Staphylococci can infect both mammals and humans; they are one of the most common bacterial pathogens and one of the most important factors for nosocomial infections. Therefore, the purpose of this study was to compare human and animal samples in relation to Staphylococci and to examine their resistance to antibiotics in Thessaly, Greece.
Method: A total of 344 samples were collected; 74 from humans and 270 from domestic animals (cats, dogs, goats, horses, sheep). Sampling was performed using cotton swabs. All Staphylococci were isolated, identified and tested for their susceptibility to various antimicrobial agents using an automated system.
Result: The majority of the samples collected were positive to a bacterium. The bacterium, which was isolated was mainly Staphylococcus sciuri. The isolates of S. aureus were detected in the human samples, as well as in the samples from dogs, and horses. Regarding the resistance to antibiotics, the highest rates were recorded against penicillin. In conclusion, humans, domestic animals and people who are taking care of them carried S.aureus.
The bacterium, which was isolated to a greater extent was Staphylococcus sciuri (12,2%). A really high percentage of Staphylococcus spp was found in human and in dog. Thus, we can conclude that there is a close contact between human and dog which allows the transmission of Staphylococcus.spp between them. The isolates of S. aureus were detected only in the samples of the human, dog, horse. Regarding the resistance to antibiotics, the highest rates were recorded against penicillin. The resistant rate of cat and dog to penicillin was the highest recorded (100%). The resistance rate of goat was also remarkable (20%). Moreover, pig, was resistant to all antibiotics apart from kanamycin, in which it was susceptible. Most of these animals’ owners were asked and told that the animals had been given antibiotics at the moment in which the samples were taken or even earlier. These high rates of resistance, therefore, may occur due to the excessive use of penicillin that had been given by vets as a therapy to these animals. S. aureus isolates exhibited resistance to oxacillin, cefoxitin and penicillin.
Until now, 47 species of bacteria belonging to the genus Staphylococcus have been identified, which can affect both humans and other kinds of mammals. The bacteria isolated in most cases from humans are S.aureus and Staphylococcus epidermidis [1-3]. S.aureus is the most pathogenic of the Staphylococcus genus [4,5]. It is characterized by its high resistance to antibiotics [6,7].
Bacteria of the Staphylococcus genus can also cause food poisoning, and S. aureus is the major factor. S. aureus food poisoning incidents are the most common food-borne diseases worldwide [8,9]. The aim of this research is to examine the nasal carriage of S.aureus in domestic animals and the people involved in their care in Thessaly, Greece
Sample collection and S. aureus identification
The survey was conducted in 2016 on a total of 344 samples, 74 of which were from humans and the remaining 270 from domestic animals such as cats, dogs, goats, horses and sheep. All samples were collected from houses that bred and kept domesticated animals and from the people who were taking care of them in Thessaly, Greece.
Sampling was performed using cotton swabs, while samples were collected from the participants' nasal cavity. The laboratory experiment was performed in the Microbiology Department of the University Hospital of Thessaly, Larissa.
The swab of each coating was initially inoculated into fresh nutrient broth (Tryptic Soy Broth) at 37°C for 24 hours in order to enrich the sample. A new cultivation of the broth in blood agar containing 5% sheep blood followed. From each culture, one or more colonies with different morphology (size, colour, haemolysis etc) were sub-cultured and were collected for further analysis.
Gram staining and catalase production was performed in all bacteria isolates. Gram-positive cocci, catalase-positive were selected. Identification to species level and antibiotic susceptibility testing was performed using an automated system.
A total of 196 out of 344 samples (57.0%) were positive for Staphylococcus spp. 94.6% of horse samples studied were positive to one or more different staphylococcal species, as well as 60.8% of human samples (Table 1). The most common isolate which was identified was Staphylococcus sciuri (12.2%), followed by S. aureus and Staphylococcus lentus (5.6% for each other) (Table 2). S. intermedius was found in a higher percentage in cats, S. aureus in dogs, humans and horses, S. lentus in goats and S. sciuri in sheep (Table 3).
