Background: Cervical cancer is a high incidence cancer in India, although it is a preventable cancer. We proposed a public private participation (PPP) strategy between Private/Corporate Organizations and Non-governmental organizations for HPV vaccination and screening of cervical cancers.
Methods: The pilot project was conducted in Maharashtra State, India. The Private/Corporate Organizations with a Corporate Social Responsibility (CSR) Cell/Committee were identified for the project.
The HPV vaccination camp sites and schools were identified and confirmed. The bivalent HPV vaccine – Cervarix, was administered in two doses in the deltoid muscle, to socio-economically under privileged school girls, aged 11-15 years, from seven villages in Maharashtra.
The cervical cancer screening camp sites were identified, post identification and confirmation of the screening camps, HR-HPV was assayed in 8286 of 9353 recruited women. The women were 30-65 years old and residents of rural villages and urban slums in the state. HPV DNA PCR using the MY09 and MY11 primers, followed by nucleotide sequencing using Big Dye terminator kit and Genetic Analyzer ABI 3130XL system in HPV positive samples, or the Hybrid Capture 2 assay, were the methods of choice to detect HR-HPVs in the cervical smears.
Results: We administered 4008 total doses of the bivalent HPV vaccine in the school girls, and 82-100% compliance for the second dose of the vaccine was recorded. Mild adverse reactions included headache, weakness or fainting in 1-2% of the school girls.
The HPV DNA PCR assay with nucleotide sequencing and Hybrid Capture 2 assay demonstrated an average of 4.1% women were positive for HR-HPV with HPV16 being the most common infection.
Conclusions: Our study demonstrated the feasibility of the Public Private Participation approach with collaboration between Non-governmental organizations and corporate organizations, for prevention and control of cervical cancer.
Cervical Cancer, India, Human Papillomavirus, Screening, Vaccination
HPV: Human Papilloma Virus; HR-HPVs: High risk Human Papilloma viruses; PPP: Private Public Partnership; NGO: Non-Governmental Organization; CPAA: Cancer Patients Aid Association; CSR: Corporate Social Responsibility.
Cervical cancer ranks as the second most common cancer in females in India with annual age-standardized incidence rate (AAR) of 14.7 per 100,000 women, and 96,922 new cases and 60,078 deaths annually due to cervical cancer [1,2]. Oncogenic High Risk Human Papilloma viruses (HR-HPVs) are the etiologic agents of cervical cancers [3], and about 70-80% of cervical cancers globally are caused by HR-HPV16 and 18 [4]. A prolonged latent period of 10-30 years is observed between HR-HPV infection, precancerous lesions and cervical cancer [5].
Cervical cancer is a preventable cancer, with simple modalities of primary prevention of HPV vaccination, and secondary prevention through screening of cervical smears. HPV vaccines are safe, efficacious and licensed by regulatory authorities including Food and Drug administration, USA, several European Medicines Agency, and Drug Controller of India, for vaccinating school girls 9-18 years, and women >18-45 years. Three globally approved vaccines are the bivalent vaccine against HPV16/18 (Cervarix), quadrivalent vaccine against HPV6/11/16/18 (Gardasil), and nonavalent vaccine against HPV6/11/16/18/31/33/45/52/58 (Gardasil-9). Globally 270 million doses of the vaccines have been administered worldwide since 2007, with absence of severe adverse events to the participants [6]. The bivalent and quadrivalent vaccines are also available in India. Countries with high vaccination coverage have demonstrated 73–85% decrease in vaccine-type HPV prevalence and decline of 41–57% in high grade lesions among young women, and 60% reduction in cervical cancer cases and mortality in less than 10 years after implementation of HPV vaccination [7,8]. HPV vaccination has been adopted by 85% of high developed index countries in the world, although only a small number of low-to-middle income countries have introduced HPV vaccination due to lack of financial resources and infrastructure facilities [9]. Cavaljuga and colleagues in their extensive analysis on 57 studies from 64 countries concluded that HPV vaccination is cost-effective [10].
