A decline in the ability to process facial expression of emotions has been reported in individuals with Alzheimer’s disease (AD). However, the low number of participants and the lack of diversity in the tasks being used in previous studies leaves a gap in our knowledge about this issue. Gap, we recruited 169 participants including healthy older adults (HOA), participants with mild cognitive impairment (MCI) and AD patients at different stages of the disease (mild to moderate). Four tasks including recognition, selection, matching and declarative knowledge about facial expression of emotions were used. Face identification was used as a control task. Our results revealed that compared with HOA, MCI participants did not show any significant deficits in none of the tasks. AD patients did not show any impairment in the control task. However, they were impaired in the processing of facial expression of negative emotions across all four affective tasks. Interestingly, recognition and selection of happiness were intact in AD patients at the mild stage of the disease. Our findings suggest that despite the pathology affecting distributed areas in the brain, the less challenging aspects (recognition and selection) of the ability to process the facial expression of happiness were preserved at the early stage of the disease. By recruiting a large number of participants together using with several different tasks our study provides a comprehensive picture of the disorders of facial emotion processing in Alzheimer's Disease. Our findings have significant implications for improving the AD patients’ quality of life and the quality of their social interaction with others. Future studies might start to investigate the processing of emotions in AD patients in other modalities rather than visual
Alzheimer’s Disease, Mild Cognitive Impairment, Facial Expression, Emotions, Happiness
Faces are of great importance in our everyday social interactions. Previous studies have shown that humans spend more time looking at other peoples’ faces than any other parts of the body [1]. The dynamic and highly visible nature of the faces allows us to socially communicate via facial expression of emotions [2].
Impairments in the perception of facial expression of emotions (FE) across various neurodegenerative conditions including Alzheimer’s Disease (AD) have been reported by previous studies [3-6]. Deficits in the perception of FE in AD patients have been shown to have devastating negative effects on patients’ social life leading to the increased burden on caregivers [7,8].
Previous studies in AD patients have shown inconsistent findings regarding the processing of FE in these patients [9-13]. For example, Lavenu and colleagues (1999) showed that AD patients had a selective deficit in recognition of fear. Another study using three tasks including recognition, matching, and differentiation of facial expression of emotions revealed that AD patients performed worse than healthy older adults (HOA) across all tasks. Furthermore, this study showed that the AD patients had selective deficits in recognition of sadness [14]. Another experiment by Henry and colleagues (2008) found preserved recognition of disgust in AD patients. However, using similar tasks some studies did not observe any significant differences between AD and HOA in FE processing tasks [15,9,16]. A recent review on the deficits of FE processing associated with AD confirmed the inconsistency in the findings. But, based on previous results this review suggests overall poorer recognition of facial expression of emotions, with particular difficulty with the expression of negative emotions especially sadness [17].
The literature regarding the deficit in recognition of facial expression of positive emotions such as happiness in AD patients is also inconsistent. For example, Burnham and Hogervorst (2004) tested the AD patients on FE recognition as well as matching tasks. They found that compared with HOA, AD patients were not impaired in the recognition task. However, AD patients showed a deficit in matching task for fear, sadness, and happiness. However other studies did not report any impairment in recognition of facial expression of happiness in AD patients [14,18]. The inconsistent findings in FE processing in AD patients may be related to the heterogeneity of patient population. Such a heterogeneity might be due to the fact that different areas have been affected by pathological changes in the brain in these patients [19]. Despite the previous attempts to understand the deficits of FE processing in AD patients, there remain some shortcomings. Here, we point to two main issues.
The first issue is related to the scope and the diversity of the FE processing tasks used in previous studies. Most of the earlier studies did not conduct a comprehensive assessment of FE processing in AD patients. Using a broader set of tasks will help to better understand the extent of FE processing impairments in AD patients. The second issue concerns the small sample size and the small number of trials and therefore less variance in the data reported by previous studies [10,11]. Only, few studies used a significantly large sample size of AD patients together with MCI and HOA. These studies are more reliable for future replications compare to the most of the previous experiments [16,20 &18].