Table 1. Number of samples studied and positive samples to Staphylococcus spp
|
|
Number
of samples
|
Number
of positive
samples
|
Rate
of positive
samples
|
|
Human
|
74
|
45
|
60,8
|
|
Sheep
|
117
|
40
|
34,2
|
|
Goat
|
49
|
42
|
85,7
|
|
Dog
|
33
|
10
|
30,3
|
|
Cat
|
22
|
14
|
63,6
|
|
Horse
|
37
|
35
|
94,6
|
|
Pig
|
37
|
10
|
27,0
|
|
Total
|
344
|
196
|
57,0
|
Table 2. Data regarding the positive samples to Staphylococcus spp
|
|
n
|
%
|
|
Origin
|
Cat
|
14
|
7,1
|
|
Dog
|
10
|
5,1
|
|
Goat
|
42
|
21,4
|
|
Horse
|
35
|
17,9
|
|
Human
|
45
|
23,0
|
|
Pig
|
10
|
5,1
|
|
Sheep
|
40
|
20,4
|
|
Catalase
|
No
|
None
|
None
|
|
Yes
|
195
|
100,0
|
|
Coagulase
|
No
|
100
|
52,9
|
|
Yes
|
89
|
47,1
|
|
Identification
|
Not found
|
129
|
65,8
|
|
Aerococcus viridans
|
1
|
0,5
|
|
CoNS
|
6
|
3,1
|
|
Kocuria kristinae
|
1
|
0,5
|
|
S. aureus
|
11
|
5,6
|
|
S. epidermidis
|
1
|
0,5
|
|
S. gallinarum
|
3
|
1,5
|
|
S. lentus
|
11
|
5,6
|
|
S. sciuri
|
24
|
12,2
|
|
S. simulans
|
1
|
0,5
|
|
S. xylosus
|
5
|
2,6
|
|
S.intermedius
|
3
|
1,5
|
Τable 3. Percentages of positive coagulase samples and identification of samples depending on their origin
|
|
Origin
|
P Fisher's exact test
|
|
Cat
|
Dog
|
Goat
|
Horse
|
Human
|
Pig
|
Sheep
|
|
n (%)
|
n (%)
|
n (%)
|
n(%)
|
n (%)
|
n(%)
|
n(%)
|
|
Coagulase
|
No
|
3 (30,0)
|
5 (55,6)
|
17 (40,5)
|
27 (77,1)
|
25 (58,1)
|
10 (100)
|
13 (32,5)
|
<0,001
|
|
Yes
|
7 (70,0)
|
4 (44,4)
|
25 (59,5)
|
8 (22,9)
|
18 (41,9)
|
None
|
27 (67,5)
|
|
Identification
|
Not found
|
10 (71,4)
|
8 (80)
|
25 (59,5)
|
33 (94,3)
|
27 (60)
|
9 (90)
|
17 (42,5)
|
<0,001
|
|
Aerococcus viridans
|
None
|
None
|
None
|
None
|
1 (2,2)
|
None
|
None
|
|
CoNS
|
None
|
None
|
None
|
None
|
6 (13,3)
|
None
|
None
|
|
Kocuria kristinae
|
None
|
None
|
None
|
1 (2,9)
|
None
|
None
|
None
|
|
S. aureus
|
None
|
2 (20)
|
None
|
1 (2,9)
|
8 (17,8)
|
None
|
None
|
|
S. epidermidis
|
None
|
None
|
None
|
None
|
1 (2,2)
|
None
|
None
|
|
S. gallinarum
|
1 (7,1)
|
None
|
1 (2,4)
|
None
|
None
|
None
|
1 (2,5)
|
|
S. lentus
|
None
|
None
|
8 (19)
|
None
|
1 (2,2)
|
1 (10)
|
1 (2,5)
|
|
S. sciuri
|
None
|
None
|
3 (7,1)
|
None
|
1 (2,2)
|
None
|
20 (50)
|
|
S. simulans
|
None
|
None
|
1 (2,4)
|
None
|
None
|
None
|
None
|
|
S. xylosus
|
None
|
None
|
4 (9,5)
|
None
|
None
|
None
|
1 (2,5)
|
|
S.intermedius
|
3 (21,4)
|
None
|
None
|
None
|
None
|
None
|
None
|
A major difference was demonstrated in the rates of susceptibility to clindamycin, fusidic acid, penicillin, cefoxitin and tetracycline. In particular, in sheep and pigs the rates of sensitivity to clindamycin were the lowest ones, while the highest ones were demonstrated to cats, dogs and horses (100%). Similarly, in sheep and pigs the rates of susceptibility to fusidic acid, were the lowest ones, whereas the highest ones were demonstrated to cats, dogs and horses (100%). Moreover, the goats and the horses were more susceptible to penicillin, the pigs where less susceptible to cefoxitin and to tetracycline (Table 4).