Secondary prevention of cervical cancer comprises organized screening of cervical smears using Papanicolaou test (Pap test) with high specificity (96%) but low sensitivity (28-53%); and HPV testing with high specificity (90.7%), and high sensitivity (>80%) for identification of precancerous lesions and cancer [11-13]. Further, the negative predictive value of HR-HPV test is 100% [14].
In India, besides the financial constraints and infrastructure limitations, the number of women in the cervical cancer susceptibility age group is large (469.1 million) [15]. Hence, these preventive measures are not affordable and accessible to the women. Our study proposed a public private partnership (PPP) between Non-Government Organizations (NGOs) and Corporate organizations for primary prevention through HPV vaccination and secondary prevention through HPV screening, in the socio-economically underprivileged women in rural regions and urban slums in the state of Maharashtra.
The study for HPV vaccination and screening was approved by the Institutional Review Board of Cancer Patients Aid Association [CPAA] [CPAA Ethics Committee Approval No. 2017/1 Project - HPV Vaccination and detection of high-risk oncogenic HPVs as a primary screening tool for risk to cervical cancer].
Primary Prevention by HR-HPV Vaccination
Identification of Corporate Partners
The collaborative partners in the project constituted our NGO – Cancer Patients Aid Association, and Corporate Organizations. In India, it is mandatory that every company having net worth of rupees five hundred crore or more, or turnover of rupees one thousand crore or more or a net profit of rupees five crore or more during any financial year, have to institute a Corporate Social Responsibility (CSR) Committee or Cell in their management. The purpose of the CSR Cell is philanthropy, donating to charity as a management concept, adopting ethical labor practices. Our organization team personally approached the CSR Cell of such Corporate Organizations with a written project and presented the project to the CSR Cell. On approval of the project by the CSR committee members, the project was sent to the Board of Directors of the company. Post discussion and approval of the project and the proposed budget, our NGO was informed, and the project was initiated. Quarterly reports were sent to the collaborating Corporation(s), and on completion of the project a final report along with audited utilization certificate for the expenses incurred was submitted.
HPV Vaccination:
The bivalent HPV vaccine Cervarix (Glaxo SmithKline Pharmaceuticals Ltd) was administered to school girls in rural districts in Maharashtra. The districts with Schools in the region certified by the State Education Board, were identified by our team in consultation with local social workers. The vaccination camps were organized by our NGO in concert with the social worker teams and their coordinators, volunteers, medical and paramedical staff. Our NGO (CPAA) procured the vaccines and transported this to the camp sites maintaining cold chain (2-8°C).
An initial interactive cervical cancer awareness session was conducted with the school girls/ parents/ guardians/ teachers/ administrators. The participants were registered, and necessary documentation was completed. Demographic data including age, standard or class of study, date of birth, profession and income of parents, and identification parameters including name, phone contact number and Aadhar Card (national identification card) details were noted for each student. Medical history included current or past disease(s), allergy and menstruation history, was recorded. Informed consent for voluntary participation was signed by the parent. The vaccine was administered in the deltoid muscle in the arm. Post vaccination the school girls were under observation for an hour by the medical staff. Adverse reactions were monitored and attended to by the medical staff. Relevant health records were updated and documented annually, and monitored by the class teacher, internal and external auditors.
HR-HPV Screening:
The target populations for HR-HPV testing were socio-economically under privileged women from rural regions and urban slums of Maharashtra and were in the 30-65 age group. Information campaigns were conducted in the rural and urban settlements with the aid of the local social workers and coordinators. We recruited 9353 females, and analyzed 8286 samples for cervical cancer screening, with DNA samples inadequate in 1067 (11.4%) samples. Age, marital status, number of children, medical history of gynecological diseases, hysterectomy, personal and family cancer history, tobacco and alcohol habits were noted. Those who had undergone hysterectomy or were pregnant were excluded from the study. The participants were directed for gynecologic examination and cervical sample collected.