In the present study, we aimed to extend the existing findings as well as compensating for the previous shortcomings by recruiting a wide range of participants including HOA, MCI, and AD patients (mild to moderate). We further aimed to evaluate multiple aspects of FE processing in these groups by using four different tasks related to FE processing as well face identity discrimination as a control task to asses the general aspects elements of face processing. The main tasks included recognition, selection, matching and declarative knowledge about the facial expression of emotions. To the best of our knowledge, this latter task has not been used before in association with FE processing in AD patients. This task will help us to better understand the scope of FE processing deficits in the AD. Based on previous findings, we expected the AD patients to experience difficulty in recognizing the facial expressions representative of negative emotions. Also, based on the past literature we did not expect to observe any significant deficit in AD and MCI participants on the control task of face identity discrimination. Our study is valuable for it sheds new light on different aspects of facial expression processing in AD patients by using a variety of tasks in a large sample size of participants. Our study could potentially help to replicate the findings of the previous studies as well as adding to the current knowledge about the different aspects of facial expression processing in AD patients.
Participants
In total, 169 participants took part in this study. Four participants were excluded due to depression. The remaining participants (165) including 76 patients with a probable AD (ranging from mild to moderate), 40 HOA, and 49 MCI gave their written consent to take part in the study.
The diagnosis of the AD was based on the results of extensive neuropsychological testing and on the clinical examination conducted by a neurologist in accordance with the DSM-IV-TR criteria (American Psychiatric Association, 2000) which is an accepted tool in the clinical diagnosis of the AD [21]. The exclusion criteria for AD patients were the prevalence of disorders such as prosopagnosia, head trauma, hearing/visual problems, clinically severe depression/anxiety, alcoholism, or other neurological diseases such as Parkinson's Disease, stroke, and small vessel disease. Patients at the different stages of the AD were on glutaminergic inhibitors (Memantine) or acetylcholinesterase inhibitors (Rivastigmine). For both HOA and MCI participants the presence of cognitive impairments, being diagnosed with any neurological or psychiatric disorders or being on any regular medication resulted in exclusion from the study. MCI participants were classified according to the criteria of Peterson into amnestic MCI single domain [22]. All MCI participants reported that their activity of daily living was not significantly affected by their memory problem [23]. All participants (HOA, MCI, and AD) required to have normal or corrected to normal vision and hearing to be able to perform the tasks.
Cognitive impairment severity rating was derived according to MMSE scores [24]. The following cut-off points were used for classification of the participants: normal cognitive function and MCI = 30-25, mild probable AD (MMSE = 24–20), moderate probable AD (MMSE 19–10). Similar criteria for subdividing AD patients was used in previous studies [18,25].
The patients with probable AD and participants with MCI were recruited through an outpatient dementia clinic based at the Iranmehr Hospital in Tehran. The HOA participants were recruited in response to an advertisement which offered a free neuropsychological assessment for individuals aged between 60 to 80.
The ethics committee of Beheshti School of Medical Science approved the study. All participants were informed about the aims of the study and were given a chance to ask questions. Participants were then consented to take part in the study. If some patients (for example, some of the Moderate AD patients) were unable to give consent, the next of kin/caregivers consented on behalf of the patients.
Stimuli and procedure
In addition to MMSE, all participants were tested on comorbid depression and anxiety using the Persian version of the Beck’s Depression Inventory and Beck’s Anxiety Inventory. Furthermore, to verify if the patients had any language related deficits that could affect their performances at the FE tasks especially the recognition task participants also completed the verbal fluency part of the Persian version of MoCA test [26-28].