Table 4. Resistance of samples to different antibiotics, depending on their origin
|
|
Origin
|
P Fisher's exact test
|
|
Cat
|
Dog
|
Goat
|
Horse
|
Human
|
Pig
|
Sheep
|
|
n (%)
|
n (%)
|
n (%)
|
n (%)
|
n (%)
|
n (%)
|
n (%)
|
|
Erythromycin
|
Susceptibility
|
3 (100)
|
2 (100)
|
13 (65)
|
2 (100)
|
6 (75)
|
None
|
21 (95,5)
|
0,130
|
|
Intermediate
|
None
|
None
|
1 (5)
|
None
|
1 (12,5)
|
None
|
None
|
|
|
Resistance
|
None
|
None
|
6 (30)
|
None
|
1 (12,5)
|
1 (100)
|
1 (4,5)
|
|
|
Clindamycin
|
Susceptibility
|
3 (100)
|
2 (100)
|
13 (68,4)
|
2 (100)
|
7 (87,5)
|
None
|
4 (19)
|
0,001
|
|
Intermediate
|
None
|
None
|
5 (26,3)
|
None
|
1 (12,5)
|
None
|
15 (71,4)
|
|
|
Resistance
|
None
|
None
|
1 (5,3)
|
None
|
None
|
1 (100)
|
2 (9,5)
|
|
|
Fusidic acid
|
Susceptibility
|
3 (100)
|
2 (100)
|
13 (65)
|
2 (100)
|
6 (75)
|
None
|
2 (9,1)
|
<0,001
|
|
Intermediate
|
None
|
None
|
6 (30)
|
None
|
2 (25)
|
1 (100)
|
18 (81,8)
|
|
|
Resistance
|
None
|
None
|
1 (5)
|
None
|
None
|
None
|
2 (9,1)
|
|
|
Penicillin
|
Susceptibility
|
None
|
None
|
16 (80)
|
2 (100)
|
5 (62,5)
|
None
|
4 (18,2)
|
<0,001
|
|
Intermediate
|
None
|
0 (0)
|
0 (0)
|
None
|
None
|
None
|
None
|
|
|
Resistance
|
3 (100)
|
2 (100)
|
4 (20)
|
None
|
3 (37,5)
|
1 (100)
|
18 (81,8)
|
|
|
Oxacillin
|
Susceptibility
|
3 (100)
|
2 (100)
|
20 (100)
|
2 (100)
|
6 (75)
|
None
|
19 (86,4)
|
0,074
|
|
Intermediate
|
None
|
None
|
None
|
None
|
None
|
None
|
None
|
|
|
Resistance
|
None
|
None
|
None
|
None
|
2 (25)
|
1 (100)
|
3 (13,6)
|
|
|
Cefoxitin
|
Susceptibility
|
3 (100)
|
2 (100)
|
20 (100)
|
2 (100)
|
6 (75)
|
none
|
21 (100)
|
0,009
|
|
Intermediate
|
None
|
None
|
None
|
None
|
None
|
None
|
None
|
|
|
Resistance
|
None
|
None
|
None
|
None
|
2 (25)
|
1 (100)
|
None
|
|
|
Tetracycline
|
Susceptibility
|
1 (33,3)
|
2 (100)
|
13 (65)
|
2 (100)
|
7 (87,5)
|
None
|
21 (95,5)
|
0,015
|
|
Intermediate
|
None
|
None
|
None
|
None
|
None
|
None
|
None
|
|
|
Resistance
|
2 (66,7)
|
None
|
7 (35)
|
None
|
1 (12,5)
|
1 (100)
|
1 (4,5)
|
|
|
Kanamycin
|
Susceptibility
|
1 (100)
|
1 (100)
|
5 (100)
|
2 (100)
|
5 (100)
|
1 (100)
|
3 (100)
|
-*
|
|
Intermediate
|
None
|
None
|
None
|
None
|
None
|
None
|
None
|
|
|
Resistance
|
None
|
None
|
None
|
None
|
None
|
None
|
None
|
|
*not calculated
S. sciuri: All the samples of S. sciuri, that were examined, were susceptible to cefoxitin and tetracycline. Also, 91.3% of the samples were susceptible to erythromycin, 9.1% to clindamycin, none of them to fusidic acid, 8.7% to penicillin and 87.0% to oxacillin.
S. aureus: All S.aureus samples were susceptible to erythromycin, clindamycin, fusidic acid, tetracycline and kanamycin. Half of the samples were resistant to penicillin and 75% of the samples were susceptible to oxacillin and cefoxitin.
