved articles were also reviewed. If necessary, the corresponding authors of each one of the included studies were contacted by e-mail for additional information.
Selection of studies
Two investigators independently carried out the computerized literature search and local the potentially eligible articles. The inclusion criteria in this meta-analysis is that critically ill patients with sepsis managed with a PCT-guided algorithm vs. Routine practice. No limitations regarding the country, time or language of publications. We excluded trials performed outside the intensive care unit(ICU).
Outcome measures
The duration of antibiotic therapy, length of ICU and hospital stay, 28-day all-cause mortality, ICU and in hospital mortality, the rates of clinical cure.
Data extraction and quality assessment
Two investigators dependently reviewed the full text of the retrieved articles and reported the results in a structured dataset. Disparities between investigators regarding the inclusion of each trial were resolved by consensus by a third independent investigator. The data’s included first author, year and country of publication, ICU type, study design, number of patients managed with Procalcitonin-guided algorithmic vs. routine practice, details of the PCT-implemented algorithm. The assessment of the methodologic quality of the included RCTs were followed the recommendations exemplified in the Cochrane handbook for systematic reviews of interventions and summarized in a domain-based evaluation of the following components: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other bias [8].
Data synthesis and analysis
We used Review Manager 5.3 to conduct the statistical analysis. The between-study heterogeneity was assessed by the chi-squared test and its extent was quantified by the I2 statistic (I2 values of 25%,50%, and 75% were considered to represent low, moderate and severe statistical inconsistency [9]. If significant heterogeneity could be detected, subgroup analysis and sensitivity analysis will be estimated. Continuous outcomes were analyzed using mean differences(MD) and 95% confidence intervals(CIs). Risk ratio(RR) and 95% CI were calculated by implementing the Mantel-Haenszel fixed effect model(FE) and the Mantel-Haenszel random effect model(RE). A p<0.05 was thought to indicate statistical significance in this meta-analysis.
Results
Study selection
Of the 829 citations found in MEDLINE, the Cochrane Library, EMbase and Web of Science. The majority were excluded for reasons presented in (Figure 1). Thus, thirteen RCTs [10-22] met the inclusion criteria for this Meta-analysis.
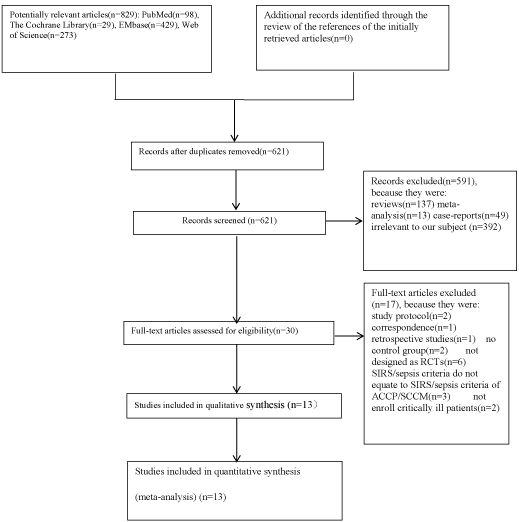
Figure 1: Flow diagram of the literature search and selection process of the studies.
Study description
In Table 1, we list the main characteristics of the included studies that meet our eligibility criteria. All studies [10-22] were published from 2007 to 2016. Their individual sample size ranged from 27 to 1546 patients. Eight single-center studies and Five multi-center studies. All studies had been conducted in ten different countries: Czech Republic, Switzerland, Germany, France, Belgium, China, Brazil, Iran, Australian, Netherlands. Twelve studies were published in the English language and one study was published in the Chinese language. Eleven studies included medical, surgical, and mixed ICUs [10-13,15-19,21-22], two focused on surgical ICUs [14,20].
Table 1: Main characteristics of the studies included in the Meta-analysis: PCT-group vs. routine practice
First Author/Year of publication (Reference) |
Svoboda/2007 [22] |
Nobre/2008
[19] |
Schroeder/2009
[20] |
Hochreiter/2009
[14] |
Bouadma/2010
[12] |
Layios/2012
[16] |
Country/Type of ICU |
Czech Republic/4 ICUs in the same hospital |
Switzerland/I ICU in university hospital |
Germany/1 surgical ICU |
Germany/1 surgical ICU |
France/7 (5 medical,2 surgical) ICUs in 5 university-affiliated hospitals, and 1 medicosurgical ICU in a general hospital. |
Belgium/5 ICUs of the univeristy hospital |
Study Design |
P,PG,OL,SC |
P, PG, OL, SC |
P,PG,OL,SC |
P,PG,OL,SC |
P,PG,OL,MC |
P,PG,SB,SC |
Inclusion Criteria |
All ICU patients 18 years or older who developed severe sepsis |
All ICU patients with suspected severe sepsis or septic shock |
All ICU Patients after abdominal surgery and after start of antibiotic treatment with the diagnosis of severe sepsis |
All ICU patients requiring antibiotic therapy based on confirmed or highly suspected bacterial infections and at least
two concomitant systemic inflammatory response syndrome criteria |
All ICU adult patients with suspected infections if they were not receiving
antibiotics before inclusion in the study or if they had received antibiotics for less than 24 h. |
All ICU adult patients hospitalized for > 48 hrs and suspected of developing infection either on admission or during ICU stay. |
Exclusion Criteria |
chemical or burn trauma, moribund patients, and patients who have been designated as "not full support" or "do not resuscitate" |
Infections caused by Pseudomonas aeruginosa, Acinetobacter baumanni, Listeria spp., Legionella pneumophila, Pneumocystis jiroveci, or Mycobacterium tuberculosis, viruses or parasites or infectious conditions requiring prolonged ABx; ABx started >48 hrs before enrollment; chronic, localized infections; severely immunocompromised patients; withholding of life support; or absence of antimicrobial treatment despite clinical suspicion of sepsis. |