Processing of facial expression of emotions was assessed using three subtests of the Florida Affect Battery (FAB) including facial emotion recognition, facial emotion matching and facial emotion selection [29]. FAB test has been shown to be sensitive in detecting the impairments of FE recognition in patients with neurodegenerative disorders [5]. For the facial expression of emotions tasks, we included 10 repetitions for each of target emotions including happiness, fear, anger, sadness and neutral. This design was the same as what Rosen and colleagues used (2004) except that we additionally used the declarative knowledge on emotions to evaluate whether or not the AD patients could relate the knowledge about the emotional situation to the corresponding facial expression of emotions. Facial identity discrimination was used as a control task. All of the tests and questionnaires were conducted in pen and paper (card) format.
FAB subtests
Three FAB subtests were used. The methodology is the same as described in Rosen et al. (2004). However, there were two main differences compared with the method that Rosen et al. (2004) used: 1-In our study, we used 10 trials per emotion 2- for the recognition task instead of collecting free response from the participants for each trial participants were given five options to choose.
Facial emotion recognition
This task measured the recognition of facial emotional expressions. During each trial, participants were presented with a single photograph of a face depicting a specific emotion. For this task, instead of asking the participants to simply identify the emotion on each trial we asked them to choose their response from one of the five options they were given. For emotion recognition task different options were provided for each trial.
Facial emotion selection
This task measured participants’ability to select a specific facial expression of emotions among other expressions. On each trial, five photographs of the same individual were presented to the participants. Each picture depicted a different facial expression. Participants were required to select the face portraying the emotion requested by the examiner.
Facial emotion matching
This task measured participants’ ability to match the facial expression of emotions. Participants viewed a card. On the left side of the card, there was a single photograph of a target emotional face. Participants were then asked to choose the picture that showed the same emotion from five images located on the right side of the same card.
Declarative knowledge about facial expression of emotions
In this task, we measured participants’ ability to associate their understanding about a specific emotional situation to its corresponding facial expression. The experimenter read a series of short sentences about different situations in which someone experienced a certain emotion. Participants were then shown five photographs of the same individual with each photograph depicting one of the five different facial expressions of emotions (the face stimuli were the same as those used in other subtest of FAB). Participants were asked to choose the facial expression that matched the situation read to them by the experimenter. For example, the experimenter read the following sentence to the participant: "Mary found the ring she lost yesterday." How do you think Mary would feel? Show me by choosing (pointing to) one of these five photos. The correct response for this example would be the happy face.
To validate the sentences that we used for this task we initially conducted this task in an independent group of participants (30 participants with similar age range and education level as to the participants taking part in the current study). We conducted the same task using a series of 40 (Persian) sentences depicting different emotional situations (8 different sentences per emotion). The length of the sentences was between 5-8 words. Based on the performance of the independent participants 2 sentences per emotional situations were chosen to which more than 90 percent of the participants responded correctly. Therefore, for the current study, there were 2 sentences per emotion (happy, neutral, angry, scared and sad) with each sentence being repeated five times. Therefore, in total, there was 10 (2 x 5) trial per emotional situation.
Facial identity discrimination
This task was used as a control task to evaluate participants’ visuospatial and perceptual ability to differentiate between the identity of same or different faces. In this task, all the faces showed neutral expression. On each trial, two photographs of faces of individuals (same or different persons), both with the neutral expression, were presented to the participants at each trial. Participants were instructed to indicate whether the faces were the same or different people. There were 10 trials per face (5 trials using the same face and 5 trials using two different faces) in a pseudorandom order.
Seven AD patients were excluded from the study due to poor performance on the verbal fluency of the MoCA test. These patients had poor performance on the language subdomain of the MMSE test. The remaining 69 patients with the probable AD, the HOA (40) and MCI participants (49) were tested on the four FE tasks and one general face processing task. The demographic details of all participants and the scores on neuropsychological assessments can be found in Table 1. As it is shown in Table 1. the groups were not different regarding age, education, gender proportion, anxiety and depression scores.
Neuropsychological assessments
HOA group significantly differed from both MCI and AD patients on MMSE scores. In all the other demographic characteristics, such as age, years of education, handedness, and gender, the HOA did not significantly differ from the MCI and AD (Mild and Moderate) groups. See Table 1. for more details.