S.epidermidis: The S. epidermidis sample was susceptible to clindamycin, penicillin, oxacillin, cefoxitin and tetracycline.
S.lentus: All S. lentus samples were susceptible to oxacillin and cefoxitin. Moreover, 60.0% of the samples were susceptible to erythromycin, 66.7% to clindamycin, 90.0% to fusidic acid, 80.0% to penicillin and tetracycline.
S. intermedius: All S. intermedius samples tested were susceptible to erythromycin, clindamycin, fusidic acid, oxacillin, cefoxitin and kanamycin. Finally, 33.3% of the samples were susceptible to tetracycline and all samples were resistant to penicillin.
S.xylosus:All samples of Staphylococcus xylosus tested were susceptible to clindamycin, penicillin, oxacillin and cefoxitin.
S.simulans:The Staphylococcus simulans sample was susceptible to all antibiotics apart from kanamycin in which it was not tested (Table 4-12).
Table 5.Rates of contaminated samples per organism by S. aureus. Comparison with other surveys
|
Research
|
Dog
|
Human
|
Cat
|
Horse
|
|
Current Research/ Greece
|
20%
|
17,8%
|
0%
|
2,9%
|
|
Faires/Canada [13]
|
8,3%
|
18%
|
10%
|
-
|
|
Hanselman/ -Canada [14]
|
68%
|
27%
|
57%
|
-
|
|
Drougka/
Greece [15]
|
37%
|
38,9%
|
30%
|
-
|
|
Weese/
Canada [16]
|
-
|
13%
|
-
|
4,7%
|
|
Gómez-Sanzetal/Spain [12]
|
9,3%
|
41,8%
|
25%
|
-
|
|
El-Jakee/Egypt [17]
|
16%
|
20%
|
-
|
-
|
|
Vincze/ Germany [18]
|
5,8%
|
-
|
12,2%
|
22,2%
|
|
Abdel-moein al/Egypt [19]
|
4,5%
|
3,6%
|
0%
|
-
|
Table 6. Resistance of S.lentus samples to different antibiotics (n=11)
|
|
Susceptibility
|
Intermediate
|
Resistance
|
|
|
n (%)
|
n (%)
|
n (%)
|
|
Erythromycin
|
6 (60,0)
|
None
|
4 (40,0)
|
|
Clindamycin
|
6 (66,7)
|
3 (33,3)
|
None
|
|
Fusidic acid
|
9 (90,0)
|
1 (10)
|
None
|
|
Penicillin
|
8 (80,0)
|
None
|
2 (20,0)
|
|
Oxacillin
|
10 (100,0)
|
None
|
None
|
|
Cefoxitin
|
10 (100,0)
|
None
|
None
|
|
Tetracycline
|
8 (80,0)
|
None
|
2 (20,0)
|
|
Kanamycin
|
None
|
None
|
None
|
Table 7. Resistance of S.epidermidids samples to different antibiotics(n=1)
|
|
Susceptibility
|
Intermediate
|
Resistance
|
|
|
n (%)
|
n (%)
|
n (%)
|
|
Erythromycin
|
None
|
1 (100,0)
|
None
|
|
Clindamycin
|
1 (100,0)
|
None
|
None
|
|
Fusidic acid
|
None
|
1 (100,0)
|
None
|
|
Penicillin
|
1 (100,0)
|
None
|
None
|
|
Oxacillin
|
1 (100,0)
|
None
|
None
|
|
Cefocitin
|
1 (100,0)
|
None
|
None
|
|
Tetracycline
|
1 (100,0)
|
None
|
None
|
|
Kanamycin
|
None
|
None
|
None
|
Table 8. Resistance of S.aureus samples to different antibiotics(n=11)
|
|
Susceptibility
|
Intermediate
|
Resistance
|
|
|
n (%)
|
n (%)
|
n (%)
|
|