refused informed consent, or already had received antibiotic treatment prior to admission to the ICU. |
Patients who refused study consent, whose antibiotic treatment had been initiated before intensive care admission, or who had therapy limitations |
age under 18 years; known pregnancy; expected stay in the ICU of less than 3 days; bone-marrow transplant or chemotherapy induced neutropenia; infections for which long-term antibiotic treatment is strongly recommended ;poor chance of survival, defined as a simplified acute physiology score (SAPS II) of more than 65 points at screening; and do not-resuscitate orders. |
no informed consent, stay less than 48 hours |
Description of PCT-Guided Algorithms |
ABx were encouraged when severe sepsis with PCT value> 2μg/L, severe sepsis with PCT value≤2μg/L prompted to ultrasonography and/or CT |
ABx were stopped when PCT levels had decreased>90% from the initial value but not before Day 3(if baseline PCT<1μg/L) or Day 5 (if baseline PCT>1 μg/L). |
ABx were discontinued if clinical signs of infection improved and the PCT value was either <1μg/Ll or decreased to <35% of the initial concentration within three consecutive days |
ABx were discontinued if clinical signs and symptoms of infection improved and PCT decreased to <1 μg/L or the PCT>1 μg/L but had dropped to 25 to 35% of the initial value over
three days. |
ABx were discontinued when PCT<80% of the peak concentration or an absolute value< 0.5 μg/L |
ABx were discouraged if PCT <0.25 μg/L or
0.50 μg/L, and more or less recommended
if PCT> 1 μg/L or 0.50 μg/L |
Description of Routine practice |
ABx were based on consultant surgeon according to contemporary treatment protocol of their institute |
ABx were decided by the clinicians based on empirical rules. |
ABx were directed by clinical signs and empirical rules. |
ABx were applied as standard regimen over eight days. |
ABx were based on international
and local guidelines. However, investigators were free to decide the optimum duration of antibiotic treatment based on their own assessment of the infection’s clinical
course. |
ABx were based on the physician own assessment |
Number of Patients, CT-group/Control-group |
38/34 |
31/37 |
14/13 |
57/53 |
307/314 |
258/251 |
ICU, intensive care unit; PCT, procalcitonin; P, prospective; PG, parallel group; OL, open label; SB, single-blind; MC, multi-center; SC, single-center; ABx, antibiotics.
Liu BH/2013
[17] |
Deliberato/2013
[13] |
Annane/2013
[10] |
Najafi/2015
[18] |
Shehabi/2014
[21] |
Jong/2016
[15] |
Bloos/2016
[11] |
China/1 ICU |
Brazil/1 ICU of a tertiary care,private hospital |
France/8 ICUs |
Iran/1 mixed ICU |
Australian/11 ICUs |
Netherlands/15(3 university medical centres,12 teaching hospitals) ICUs |
Germany/33 multidisciplinary ICUs |
P, PG,OL,SC |
P, PG,SB,SC |
P, PG,SB,MC |
P, PG, SB,SC |
P, PG, SB,MC |
P, PG, OL,MC |
P, PG, OL,MC |
All ICU patients aged at least 18 years and suspected of developing infection |
All ICU Patients with microbiologically confirmed infections with sepsis, severe sepsis, and septic shock |
All ICU adult patients with the systemic
inflammatory response syndrome, acute dysfunction of at least one organ, absence of indisputable clinical
infection and negative microbial cultures |
All ICU Patients with at least two of the four criteria including body temperature above 38? or below 36?,
tachycardia >90/min, tachypnea >20/min and leucocytosis >12×109/L or a leftward shift with more than 10% band cells or leukopenia <4×109/L were
defined as subject with SIRS |
All ICU Patients older than 18 years of age, admitted to ICU within the previous 72 hours, receiving parenteral and/or enteral antibiotics for a suspected bacterial infection and expected to remain in the ICU for longer than 24 hours |
All ICU patients aged at least 18 years, and received their first dose of
antibiotics no longer than 24 h before inclusion in the study |
All ICU adult patients with sepsis or septic shock |
onset of antibiotic therapy more
than 48 hours before the date when the cultures were performed; severe infection caused by viruses, Acinetobacter baumannii or mycobacteria;chronic localized infections,and refused study consent |
onset of antibiotic therapy more
than 48 hours before the date when the cultures were performed; patients under 18 years old; known pregnancy; infections requiring prolonged antibiotic therapy, and osteomyelitis; severe infection caused by viruses, parasites, fungi, or mycobacteria; chronic localized infections, and negative cultures in patients with suspected sepsis, severe sepsis, or septic shock. |
pregnancy, burns over ≥15% of body surface area, trauma, outpatient or inpatient cardiac arrest, postorthopaedic surgery status, drug-related neutropenia, withdrawal of life-supportive therapies or a decision to withhold them, indisputable clinical infection or antibiotic exposure ≥48 h during the time shortly before ICU admission. |