DNA PCR and Genotyping
The digene HC2® DNA Collection Device (QIAGEN, Venlo, Netherlands) was used to collect cells from the exo-/endocervix and dislodged into Specimen Transport Medium. DNA was extracted using Invitrogen PureLink Mini KitTM, as per the instructions of the manufacturer (Invitrogen, Waltham, Massachusetts, USA). About 30% of the samples were analysed by HPV DNA PCR and Hybid Capture 2 was used for analysis of 70% samples. HPV DNA PCR was performed using MY09/MY11 primers, with beta globin as an internal control (Table 1). The HPV positive samples were outsourced for nucleotide sequencing using Big Dye terminator kit and Genetic Analyzer ABI 3130XL system (Applied Biosystems, Waltham, Massachusetts, USA).
Table 1: DNA PCR Primers – HPV and Internal Control
Sr. No. |
Primers |
Sequence 5' to 3' |
PCR Cycling Conditions |
1 |
Forward primer Internal Control Beta Globin |
CAA TGT ATC ATG CCT CTT TGC ACC |
Initial Denaturation: 950C/5 min
Cycles (40 cycles): Denaturation: 950C / 30 sec
Annealing: 600C / 30 sec Extension: 720C / 45 sec
Final Extension: 720C/7 min |
2 |
Reverse primer Internal Control Beta Globin |
GAG TCA AGG CTG AGA GAT GCA GGA |
3 |
Forward primer HPV MY09 |
CGT CCM ARR GGA WAC TGA TC |
Initial Denaturation: 950C / 5 min
Cycles (40 cycles):
Denaturation: 950C/ 30 sec
Annealing: 500C / 45 sec
Extension: 720C / 60 sec
Final Extension: 720C/7 min |
4 |
Reverse primer HPV MY11 |
GCM CAG GGM CAT AAY AAT GG |
The sequences of the primers for HPV DNA PCR are given in the Table 1 [11,12].
Hybrid Capture 2 Assay for detection of HR-HPVs
was used to detect 13 HR-HPVs - HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68, according to the manufacturer’s instructions (Digene, Gaithersburg, Maryland, USA).
HPV Vaccination
During the period from August 2018 to March 2021, 4008 doses were administered to 11-15-year school girls. The female population in the villages ranged from 597 to 23,914, with the 11-15-year-old school girls comprising about 30% of the total female population. An average of 92% compliance for the second dose in the various vaccination camps was recorded. Details of the vaccination camps and the camp sites are outlined in Table 2 and Figure 1, respectively. Minor adverse reactions included redness and swelling at the site of infection, headache, and fainting in 1-2% school girls. Quarterly reports were provided to our corporate partners, maintaining anonymity, confidentiality and privacy of the participants. Follow-up of the participants by local coordinators, class teachers, principal of the schools, and medical officers was discussed with the NGO project director on annual basis.
Figure 1: Representative Vaccination camp locations in Maharashtra
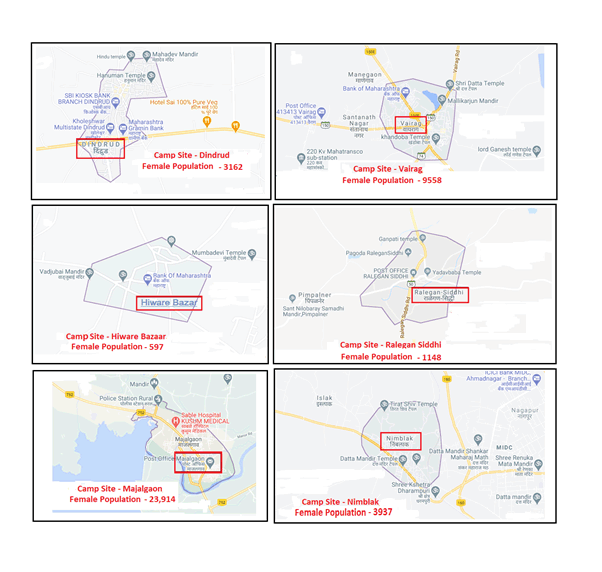
Legend to Figure 1: Representative Vaccination Camp locations in Maharashtra, highlighting the female population of the village.
Table 2: HPV Vaccination Details.