Erythromycin
|
8 (100,0)
|
None
|
None
|
|
Clindamycin
|
8 (100,0)
|
None
|
None
|
|
Fusidic acid
|
8 (100,0)
|
None
|
0 (0,0)
|
|
Penicillin
|
4 (50,0)
|
None
|
4 (50,0)
|
|
Oxacillin
|
6 (75,0)
|
None
|
2 (25,0)
|
|
Cefoxitin
|
6 (75,0)
|
None
|
2 (25,0)
|
|
Tetracycline
|
8 (100,0)
|
None
|
None
|
|
Kanamycin
|
7 (100,0)
|
None
|
None
|
Table 9. Resistance of S.sciuri samples to different antibiotics (n=24)
|
|
Susceptibility
|
Intermediate
|
Resistance
|
|
|
n (%)
|
n (%)
|
n (%)
|
|
Erythromycin
|
21 (91,3)
|
1 (4,3)
|
1 (4,3)
|
|
Clindamycin
|
2 (9,1)
|
18 (81,8)
|
2 (9,1)
|
|
Fusidic acid
|
None
|
21 (91,3)
|
2 (8,7)
|
|
Penicillin
|
2 (8,7)
|
None
|
21 (91,3)
|
|
Oxacillin
|
20 (87,0)
|
None
|
3 (13)
|
|
Cefoxitin
|
22 (100,0)
|
None
|
None
|
|
Tetracycline
|
23 (100,0)
|
None
|
None
|
|
Kanamycin
|
2 (100,0)
|
None
|
None
|
Table 10. Resistance of S.simulans samples to different antibiotics (n=1)
|
|
Susceptibility
|
Intermediate
|
Resistance
|
|
|
n (%)
|
n (%)
|
n (%)
|
|
Erythromycin
|
1 (100,0)
|
None
|
None
|
|
Clindamycin
|
1 (100,0)
|
None
|
None
|
|
Fusidic acid
|
1 (100,0)
|
None
|
None
|
|
Penicillin
|
1 (100,0)
|
None
|
None
|
|
Oxacillin
|
1 (100,0)
|
None
|
None
|
|
Cefoxitin
|
1 (100,0)
|
None
|
None
|
|
Tetracycline
|
1 (100,0)
|
None
|
None
|
|
Kanamycin
|
None
|
None
|
None
|
Table 11. Resistance of S.xylosus samples to different antibiotics(n=5)
|
|
Susceptibility
|
Intermediate
|
Resistance
|
|
|
n (%)
|
n (%)
|
n (%)
|
|
Erythromycin
|
4 (80,0)
|
None
|
1 (20,0)
|
|
Clindamycin
|
5 (100,0)
|
None
|
None
|
|
Fusidic acid
|
1 (20,0)
|
3 (60,0)
|
1 (20,0)
|
|
Penicillin
|
5 (100,0)
|
None
|
None
|
|
Oxacillin
|
5 (100,0)
|
None
|
None
|
|
Cefoxitin
|
5 (100,0)
|
None
|
None
|
|
Tetracycline
|
2 (40,0)
|
None
|
3 (60,0)
|
|
Kanamycin
|
1 (100,0)
|
None
|
None
|
Table 12. Resistance of S.intermedius samples to different antibiotics(n=3)
|
|
Susceptibility
|
Intermediate
|
Resistance
|
|
|
n (%)
|
n (%)
|
n (%)
|
|
Erythromycin
|
3 (100,0)
|
None
|
None
|
|
Clindamycin
|
3 (100,0)
|
None
|
None
|
|
Fusidic acid
|
3 (100,0)
|
None
|
None
|
|
Penicillin
|
None
|
None
|
3 (100,0)
|
|
Oxacillin
|
3 (100,0)
|
None
|
None
|
|
Cefoxitin
|
3 (100,0)
|
None
|
None
|
|
Tetracycline
|
1 (33,3)
|
None
|
2 (66,7)
|
|
Kanamycin
|
1 (100,0)
|
None
|
None
|
57% of the samples were positive to Staphylococcus.spp, while 8 of the 11 identified bacteria belonged to the Staphylococcus genus. The most common strain identified was S.sciuri (12.2%), followed by S.aureus and S.lentus (5.6% for each other) (Tables 1 and 2). Although most of the samples were taken from sheep, these animals showed less (34.2%) infected samples than horses (94.6%), humans (60.8%) and goats (85.7%) (Table 1). The above results are consistent with the results of the research by Loeffler et al [10] where the most isolated bacteria were S. aureus and S. intemedius.