documented infection, pus from wound or abscess,empyema, thrombophlebitis, infection due to viral or parasites, hypoxemia (PO2<60 mmHg), oliguria, Glasgow coma scale (GCS) 3 without sedation, parenteral antibiotic usage 24 hours before admission to ICU, hospitalization 48 hours before enrollment, conditions requiring prolonged antibiotic therapy such as endocarditis, chronic localized infection such as osteomyelitis and severely immunocompromised patients. |
patients receiving antibiotics for surgical prophylaxis or with proven bacterial infection requiring more than 3 weeks’ antibiotic therapy,
isolated systemic fungal or systemic viral infection in the absence of bacterial infection, neutropenia with a neutrophil count less than 1,000 cells/ml, receiving immunosuppressive agents, cardiac surgery or trauma or heat stroke within 48 hours, medullary thyroid or small cell lung cancer, subject not expected to survive to hospital discharge, or known regnancy |
in cases of systemic antibiotics as
prophylaxis only, antibiotics solely as part of selective decontamination of the digestive tract, prolonged therapy (eg, endocarditis), expected ICU stay of less than 24 h, severe immuno suppression, severe infections (due to viruses, parasites,or Mycobacterium tuberculosis),and moribund patients. |
Pregnant or lactating women, patients with selenium intoxication, individuals with
infections for which guidelines recommend a longer duration of antimicrobial therapy, immunocompromised patients, and those without commitment for full therapy or where death was imminent owing to coexisting diseases |
ABx were discontinued when PCT <90% of the peak value or an absolute value<0.25 μg/L |
ABx were discontinued when PCT<90% of the peak value or an absolute value<0.5 μg/L |
ABx were not to be started or was to be halted when PCT<0.25μg/L, strongly discouraged when PCT was ≥0.25 to<0.5 μg/L, recommended when PCT≥0.5 to<5μg/L, strongly recommended when PCT≥5μg/L |
ABx were discontinued when PCT value≤0.5 μg/L,
ABx were continued when PCT value ≥ 2μg/L |
ABx were discontinued when PCT level<0.10 μg/L or <90% of the peak concentration |
ABx were discontinued when PCT value<80% of the peak value or an absolute value≤0.5 μg/L |
ABx were discontinued when PCT value<50% of the peak value or an absolute value≤1.0 μg/L |
ABx were based on the national guidelines and the discretion of attending physicians. |
ABx were based on the physician following the PCTprotocol after a lecture about PCT and the evidence about it. |
ABx were based on the physician' own assessment |
ABx were based on the physician' antibiotic empiric therapy |
ABx were based on the local guidelines a treating clinician were allowed
to overrule the algorithm as clinically
indicated. |
ABx were based on the local or national guidelines and according to the discretion of attending physicians. |
ABx were based on the local Guidelines |
42/40 |
42/39 |
30/28 |
30/30 |
196/198 |
761/785 |
552/537 |
Data regarding the impact of PCT-guided algorithms on the antibiotic therapy are presented in [Table 2]. Data on the quality of the included studies are presented in Table 3. Eight studies are considered of high quality [10-13,15,18-19,21] and five of low quality [14,16-17,20,22].
Table 2: Outcome data of the studies included in the Meta-analysis: Procalcitonin-guided algorithms vs. Routine practice
Study (reference) |
Total duration of antibiotic treatment, days, Mean (SD) |
Length of ICU stay, days, Mean (SD) |
Length of hospital stay, days, Mean (SD) |
ICU mortality, n (%) |
Hospital mortality, n (%) |
28-day mortality, n (%) |
Rate of clinical cure, n (%) |
Svoboda
[22] |
NA |
16.1±6.9 vs. 19.4±8.9 |
NA |
NA |
NA |
10(26%) vs. 13(38%) |
NA |
Nobre
[19] |
NA |
4(1-21) vs. 7(1–91)a |
17(3-96) vs. 23.5(5-44)a |
NA |
9(23.1%) vs. 9(22.5%) |
8(20.5%) vs. 8(20%) |
31(79.4%) vs. 32(80%) |
Schroeder
[20] |
6.6±1.1 vs. 8.3±0.7 |
16.4±8.3 vs. 16.7±5.6 |
NA |
NA |
3(21.4%) vs. 3(23.1%) |
NA |
NA |
Hochreiter
[14] |
5.9±1.7 vs. 7.9±0.5 |
15.5±12.5vs. 17.7±10.1 |
NA |
NA |
15(26.3%)vs. 14(26.4%) |
NA |
NA |
Bouadma
[12] |
10.3±7.7 vs. 13.3±7.6 |
15.9±16.1vs. 14.4±14.1 |
26.1±19.3 vs. 26.4±18.3 |
NA |
NA |
65(21.2%) vs. 64(20.4%) |
NA |
Layios
[16] |
NA |
7(4-16) vs. 7(4-18)a |
NA |
56(21.7%)vs. 53(21.1%) |
NA |
|
NA |
Liu BH
[17] |
8.1±0.3 vs. 9.3±0.3 |
12.0±2.9 vs. 14.0±2.7 |
27.0±4.9 vs. 32.0±5.4 |
NA |
NA |
6(14.3%) vs. 5(12.5%) |
33(78.5%)vs. 34(85%) |
Deliberato
[13] |
10(3-39) vs. 11(2-45)a |
3.5(1–57) vs. 3(1–28)a |
11(3-547) vs. 11(2-228)a |
1(2.4%) vs. 4(10.3%) |
2(4.8%) vs.4(10.3%) |
NA |
NA |
Annane
[10] |
5(2-5) vs. 5(3-5)a |
22(8-42) vs. 23(10-60)a |
27(9-49) vs. 33(11–69)a |
7(23%) vs. 10(33%) |
7(23%) vs.10(33%) |
NA |
NA |
Najafi
[18] |
128 vs. 320 |
4(2-20) vs. 6(2-28)a |
20(8-44) vs. 22(6-65)a |
NA |
5(16.6%) vs.4(13.3%) |
NA |
27(90%)vs. 26(86.6%)
|
2021 Copyright OAT. All rights reserv
Shehabi
[21] |
9(6–20) vs. 11(6–22)a |
6(3–9.5) vs. 6(4–10)a |
15(9–29)vs. 17(10–32)a |
21(11%)vs. 15(8%) |
30 (16%) vs. 26 (13%) |
NA |
NA |
Jong
[15] |
5(3-9) vs. 7(4-11)a |
8.5(5-17) vs. 9(4-17)a |
22(13-39.3) vs. 22(12-40)a |
NA |
NA |
149(19.6%) vs. 196(25.0%) |
NA |
Bloos
[11] |
NA |
12(6-24)vs. 11(6-21)a |
29(18-46)vs. 26(16-44)a |
NA |
NA |
140(25.6%)vs. 149(28.2%) |
NA |
ICU, intensive care unit; NA, not available/applicable; aThese values are referred to median (range) instead of mean(SD).