Camp Site -
Village or Taluka, District |
Dose One Numbers Vaccinated |
Second Dose Numbers Vaccinated (% Compliance) |
Total Vaccine Doses Administered |
Total Female
Population |
Dindrud, Beed |
432 |
357 (82%) |
789 |
3162 |
Vairag, Solapur |
372 |
369 (99%) |
741 |
9558 |
Hiware Bazaar, Ahmednagar |
202 |
133 (67%) |
335 |
597 |
Ralegan Siddhi, Ahmednagar |
346 |
330 (95%) |
676 |
1148 |
Majalgaon, Beed |
539 |
539 (100%) |
1078 |
23,914 |
Nimblak, Ahmednagar |
189 |
189
(To be given in June 2021) |
189 (378 total with 189 second doses to be given) |
3937 |
Usgaon, Palghar |
200 |
200 (To be given in October 2021) |
200 (Total 400 with 200 second doses to be given) |
1379 |
Total |
2111 |
1948 (92%) |
4008
(Total 4397 with 389 to be given) |
39,758 |
HR-HPV Screening
Women for cervical cancer screening were recruited in 120 camps and constituted 9353 women, between June 2012 to March 2020. About 11.4% (n=1067) DNA samples were inadequate (Quantity not Sufficient) for the HPV tests as indicated by absence of amplification of the control beta globin gene in the PCR assay. Thus about 88.6% (n=8286) women were examined for HPVs (Table 3). After electrophoresis of the PCR products on 1.5% agarose gels, HPV positivity was indicated by presence of a distinct 450 bp HPV specific amplimer, whereas absence of the 450 bp amplimer indicated HPV negativity (Figure 2). The HPV specific amplimers were subjected to DNA sequencing and the HPV types were identified using NCBI BLAST search.
Figure 2: Agarose gel electrophoresis of HPV DNA PCR
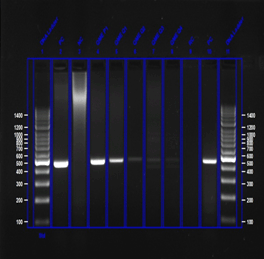
Legend to Figure 2: Agarose Gel (1.5%) Electrophoresis for HPV DNA PCR indicated by a distinct 450 bp fragment; 450 bp HPV specific fragment is not observed in HPV negative samples. Molecular weight markers are loaded and marked on the RHS and LHS lanes.
Table 3: Cervical Cancer Screening by HPV detection
No |
Vaccination dates |
Camps conducted |
Samples Collected |
QNS |
Samples Tested |
HPV Positive |
HPV Negative |
1 |
June 2012 - May 2013 (Urban slums) |
21 |
1000 |
- |
1000 |
27 (2.7%) |
973 |
2 |
July 2013 - February 2017 (Urban slums) |
42 |
3918 |
675 |
3243 |
124 (3.8%) |
3119 |
3 |
December 2017 - September 2018 (Urban slums) |
25 |
2197 |
392 |
1805 |
59 (3.3%) |
1746 |
4 |
October 2018 - March 2020 (Rural regions) |
32 |
2238
(100 samples were from professional sex workers) |
- |
2238 |
131(5.95%) |
2107 |
Total |
120 |
9353 |
1067 (11.4%) |
8286 |
341 (4.1%) |
7945 (95.9%) |
The virus was identified in 341 (4.1%) women and 7945 (95.9%) were HPV negative. Samples with weak bands were repeated and the PCR product subjected to nucleotide sequencing to confirm the presence of HPV.
The HR-HPV identified in the women, by nucleotide sequencing, were HPV16 (54.5%), HPV66 (23%), HPV58 (13%), HPV18 (6.5%) and HPV31 (3%).
The main finding of the study was application of public private partnership strategy towards primary prevention by HPV vaccination and secondary prevention by HPV screening of cervical cancer in India. The financial resources provided by Corporate Organizations and the NGOs providing the technical and functionally operative aspects towards primary and secondary prevention of cervical cancer provided a feasible strategy. The strategy could be replicated in several low-to-middle income countries. Further, policies to support HPV vaccination and cervical screening from the Government Union and State Health Ministries, and financial support from private organizations for scale up and sustainability is recommended and will result in reduction in annual incidence of cervical cancer, early detection and decrease in mortality.