Table 1. Demographic details (Mean ± SD) of the participants. Notes: HOA: healthy older adults, MCI: mild cognitive impairment, AD: Alzheimer’s Disease, MMSE: Mini-Mental State Examination, BDI: Beck's Depression Inventory, BAI: Beck’s Anxiety inventory, NA: not applicable. P<.05 is considered as statistically significant.
Characteristics |
HOA
(N=40) |
MCI
(N=49) |
Mild AD
(N=41) |
Moderate AD (N=28) |
ANOVA/Kruskal-Wallis
F3,154 |
P (<.05) * |
Age |
74(6) |
74(6) |
73(6) |
74(7) |
0.119 |
.949 |
Gender (%male) |
52 |
49 |
46 |
43 |
- |
0.878 |
Handedness (%Right handed) |
87 |
89 |
92 |
85 |
- |
0.161 |
Education (years) |
15(2) |
14(2) |
15(2) |
14(2) |
0.246 |
0.864 |
BDI(max=63) |
4(3) |
4(3) |
4(3) |
3(3) |
1.33 |
0.264 |
BAI(max=63) |
4(4) |
5(5) |
5(4) |
3(4) |
1.13 |
0.338 |
MMSE(max=30) |
29(2) |
26(3) |
22(4) |
15(3) |
496.78 |
0.001 |
Medication (%cholinesterase inhibitors) |
NA |
NA |
55 |
45 |
- |
- |
Statistical analysis of main tasks (recognition, selection, matching and declarative knowledge)
Statistical analysis regarding the performance of each task was conducted separately in SPSS. For all the analyses, a mixed-effects 4x5 ANOVA was performed, with the between-subject independent variable of the group (HOA/MCI/Mild AD/Moderate AD) and within-subject independent variable of emotion (happiness, neutral, anger, fear, and sadness). Post-hoc comparisons were then performed to describe the key findings in more details. These analyses were repeated for each task.
Facial emotion recognition
Our results showed that on this task, there was a significant main effect of the group, F3,154= 334.55, P < .001, η2 =.867. The overall accuracy was significantly higher in HOA and MCI compared with the Mild and Moderate AD patients on facial emotion recognition task. Pairwise comparison showed that MCI participants did not significantly differ from HOA [mean difference± SE=.009 ±.008, P<.269]. Both HOA and MCI performed significantly better than Mild AD patients [mean difference± SE=.154 ±.008, P <.001; mean difference± SE=.144 ±.008, P<.001]. Moderate AD patients also significantly performed worse in comparison with both HOA [mean difference± SE=.242 ±.009, P<.001] and MCI individuals [mean difference± SE=.233 ±.009, P<.001]. The overall performance of the Mild AD patients was also better than Moderate AD patients [mean difference± SE=.089 ±.008, P <.001]
The main effect of emotion was also significant, F4,616= 140.43, P < .001, η2= .477. On average participants across all groups were more accurate in recognition of facial expression of happiness. The interaction between group and emotion was also significant, F12,616= 32.22, P < .001, η2=.414. To decompose the interaction between emotion and group, accuracy for recognition of each emotion was compared for each possible pair of groups. The results revealed that mild AD patients performed significantly worse on all emotions, except for happiness recognition. The performance of Mild AD patients was not significantly different from the HOA and MCI participants on recognition of happiness. Although, this was not the case for Moderate AD patients. Table 2. shows the mean accuracy (percent correct) on recognition of different emotions across different groups of participants.
Table 2. Percentage correct response (Mean ± SD) on recognition of different facial expression of emotions across HOA, MCI, Mild and Moderate AD participants. HOA: Healthy Older Adults, MCI: Mild Cognitive Impairment, AD: Alzheimer’s Disease.