Study (reference) |
Annane
[10] |
Bloos
[11] |
Bouadma
[12] |
Deliberato
[13] |
Hochreiter
[14] |
Jong
[15] |
Bias component Sequence generation and allocation concealment |
Randomised in a 1:1 ratio according to a computer-generated list. Randomisation was centralised through a secured website and performed by an independent statistician, and was stratified by the centre and according to whether patients underwent surgery in the past 48 h. |
An allocation ratio of 1:1 by use of a central randomization web server, Randomization was stratified by study
centre, sex, and sepsis severity. |
Independent, centralised,
computer-generated randomisation sequence was used to randomly assign patients in a 1:1 ratio,Patients were stratified by centre with random block sizes of 2, 4, or 6; |
2 of the authors
would randomly draw 1 folder from a black box containing 100 folders (50 “PCT group” and 50 “control group”). |
Patients were randomly assigned to either a PCT-guided or control group without further reported details |
Randomised in a 1:1 ratio according to a computer-generated list. Randomisation was stratified according to treatment centre |
Blinding |
Using permutation blocks, the size of which remained unknown to the investigators. In the control arm, patients, physicians, nurses, investigators, study coordinators, the statistician and the sponsor remained blinded to PCT levels throughout the study. |
No |
Investigators were masked
to assignment before, but not after, randomisation, treatment assignments were not masked, all investigators were unaware of aggregate outcomes during the study. |
No |
No |
No |
Incomplete outcome data |
3 patients withdrew informed consent,1 from PCT-Group,2 from Control-Group |
Almost 1 every 5 screened patients were excluded from the study |
2 patients withdrew informed consent,1 from each group |
No patient was lost to follow-up |
Almost 1 every 4 screened patients were excluded from the study |
29 patients withdrew from the study,15 from PCT-group,14 from control-group. |
Selective outcome reporting |
Extensive reporting |
Extensive reporting |
Extensive reporting |
Extensive reporting |
Limited reporting |
Extensive reporting |
Other sources of bias |
In control-group, the physicians were free to decide to continue or change the antibiotics on clinical judgment |
No data on adequacy of antimicrobial therapy |
In control group, investigators were free to decide the optimum duration of antibiotic treatment based on their own assessment of the infection’s clinical course, |
not all attending
physicians agreed to participate. |
No data on adequacy of antimicrobial therapy |
It is unclear if there was crossover between the two groups |
Quality score |
High |
High |
High |
High |
Low |
High |
Table 3: Assessment of the methodologic aspects of the included studies
Layios
[16] |
Liu BH
[17] |
Najafi
[18] |
Nobre
[19] |
Schroeder
[20] |
Shehabi
[21] |
Svoboda
[22] |
Patients were randomly assigned to either a PCT-guided or control group antibiotic regimen without further reported details |
Randomised in a 1:1 ratio according to a computer-generated list. |
Randomly divided into two groups by computer based
random number generation, |
The randomization was performed using a computer-based random
number generation. Allocation was issued using opaque, sealed, numbered
envelopes. |
Patients were randomly assigned to either a PCT-group or a control group without further reported details |
variable block randomized
1:1 via a secured central study website into either a PCT group) or control
group. Randomization was stratified according to the presence of septic shock |
The randomization was performed by means of a computer generated random number table to generate a random treatment list, treatment regimens were included in opaque sealed numbered envelopes and envelope with the lowest number was always used for consecutive patients |
Physician was blinded to PCT result, CT results in the control group were eventually
unblinded for the statistical analysis |
No |
Single-blind without further reported details |
No |
No |
In control group, clinicians were blinded
to the PCT levels, and results were faxed directly to the Clinical Informatics and Data
Management Unit. |
No |
No patient was lost to follow-up |
No patient was lost to follow-up |
No patient was lost to follow-up |
12 patients withdrew from the study,8 from PCT-group,3 from control-group. |
Almost 1 every 5 screened patients were excluded from the study |
6 patients withdrew from the study,4 from PCT-group,2 from control-group. |
Almost 1 every 6 screened patients were excluded from the study |
Limited reporting |
Limited reporting |
Limited reporting |
Extensive reporting |
Limited reporting |
Extensive reporting |
Limited reporting |
No serial determinations of PCT throughout the antibiotic course were programmed upfront in the design of the study |
No data on adequacy of antimicrobial therapy |
In control-group,the physicians were free to decide to continue or change the antibiotics on their own empiric |
"Algorithm overruling" in the PCT group
occurred in 6 of 31 (19%), The study investigators did not interfere with the duration of antibiotic therapy in control-group |
Physician in charge was always free to decide to continue or change the antibiotic regimen upon clinical judgement. |
Treating clinicians could overrule the algorithm as clinically
indicated. |
It is unclear if there was crossover between the two groups |
Low |
Low |
High |
High |
Low |
High |
Low |
Evidence synthesis
The total duration of antibiotic therapy
Eight RCTs (10, 12-15, 17, 20-21) in this Meta-analysis provided data on the duration of antibiotic therapy. Significant heterogeneity could be detected between these studies (Tau2=0.47; Chi2=182.58, df=7; P<0.00001; I2=96%). The combined estimate for the duration of antibiotic therapy based on the random-effects model showed statistically significant difference between the PCT and the routine practice group (2919 patients; REM; MD=-1.60; 95%CI, -2.14 to -1.06; Z=5.79; P<0.00001) [Figure 2].