Our results on HPV positivity are in concordance with several reports reported earlier for HPV positivity of 4.9 to 8% in Indian women, validating the methodology [16-18]. Besides HPV testing has been validated in self-collected samples in women in far-off locations as well as in women with social inhibitions with respect to collection of cervical smears by medical/paramedical staff [17-19]. Sankaranarayan and co-workers conducted a study involving 131,746 healthy women, and concluded that in low resource setting, a single round of HPV screening reduced the potential incidence and mortality from cervical cancer [14].
The WHO Director-General has issued a call for action to eliminate cervical cancer as a public health problem worldwide, placing it on a fast track [20,21]. Girls-only HPV vaccination is predicted to reduce the median age-standardized cervical cancer incidence from 19·8 to 2·1 cases per 100,000 women-years over the next century, leading to 89·4% reduction and prevention of 61·0 million cases of cervical cancer [22]. The threshold of four to ten cases per 100,000 women-years is predicted to result in elimination of 60% cervical cancers. Addition of twice lifetime screening, will further reduce the incidence to 0·7 (0·6–1·6) cases per 100,000 women-years, culminating in 96·7% reduction, and prevention of an additional 12·1 million cervical cancer cases. WHO states the necessity of a 90:70:90 policy with 90% school girls HPV vaccinated, 70% women screened for cervical cancer, and 90% women with cervical cancers treated early for the cancer [20].
There is a dire need for educational intervention and awareness programs to augment HPV immunization programs, screening for cervical cancer, and early treatment, for prevention and control of cervical cancer in India. The current study explored the feasibility of conducting HPV vaccination as primary prevention and an opportunistic population screening using a PPP strategy with NGOs and Corporate Organizations as partners, in a community based cervical cancer prevention program towards elimination of cervical cancer in women.
Our study demonstrated the feasibility of using the Public Private Partnership approach with collaboration between Non-governmental organizations and corporate organizations. Almost 85% of cervical cancers incidence and high mortality due to cervical cancers occurs in low-to-middle income countries and is preventable by simple means of HPV vaccination and cervical screening.
The members of Institutional Ethics Committee: Dr. Hemant Tongaonkar – Chairman, Dr. Dhananjaya Saranath - Member Secretary, Mr. Y. K. Sapru, Dr. Sumeet Shah, Dr. Shubhangi Parker, Mrs. Gulshan Hodiwalla, Dr. Shubha Maudgal and Mrs. Neeta More. Leave of absence was granted by the Chairman to Dr. Bharat Agarwal, Dr. Archana Swami, Dr. Indu Nair, & Ms. Anita Vesuwala.
Not applicable
The authors declare - No Conflict of Interest and competing interests by the Corporate Organization.
The supporting funding was from Aditya Birla Finance, Mumbai, for HPV Vaccination and there were no additional sources of funding. For HPV screening several Corporate Organizations including Deutsche Bank AG India, Thyssenkrupp Industrial Solutions (India) Pvt Ltd, Premlata Vandravan Shah Charities, Rotary Club of Bombay - Hanging Gardens contributed and have been declared. There is no role of the funding bodies in the design of the study, sample collection, analysis, interpretation of data and writing of the manuscript.
DS conceived and designed the framework of the study, organized the study parameters for HPV vaccination and screening, provided scientific guidance throughout the project and finalized the manuscript. BPS performed, supervised and monitored the experimental work. DS and BPS reviewed scientific literature, and wrote the draft manuscript, and approved the final manuscript.
We acknowledge the financial support from our primary corporate donor Aditya Birla Finance, Mumbai. Additional funds for Screening Camps and tests were provided by Thyssenkrupp Industrial Solutions (India), Pvt Ltd., IndoStar Capital Finance, JMC Projects India, Financial Software & Systems, Prestige Foundation, Asahi India Glass, Deutsche Bank, Rotarians Mrs. Kavita Kulkarni, Mr. Dilip Shah and Mr. Digant Shah and Premlata Vandravan shah Charities. We are indebted to the medical, social and political leaders, local coordinators and volunteers for smooth functioning of the camps. The government schools in the rural areas of Ralegan Siddhi, Dindrud, Vairag, Hiware Bazar, Majalgaon, Usgaon and Nimblak are acknowledged for their immense cooperation and participation.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. (2018) Global cancer statistics: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394-424. [Crossref]
- Bruni L, Albero G, Serrano B, Mena M, Gómez D, et al. (2019) ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in India.