The rate of samples found infected with S.aureus (5.6%) is close to the respective percentage found in a survey conducted in Tunisia, where 6.5% of the samples carried the particular bacterium [11].Higher rates of positive samples have been recorded in the literature, such as in the research by Gömez-Sanz et al [12], where S.aureus strains were recorded in 12% of the samples. In addition, strains of S.aureus were found in 20% of dog samples, 17,8 of human samples and only 2.9% of horse samples (Table 3). The results are quite similar to other surveys, that were carried out in different countries, as shown in Table 5.
As shown in the literature, various percentages of positive samples have been recorded. Differences from survey to survey are expected and may be due to both the different number of samples and the different region or time period (Figure 1). Experiments performed to determine the positivity of the samples to catalase and to coagulase, showed that all samples were positive to catalase (100%), while 47,1% of samples were positive to both catalase and coagulase. 65,8% of samples was not identified (Table 2). The lowest rates of coagulation positive samples were found in horses (22.9%) and the highest ones in cats (70%) and sheep (67.5%). 42% (human), 44,4% (dog) and 59.5% (goat) of the positive samples were positive to coagulase (Table 3 and Figure 2). Similar results were also shown by two more studies, where the percentage of samples from human was approximately 40%, while those from animals were 33-41%. A small deviation was found in dog samples and more than 30% deviation was found in cat samples [10-12]. Regarding the susceptibility of different samples to antibiotics, the rates recorded were quite high (47-100%).The highest rate was found against kanamycin, while 94,7% and 89,7% of the samples tested for their susceptibility to cefoxitin and oxacillin respectively were found susceptible to the corresponding antibiotic. 53,4% of the samples were resistant to penicillin. A significant difference was observed in the clindamycin, fusidic acid, penicillin and terracycline susceptibility rates. In particular, the susceptibility rate of sheep to clindamycin was the lowest recorded, while the highest occurred in cats, dogs and horses. The sheep susceptibility rate to fusidic acid was lower than that of the cat, dog and horse. Goats and horses were more susceptible to penicillin comparing with other animals (Table 4 and Figure 3).