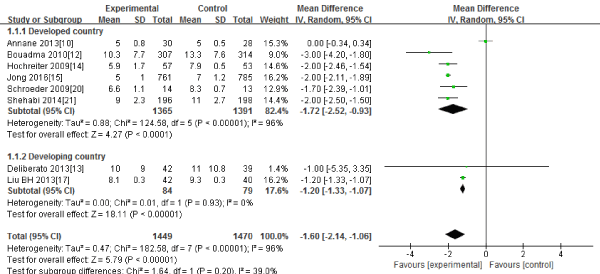
Figure 2: The duration of antibiotic therapy, days: forest plot showing the comparison of PCT-guided algorithms vs. routine practice. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the mean difference (MD) for all studies.
The subgroup analysis between the developed countries(10, 12, 14-15, 20-21) and the developing countries(13, 17) showed that the duration of antibiotic therapy had significant difference both in the developed countries(2756 patients; REM; MD=-1.72; 95%CI, -2.52 to -0.93; Z=4.27; P<0.00001) and the developing countries(163patients; REM; MD=-1.20; 95%CI, -1.33 to -1.07; Z=18.11; P<0.00001).
Sensitivity analysis including only the highest quality studies (10, 12-13, 15, 21) led to similar findings (2700 patients; REM; MD=-1.65; 95%CI, -2.78 to -0.51; Z=2.85; P=0.004).
Length of intensive care unit stay
Thirteen RCTs [10-22] in this Meta-analysis provided data on the length of ICU stay. Significant heterogeneity could be detected between these studies (Tau2=0.37; Chi2=75.61, df=12; P<0.00001; I2=84%). The combined estimate for the length of ICU stay based on the random-effects model showed no statistically significant difference between the PCT and the routine practice group (4728 patients; REM; MD=-0.23; 95%CI, -0.76 to 0.31; Z=0.83; P=0.41) [Figure 3].
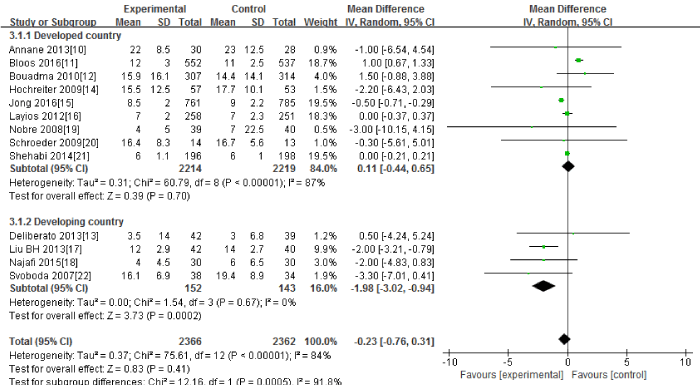
Figure 3: The length of ICU stay: forest plot showing the comparison of PCT-guided algorithms vs. routine practice. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the mean difference (MD) for all studies.
The subgroup analysis between the developed countries(10-12, 14-16, 19-21) and the developing countries [13,17,18,22] showed that the length of ICU stay had significant difference in the developing countries(295 patients; REM; MD=-1.98; 95%CI, -3.02 to -0.94; Z=3.73 P=0.0002) ,but no significant difference in the developed countries(4433patients; REM; MD=0.11; 95%CI, -0.44 to 0.65; Z=0.39; P=0.70).
Sensitivity analysis including only the highest quality studies (10-13, 15, 18-19, 21) led to similar findings (3928 patients; REM; MD=0.10; 95%CI, -0.58 to 0.78; Z=0.29; P=0.77).
Length of hospital stay
Nine RCTs (10-13, 15, 17-19, 21) in this Meta-analysis provided data on the length of hospital stay. Significant heterogeneity could be detected between these studies (Tau2=5.83; Chi2=162.36, df=8; P<0.00001; I2=95%). The combined estimate for the length of hospital stay based on the random-effects model showed no statistically significant difference between the PCT and the routine practice group (4010 patients; REM; MD=-1.43; 95%CI, -3.45 to 0.59; Z=1.39; P=0.17) [Figure 4].
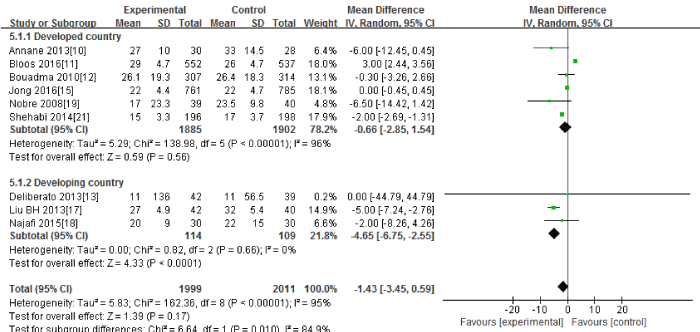
Figure 4: The length of hospital stay: forest plot showing the comparison of PCT-guided algorithms vs. routine practice. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the mean difference (MD) for all studies.