Task |
HOA (N=40) |
MCI
(N=49) |
Mild AD
(N=41) |
Moderate AD
(N=28) |
P (<.05*, <.001**) |
HOA/MCI
t(87) |
HOA/Mild AD
t(79) |
HOA/Moderate AD MCI/Mild AD
t(66) t(88) |
MCI/Moderate AD
t(75) |
Mild AD/ Moderate AD
t(67) |
Happy |
97(3) |
96(5) |
95(4) |
87(7) |
.76 |
1.84 |
6.70** |
.97 |
5.95** |
5.17** |
Neutral |
97(3) |
97(4) |
96(4) |
77(8) |
.31 |
1.54 |
11.84** |
1.30 |
11.90** |
10.13** |
Angry |
98(3) |
97(4) |
83(9) |
76(6) |
1.43 |
10.42** |
17.83** |
10.05** |
16.99** |
3.07** |
Scared |
97(5) |
96(5) |
76(9) |
63(8) |
.82 |
12.13** |
20.85** |
11.88** |
19.92** |
5.77** |
Sad |
95(5) |
94(7) |
67(8) |
59(6) |
1.04 |
16.74** |
24.55** |
15.89** |
21.94** |
4.45** |
a = Recognition of happiness was not significantly different in Mild AD patients compared with healthy older adults or MCI participants.
Facial emotion selection
Our results showed that on this task, there was a significant main effect of the group, F3,154= 103.69, P < .001, η2 =.669. The overall accuracy was significantly higher in HOA and MCI compared with the Mild and Moderate AD patients. Pairwise comparison showed that MCI participants did not significantly differ from HOA [mean difference± SE=.012 ±.011, P<.295] on their performance in the selection of facial expression of emotions. Both HOA and MCI performed significantly better than Mild AD patients on this task [mean difference± SE=.107 ±.012, P <.001; mean difference± SE=.095±.011, P<.001]. Moderate AD patients also significantly performed worse in comparison with both HOA [mean difference± SE=.196 ±.013, P <.001], MCI individuals [ mean difference± SE=.184 ±.012, P<.001] and Mild AD patients [mean difference± SE=.089 ±.013, P <.001].
There main effect of emotion was also significant, F4,616= 73.94, P < .001, η2= .324. On average participants across all groups were more accurate in recognition of facial expression of happiness. The interaction between group and emotion was also significant, F12,616= 17.14, P < .001, η2=.259. To decompose the interaction between emotion and group, the accuracy of recognition for each facial expression of emotion was compared for each possible pair of groups. Table 3. shows the mean accuracy (percentage correct) on selection task across different groups of participants.
Table 3. Percentage correct response (Mean ± SD) on the selection of different facial emotions across HOA, MCI, Mild and Moderate AD participants. HOA: Healthy Older Adults, MCI: Mild Cognitive Impairment, AD: Alzheimer’s Disease.
Task |
HOA (N=40) |
MCI
(N=49) |
Mild AD
(N=41) |
Moderate AD
(N=28) |
P (<.05*, 10.05**) |
HOA/MCI
t(87) |
HOA/Mild AD
t(79) |
HOA/Moderate AD
t(66) |
MCI/Mild AD
t(88) |
MCI/Moderate AD
t(75) |
Mild AD/ Moderate AD
t(67) |
Happy |
97(1) |
96(2) |
96(7) |
87(9) |
0.287 |
0.338 |
4.78** |
0.093 |
4.99** |
4.39** |
Neutral |
99(2) |
98(4) |
94(9) |
87(8) |
0.982 |
3.19** |
7.75** |
2.64* |
6.80** |
2.97* |
Angry |
97(4) |
97(5) |
86(10) |
82(6) |
0.191 |
6.01** |
9.56** |
6.64** |
10.21** |
1.80 |
Scared |
98(3) |
95(3) |
80(13) |
69(9) |
1.67 |
7.95** |
18.63** |
6.09** |
11.82** |
3.86** |
Sad |
96(2) |
94(2) |
77(14) |
63(9) |
1.12 |
7.64** |
13.52** |
6.81** |
12.13** |
4.12** |
a = Selection of happiness was not significantly different in Mild AD patients compared with healthy older adults or MCI participants.