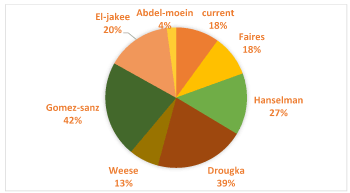
Figure 1. Percentages of contaminated human samples from S. aureus- Comparison between different surveys
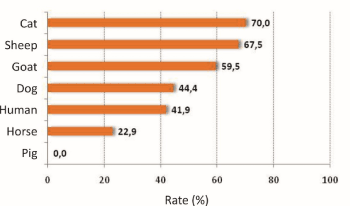
Figure 2. Rates of positive samples in coagulase.
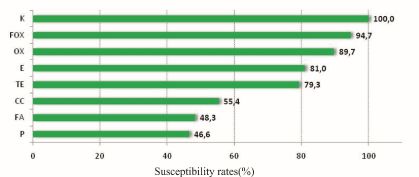
Figure 3. Susceptibility rates of samples to different antibiotics
Regarding the bacteria, S. epidermidis samples were sensitive against clindamycin, penicillin, oxacillin, cefoxitin and tetracycline antibiotics, while all S. lentus samples were susceptible to oxacillin and cefoxitin substances. 60% of the S.lentus samples showed sensitivity to erythromycin, 66.7% to clindamycin, 90% to fusidic acid, and 80% to penicillin and tetracycline. Staphylococcus aureus strains were sensitive to erythromycin, clindamycin, fusidic acid, tetracycline and kanamycin. Half of them were susceptible to penicillin and 75% to oxacillin and cefoxitin. S.sciuri strains were susceptible to cefoxitin and tetracycline. 91.3% of S.sciuri samples were sensitive to erythromycin, 87.0% of them, to oxacillin and only 9.1% and 8.7% to clindamycin and penicillin respectively. The sample that was containing S. simulans was susceptible to all antibiotics apart from kanamycin, in which it was not tested. Strains of S. xylosus were susceptible to penicillin, clindamycin, oxacillin and cefoxitin and kanamycin. 20.0% of the S.xylosus samples were susceptible to fusidic acid and 40.0% to tetracycline. Finally, strains of S.intermedius were found to be sensitive to erythromycin, clindamycin, fusidic acid, oxacillin and cefoxitin. In addition, 33.3% of the S.intermedius samples were sensitive to tetracycline and all samples were resistant to penicillin (Tables 6-12). Generally speaking, S.aureus demonstrated high rates of susceptibility and sensitivity to the most antibiotics that were used, a data which is similar with the results of other surveys. Resistance to other antibiotics such as oxytetracycline, enroflaxin, methicillin, erythromycin, amoxicillin and clindamycin was demonstrated in quite high rates. The antibiotics in which S.aureus was more susceptible were tetracycline, ciprofloxacin, cefoxerazone and cefoxitin.
S.aureus is one of the most commonly corresponding bacteria in humans and animals, while new research is being carried out for a better and more direct addressing to its health impacts on organisms. The results indicated that Staphylococcus strains were detected in the samples, with S. sciuri occurring as the most common strain, while S.aureus was identified only in dogs, horses and humans. The antibiotic against which the highest levels of resistance from both S.aureus and other Staphylococci were recorded was penicillin, however, S.aureus showed susceptibility to most antibiotics. Further studies should be conducted to provide additional information on the ecology of S.aureus in both humans and domestic animals.
The authors declare that there is no conflict of interest.