The subgroup analysis between the developed countries(10-12, 15, 19, 21) and the developing countries [13,17,18] showed that the length of hospital stay had significant difference in the developing countries(223 patients; REM; MD=-4.65; 95%CI, -6.75 to -2.55; Z=4.33; P<0.00001) ,but no significant difference in the developed countries(3787 patients; REM; MD=-0.66; 95%CI, -2.85 to 1.54; Z=0.59; P=0.56).
Sensitivity analysis including only the highest quality studies [10-19, 21] led to similar findings (3928 patients; REM; MD=-0.75; 95%CI, -2.84 to 1.35; Z=0.70; P=0.49).
28-day mortality
Six RCTs [11,12,15,17,19,22] in this Meta-analysis provided data on the 28-day mortality. No significant heterogeneity could be detected between these studies (Chi2=3.92, df=5; P=0.56; I2=0%). The combined estimate for the 28-day mortality based on the fixed-effects model showed statistically significant difference between the PCT and the routine practice group (3476 patients; FEM; MD=0.86; 95%CI, 0.76 to 0.97; Z=2.39; P=0.02) [Figure 5].
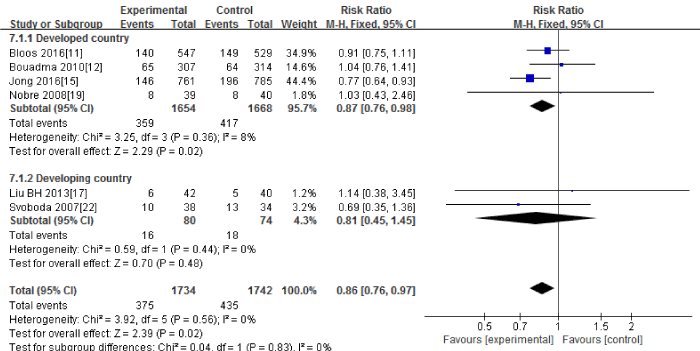
Figure 5: 28-day mortality: forest plot showing the comparison of PCT-guided algorithms vs. routine practice. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the mean difference (MD) for all studies.
The subgroup analysis between the developed countries [11,12,15,19] and the developing countries [17,22] showed that the 28-day mortality had significant difference in the developed countries (3322 patients; FEM; MD=0.87; 95%CI, 0.76 to 0.98; Z=2.29; P=0.02) , but no significant difference in the developing countries(154 patients; FEM; MD=0.81; 95%CI, 0.45 to 1.45; Z=0.70; P=0.48).
Sensitivity analysis including only the highest quality studies [11,12,15,19] led to similar findings (3322 patients; FEM; MD=0.87; 95%CI, 0.76 to 0.98; Z=2.29; P=0.02).
ICU mortality
Four RCTs [10,13,16,21] in this Meta-analysis provided data on the ICU mortality. No significant heterogeneity could be detected between these studies (Chi2=3.80, df=3; P=0.28; I2=21%). The combined estimate for the ICU mortality based on the fixed-effects model showed no statistically significant difference between the PCT and the routine practice group (1045 patients; FEM; MD=1.01; 95%CI, 0.77 to 1.33; Z=0.10; P=0.92) [Figure 6].
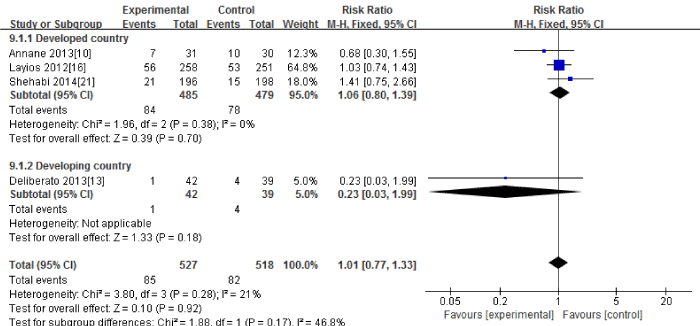
Figure 6: ICU mortality: forest plot showing the comparison of PCT-guided algorithms vs. routine practice. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the mean difference (MD) for all studies.
The subgroup analysis between the developed countries [10,16,21] and the developing countries [13] showed that the ICU mortality had no significant difference in the developed countries (964 patients; FEM; MD=1.06; 95%CI, 0.80 to 1.39; Z=0.39; P=0.70). There was only one study in the developing countries
Sensitivity analysis including only the highest quality studies [10,13,21] led to similar findings (536 patients; FEM; MD=0.99; 95%CI, 0.62 to 1.59; Z=0.04; P=0.97).
In hospital mortality
Seven RCTs [10,13,14,18-21] in this Meta-analysis provided data on the in-hospital mortality. No significant heterogeneity could be detected between these studies (Chi2=2.50, df=6; P=0.87; I2=0%). The combined estimate for the in-hospital mortality based on the fixed-effects model showed no statistically significant difference between the PCT and the routine practice group (1096 patients; FEM; MD=1.05; 95%CI, 0.80 to 1.38; Z=0.34; P=0.73) [Figure 7].