Facial emotion matching
Our results showed that on this task, there was a significant main effect of the group, F3,154= 566.24, P < .001, η2 =.917. The overall accuracy was significantly higher in HOA and MCI groups compared with the Mild and Moderate AD groups. The main effect of emotion was also significant, F4,616=53.53, P < .001, η2= .258. On average participants across all groups were more accurate in the matching of facial expression of happiness. The interaction between group and emotion was also significant, F12,616= 8.75, P < .001, η2=.146. To better understand the interaction between emotion and group accuracy of recognition of each emotion was compared for each possible pair of groups. Pairwise comparison showed that MCI participants did not significantly differ from HOA [mean difference± SE=.003 ±.008, P<.99] on the matching task. Both HOA and MCI performed significantly better than Mild AD patients [mean difference± SE=.214 ±.008, P <.001; mean difference± SE=.211 ±.009, P<.001]. Moderate AD patients performed worse compared with HOA, MCI individuals and mild AD patients [mean difference± SE=.268 ±.009, P <.001; mean difference± SE=.265 ±.008, P<.001; mean difference± SE=.054 ±.009, P <.001].
Declarative knowledge of facial emotion
Our analyses with emotion and groups as within and between independent factors showed that on this task, there was a significant main effect of the group, F3,154= 384.47, P < .001, η2 =.882. The overall accuracy was significantly higher in HOA and MCI compared with the Mild and Moderate AD patients. The main effect of emotion was also significant, F4,616= 32.36, P < .001, η2= .174. On average participants across all groups were more accurate in associating their knowledge about a happy situation to the facial expression of happiness. The interaction between group and emotion was also significant, F12,616= 12.03, P < .001, η2=.190. To decompose the interaction between emotion and group accuracy of recognition of each emotion was compared for each possible pair of groups. The results revealed that both Mild and Moderate AD patients performed significantly worse on all emotions. Pairwise comparison showed that MCI participants did not significantly differ from HOA [mean difference± SE=.001 ±.007, P<.99]. Both HOA and MCI performed significantly better than Mild AD patients [mean difference± SE=.148 ±.008, P <.001; mean difference± SE=.147 ±.007, P<.001]. Moderate AD patients performed worse compared with HOA and MCI individuals on this task [mean difference± SE=.243 ±.008, P <.001; mean difference± SE=.241 ±.008, P<.001]. The overall performance of the Mild AD patients was also better than Moderate AD patients [mean difference± SE=.095 ±.008, P <.001]. The graphical results regarding all tasks can be found in Figure 1. (A, B, C).
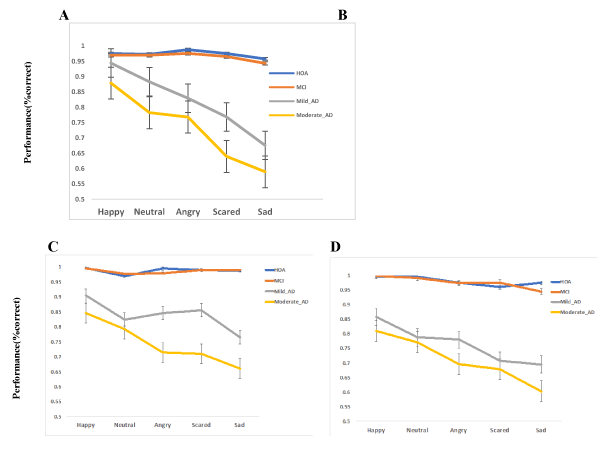
Figure 1. Effect of group and emotion on the performance in different subtests of the facial expression processing. The results showed that the groups including AD patients performed worse in all of the tasks except for the control task (identity discrimination). Both mild and moderate AD patients performed worse compared with MCI and HOA for all negative emotions. Mild AD patients’ performance was not significantly different from the MCI and HOA for the facial expression of happiness in recognition and selection tasks. (A) Mean percentage correct response in recognition task for each emotion across four groups of participants. (B) Mean percentage correct response in selection task for each emotion across four groups of participants. (C) Mean percentage correct response in matching task for each emotion across four groups of participants. HOA: Healthy Older Adults, MCI: Mild Cognitive Impairment, AD: Alzheimer’s Disease. The error bars represent standard error of the mean.