- Bosch FX, Manos MM, Muñoz N, Sherman M, Jansen AM, et al. (1995) Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst 87: 796-802. [Crossref]
- Castellsagué X (2008) Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol 110: S4-S7. [Crossref]
- Datta P, Bhatla N, Pandey RM, Dar L, Patro R, et al. (2012) Type-specific incidence and persistence of HPV infection among young women: a prospective study in North India. Asian Pac J Cancer Prev 13: 1019–1024. [Crossref]
- World Health Organization (2017) Information Sheet Observed Rate of Vaccine Reactions Human Papilloma Virus Vaccine.
- Drolet M, Bénard É, Boily MC, Ali H, Baandrup L, et al. (2015) Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 15: 565-580. [Crossref]
- Chauhan AS, Prinja S, Srinivasan R, Rai B, Malliga JS, et al. (2020) Cost effectiveness of strategies for cervical cancer prevention in India. PLoS one 15: e0238291. [Crossref]
- Foss HS, Oldervoll A, Fretheim A, Glenton C, Lewin S (2019) Communication around HPV vaccination for adolescents in low-and middle-income countries: a systematic scoping overview of systematic reviews. Syst Rev 8: 190-205. [Crossref]
- Čavaljuga S, Ćubro H, Izetbegović S (2013) Human papilloma virus vaccination – a systematic review of cost-effectiveness analyses. South Eastern Eur Health Sci J 3: 159-168.
- Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, et al. (2006) Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Intl J Cancer 119: 1095-1101. [Crossref]
- Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, et al. (2014) Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet 383: 524-532. [Crossref]
- Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, et al. (2009) HPV screening for cervical cancer in rural India. New Eng J Med 360: 1385-1394. [Crossref]
- Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, et al. (2007) Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. New Eng J Med 357: 1579-1588. [Crossref]
- Hussain S, Nasare V, Kumari M, Sharma S, Khan MA, et al. (2014) Perception of human papillomavirus infection, cervical cancer and HPV vaccination in North Indian population. PLoS One 9: e112861. [Crossref]
- Mittal S, Mandal R, Banerjee D, Das P, Ghosh I, et al. (2016) HPV detection-based cervical cancer screening program in low-resource setting: lessons learnt from a community-based demonstration project in India. Cancer Causes & Control 27: 351-358. [Crossref]
- Peedicayil A, Abraham P, Prasad J, Jeyaseelan L, Abraham S, et al. (2016) Community Prevalence of Human Papillomavirus by Self-Collected Samples in South India. Indian J Gynecol Oncol 14: 16-20.
- Cuzick J, Beverley E, Ho L, Terry G, Sapper H, et al. (1999) HPV testing in primary screening of older women. Br J Cancer 81: 554-558. [Crossref]
- Bhatla N, Dar L, Patro AR, Kumar P, Kriplani A, et al. (2009) Can human papillomavirus DNA testing of self-collected vaginal samples compare with physician-collected cervical samples and cytology for cervical cancer screening in developing countries? Cancer Epidemiol 33: 446-450. [Crossref]
- World Health organization (2018) Director-General of the World Health Organization announced a global call to action towards the elimination of cervical cancer.
- World Health organization (2020) World Health Assembly adopts global strategy to accelerate cervical cancer elimination.
- Gravitt PE, Belinson JL, Salmeron J, Shah KV (2011) Looking ahead: A case for human papillomavirus testing of self‐sampled vaginal specimens as a cervical cancer screening strategy. Int J Cancer 129: 517-527. [Crossref]
Editorial Information
Editor-in-Chief
Article Type
Research Article
Publication history
Received date: April 20, 2021
Accepted date: May 05, 2021
Published date: May 15, 2021
Copyright
©2021 Saranath D. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Sharma B, Saranath D (2021) Primary and secondary prevention of cervical cancer in Indian women using a public private participation approach: results of a pilot program. Prev Med Commun Health 4: 10.15761/PMCH.1000155.