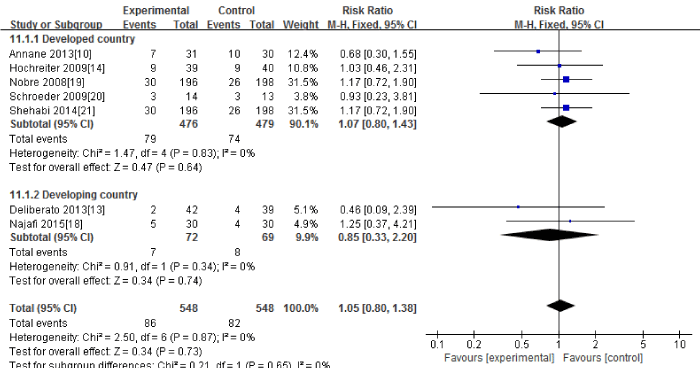
Figure 7: In hospital mortality: forest plot showing the comparison of PCT-guided algorithms vs. routine practice. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the mean difference (MD) for all studies.
The subgroup analysis between the developed countries [10,14,19-21] and the developing countries [13,18] showed that the in hospital mortality had no significant difference both in the developed countries (955 patients; FEM; MD=1.07; 95%CI, 0.80 to 1.43; Z=0.47; P=0.64) and the developing countries (141 patients; FEM; MD=0.85; 95%CI, 0.33 to 2.20; Z=0.34; P=0.74).
Sensitivity analysis including only the highest quality studies (10, 13, 18-19, 21) led to similar findings (675 patients; FEM; MD=1.00; 95%CI, 0.74 to 1.41; Z=0.00; P=1.00).
The rates of clinical cure
Three RCTs (17-19) in this Meta-analysis provided data on the clinical cure. No significant heterogeneity could be detected between these studies (Chi2=0.71, df=2; P=0.70; I2=0%). The combined estimate for the rates of clinical cure based on the fixed-effects model showed no statistically significant difference between the PCT and the routine practice group (221 patients; FEM; MD=0.98; 95%CI, 0.87 to 1.10; Z=0.33; P=0.74) [Figure 8].
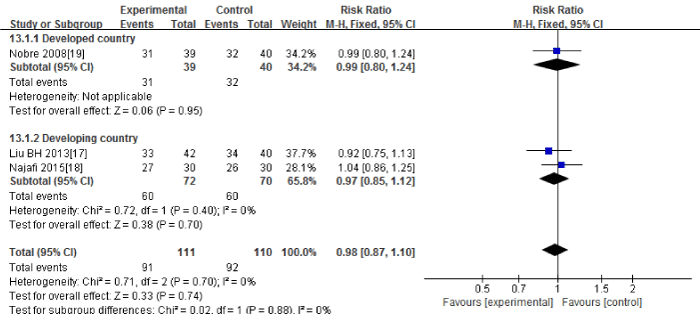
Figure 8: The rates of clinical cure: forest plot showing the comparison of PCT-guided algorithms vs. routine practice. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the mean difference (MD) for all studies.
The subgroup analysis between the developed countries [19] and the developing countries [17-18] showed that the rates of clinical cure had no significant difference in the developing countries (142 patients; FEM; MD=0.97; 95%CI, 0.85 to 1.12; Z=0.38; P=0.70). There was only one study in the developed countries.
Sensitivity analysis including only the highest quality studies [18-19] led to similar findings (139 patients; FEM; MD=1.01; 95%CI, 0.88 to 1.17; Z=0.18; P=0.85).
Publication bias
Thirteen studies [10-22] were included in this Meta-analysis. Assessment of publication bias using a funnel plot was presented in [Figure 9].
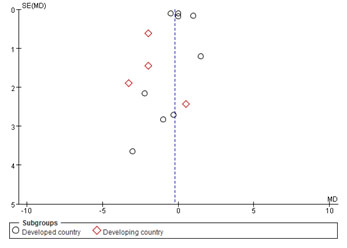
Figure 9: Funnel plot
Discussion
Our Meta-analysis included thirteen randomized controlled studies involving 4728 ICU septic patients. By synthesizing data from these studies, we found that PCT-guided algorithm decreased the total duration of antibiotic therapy which is in accordance with the former studies [23-25]. Furthermore, we also found that PCT-guided algorithm reduced the 28-day mortality of septic patients in ICU, which is different from the former studies [26-27]. These results were also retained in the sensitivity analysis. As antibiotic consumption and acquired antimicrobial resistance had been shown to be associated with increased mortality and health care costs [28], clinicians may be encouraged to tailor the antibiotic therapy of septic patients in ICU according to the guidance of PCT, rather than the empiric rules.
However, our findings do not show a significant difference between the PCT-guided algorithm and the routine practice regarding the length of ICU or hospital stay, respectively. Besides, the ICU mortality, in hospital mortality and rates of clinical cure were not different between the PCU-guided group and the routine practice group, which is in accordance with the research of Christ Crain, et al. [29]. This finding provides some reassurance that PCT-guided therapy is safe and will not compromise the septic patients’ clinical outcomes [30].
All studies in our Meta-analysis come from ten different countries. The developed countries include Switzerland, Germany, France, Belgium, Australian, Netherlands, the developing countries include Czech Republic, China, Brazil, Iran. The main finding of the subgroup showed that in the developed countries, The PCT-guided algorithm could decrease the total duration of antibiotic therapy and reduce the 28-day mortality of septic patients in ICU. While, in the developing countries, the PCT-guided algorithm could decrease the total duration of antibiotic therapy, the length of ICU and the length of hospital stay without reducing the 28-day mortality. Both in the developed countries and the developing countries, the PCT-guided algorithm may not improve the ICU mortality, the in hospital mortality and the rates of clinical cure.
This Meta-analysis has several limitations. First, not all included studies provided data on all outcomes. Second, the PCT-guided algorithms were very heterogeneous as far as the level of PCT chosen to make antibiotic therapy. Third, studies included in our Meta-analysis varied in study design and objectives.