- Harris LG, Foster SJ, Richards RG (2002) An introduction to staphylococcus aureus and techniques for identifying and quantifying S. Aureus adhesins in relation to adhesion to biomaterials: review. Eur Cell Mater 2: 39-60. [Crossref]
- Prax M, Lee CY, Bertram R (2013) An update on the molecular genetics toolbox for staphylococci. Microbiology 159: 421-435. [Crossref]
- Foster T (1996) Staphylococcus, Univ. of Texas medical branch at Galveston. Medical Microbiology 4th edition, Chapter 12. [Crossref]
- Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, et al. (2009) Eubacteria and Archaea, Essentials of Glycobiology. 2nd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2009, Chapter 20. ISBN-13: 9780879697709.
- Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, et al. (2008) Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States 2001-2004. J Infect Dis 197: 1226-1234. [Crossref]
- Grema H (2015) Methicillin Resistant Staphylococcus aureus (MRSA): A Review Advances in Animal and Veterinary Sciences 3: 79-98.
- Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, et al. (2013) National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States 2011. JAMA Inter Med 173: 1970-1978. [Crossref]
- Jablonski LM, Bohach GA (1997) Staphylococcus aureus. Food microbiology fundamentals and frontiers. Doyle MP, Beuchat LR and Montville TJ, eds, American Society for Microbiology Press, Washington, DC, 353-357.
- Hennekinne JA, De Buyser ML, Dragacci S (2012) Staphylococcus aureus and its food poisoning toxins: characterization and outbreak investigation. FEMS Microbiol Rev 36: 815-836. [Crossref]
- Loeffler A, Boag AK, Sung J, Lindsay JA, Guardabassi L, et al. (2005) Prevalence of methicillin-resistant Staphylococcus aureus among staff and pets in a small animal referral hospital in the UK. J Antimicrob Chemother 56: 692-697. [Crossref]
- Gharsa H, Ben Slama K, Gómez-Sanz E, Lozano C, Zarazaga M, et al. (2015) Molecular characterization of Staphylococcus aureus from nasal samples of healthy farm animals and pets in Tunisia. Vector Borne Zoonotic Dis 15: 109-115. [Crossref]
- Gomez-Sanz E, Torres C, Lozano C, Zarazaga M (2013) High diversity of Staphylococcus aureus and Staphylococcus pseudintermedius lineages and toxigenic traits in healthy pet-owning household members. Underestimating normal household contact?Comp Immunol Microbiol Infect Dis36: 83-94. [Crossref]
- Faires MC, Tater KC, Weese JS (2009) An investigation of methicillin-resistant Staphylococcus aureus colonization in people and pets in the same household with an infected person or infected pet. J Am Vet Med Assoc 235: 40-543. [Crossref]
- Hanselman BA, Kruth SA, Rousseau J, Weese JS (2009) Coagulase positive staphylococcal colonization of humans and their household pets.Can Vet J50: 954-958. [Crossref]
- Drougka E, Foka A, Koutinas CK, Jelastopulu E, Giormezis N, et al. (2016) Interspecies spread of staphylococcus aureus clones among companion animals and human close contacts in a veterinary teaching hospital. A cross-sectional study in Greece. Prev Vet Med 126: 190-198. [Crossref]
- Weese JS, Rousseau J, Traub-Dargatz JL, Willey BM, McGeer AJ, et al. (2005) Community-associated methicillin-resistant Staphylococcus aureus in horses and humans who work with horses. J Am Vet Med Assoc 226: 580-583. [Crossref]
- El-Jakee, Ata NS, Bakry MA, El-Said WAG (2008) Characteristics of Staphylococcus aureus strains isolated from human and animal sources. American-Eurasian J Agric Environ Sci 4: 221-229.
- Vincze S, Stamm I, Kopp PA, Hermes J, Adlhoch C, et al. (2014) Alarming proportions of methicillin-resistantstaphylococcus aureus (MRSA) in wound samples from companion animals, Germany 2010-2012. PLoS One 9: e85656. [Crossref]
- Abdel-moein ΚΑ, Elhariri M, Samir A (2011) Methicillin-Resistant Staphylococcus aureus: An Emerging Pathogen of Pets in Egypt with a Public Health Burden. Transboundary Emerging Diseases 59: 331-335.