In conclusion, the present available evidence seems to suggest that the implementation of PCT-guided algorithms for the antibiotic therapy of septic patients in ICU is associated with reduced the total antibiotic exposure and the 28-day mortality without the length of ICU and hospital stay, ICU mortality, in hospital mortality and rates of clinical cure. Further research is still necessary before the wide adoption of this strategy.
References
- Levy MM, Fink MP, Marshall JC (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31: 1250-1256. [Crossref]
- Dombrovskiy VY, Martin AA, Sunderram J (2007) Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003.Crit Care Med 35: 1244-1250. [Crossref]
- Martin GS, Mannino DM, Eaton S (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348: 1546-1554. [Crossref]
- Becker KL, Nyle´n ES, White JC (2004) Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: A journey from calcitonin back to its precursors. J Clin Endocrinol Metab 89: 1512-1525. [Crossref]
- Uzzan B, Cohen R, Nicolas P (2006) Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: A systematic review and meta-analysis. Crit Care Med 34: 1996-2003. [Crossref]
- Anna Prkno, Christina Wacker, Frank M Brunkhorst (2013) Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock - a systematic review and meta-analysis. Crit Care 17: 291.
- Petros Kopterides, Ilias I. Siempos, Iraklis Tsangaris (2010) Procalcitonin-guided algorithms of antibiotic therapy in the intensive care unit: A systematic review and meta-analysis of randomized controlled trials. Crit Care Med 38: 2229-2241. [Crossref]
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0).
- Higgins JP, Thompson SG, Deeks JJ (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557-560. [Crossref]
- Djillali Annane, Virginie Maxime, Jean Pierre Faller (2013) Procalcitonin levels to guide antibiotic therapy in adults with non-microbiologically proven apparent severe sepsis: a randomized controlled trial. BMJ Open 3: e002186. [Crossref]
- F Bloos, E Trips, A Nierhaus (2016) Effect of Sodium Selenite Administration and Procalcitonin-Guided Therapy on Mortality in Patients With Severe Sepsis or Septic Shock: A Randomized Clinical Trial. JAMA Intern Med 176: 1266-1276.
- L Bouadma, CE Luyt, F Tubach (2010) Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 375: 463-474.
- Rodrigo Octavio Deliberato, Alexandre R. Marra, Paula Rodrigues Sanches (2013) Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infec Dis 76: 266-271.
- Marcel Hochreiter, Thomas Köhler, Anna Maria Schweiger (2009) Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Critical Care 13: 83-89.
- Evelien de Jong, Jos A van Oers, Albertus Beishuizen (2016) Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 16: 819-827. [Crossref]
- Nathalie Layios, Bernard Lambermont, Jean-Luc Canivet (2012) Procalcitonin usefulness for the initiation of antibiotic treatment in intensive care unit patients. Crit Care Med 40: 2304-2309.
- Baohua, LI Haifeng, LEI Yu (2013) Clinical significance of dynamic monitoring of procalcitonin in guiding the use of antibiotics in patients with sepsis in ICU. Chin Crit Care Med 25: 690-693. [Crossref]
- Atabak Najafi, Ali Khodadadian, Mehdi Sanatkar (2015) The Comparison of Procalcitonin Guidance Administer Antibiotics with Empiric Antibiotic Therapy in Critically Ill Patients Admitted in Intensive Care Unit. Acta Medica Iranica 53: 562-567.
- Vandack Nobre, Stephan Harbarth, Jean-Daniel Graf (2008) Use of Procalcitonin to Shorten Antibiotic Treatment Duration in Septic Patients: A Randomized Trial. AM J RESP CRIT CARE 177: 498-505. [Crossref]
- Schroeder S, Hochreiter, Koehler T (2009) Procalcitonin (PCT)-guided algorithm reduces Length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of prospective randomized study. Langenbecks Arch Surg 394: 221-226.
- Yahya Shehabi, Martin Sterba, Peter Maxwell Garrett (2014) Procalcitonin Algorithm in Critically Ill Adults with Undifferentiated Infection or Suspected Sepsis: A Randomized Controlled Trial. AM J RESP CRIT CARE 190: 1102-1110.
- Petr Svoboda, Ilona Kantoroua, Peter Scheer (2007) Can Procalcitonin help us in timing of re-intervention in septic patients:after multiple trauma or major surgery? Hepato-Gastroenterol 54: 359-363. [Crossref]
- Schuetz P, Muller B, Christ-Crain M, Stolz D, Tamm M, et al. (2012) Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 8: 1297–1371. [Crossref]
- Emilio Maseda, Alejandro Suarez-de-la-Rica, Victor Anillo (2014) Procalcitonin-guided therapy may reduce length of antibiotic treatment in intensive care unit patients with secondary peritonitis: A multicenter retrospective study. Journal of Critical Care 30: 537-542. [Crossref]
- JA Carr (2015) Procalcitonin-guided antibiotic therapy for septic patients in the surgical intensive care unit. Journal of Intensive Care 3: 1-8.
- A Prkno, C Wacker, FM Brunkhorst (2013) Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock--a systematic review and meta-analysis. Crit Care 17: 291. [Crossref]
- JU Jensen, L Hein, B Lundgren (2013) Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomize trial. Crit Care Med 17: 291. [Crossref]
- Ahmad I (2009) Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis 49: 1175-1184.
- Christ-Crain M, Jaccard-Stolz D, Bingisser R (2004) Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: Cluster-randomised, single-blinded intervention trial. Lancet 363: 600-607. [Crossref]
- Schuetz P, Christ-Crain M, Thomann R (2009) ProHOSP Study Group: Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA 302: 1059-1066. [Crossref]