Facial identity discrimination
In this task, we asked whether the performance on identity discrimination significantly differed between different group of participants. Statistical analysis regarding this task was performed using an ANOVA with a between-subject independent variable of the group (HOA/MCI/Mild AD/Moderate AD). Our results showed that different groups of participants did not differ in their performance on identity discrimination task, F3,154= 32.54, P < .136. Although, the moderate AD group performed worse than the all the other groups, the difference between groups did not reach the significance level (Mean (SD) for HOA = 98(4), MCI = 98(4), Mild AD= 97(5), Moderate AD= 85(11)).
We further tested whether there was any difference between the performance on each task (on average) between the AD patients in comparison with the MCI and HOA participants. Our results showed that on average AD participants performed worse than the HOA and the MCI groups on all facial emotion processing tasks. See Table 4. for more details.
Table 4. Percentage correct response (Mean ± SD) on the four of different tasks across HOA, MCI, Mild AD and Moderate AD participants. HOA: healthy older adults, MCI: mild cognitive impairment, AD: Alzheimer's disease.
Task |
HOA
(N=40) |
MCI
(N=49) |
Mild AD
(N=41) |
Moderate AD (N=28) |
ANOVA
F3,154, P (<.05*, 10.05**) |
Recognition |
99(1) |
98(1) |
84(5) |
74(3) |
427.23** |
Matching |
98(2) |
97(2) |
76(5) |
71(4) |
566.24** |
Selection |
99(1) |
98(2) |
88(7) |
81(3) |
149.57** |
Declarative knowledge |
98(2) |
98(2) |
83(5) |
74(4) |
384.47** |
We further tested whether there was any difference between the performance on each emotion (on average) regardless of the task across four groups of participants. Our results showed that there was a significant main effect of emotion on overall performance across all main four tasks across all groups, F4,628 = 86.87, P <.001, η2= .356. On average, all participants performed better on the emotion of happiness [mean ± SD=.95 ± .05] and worse on the emotion of sadness [mean ± SD=.86 ± .14].
Interpersonal and social interaction in AD patients is likely to be influenced by impaired facial expression processing [8]. The current study was conducted to extend the existing results and explore different aspects of facial expression processing in a large sample of participants including healthy older adults, as well as MCI and AD patients at different stage of the disease. Our data offer a unique opportunity to extend the scope of findings in the field of facial expression processing in the AD.
We used four different tasks on facial expression processing including recognition, selection, declarative knowledge, and matching of facial expression of emotions together with the control task on facial identity discrimination. As expected AD patients did not show any impairment in the control task when their performance was compared with the MCI and healthy participants. However, on the facial expression processing tasks, AD patients performed significantly worse than both MCI and healthy participants on all tasks with general impairments on negative emotions. Interestingly, for the facial expression of happiness, mild AD participants did not show any impairment on recognition and selection tasks.
In recognition task, AD patients differed significantly from MCI and healthy older adults in recognition of negative emotions including anger, fear, and sadness. However, on recognition task participants in the early stage of Alzheimer’s Disease performed just as well as the HOA and MCI participants on the emotion of happiness. Similar results were found on selection task. On the other two tasks (matching and declarative knowledge) AD participants (including both mild and moderate) performed worse on all emotions in comparison with MCI and HOA participants. We further showed that across all tasks regardless of the group (HOA, MCI, Mild and Moderate AD), all participants performed better on the emotion of happiness and worse on the emotion of sadness.
The most significant finding of our study was that mild AD patients did not show impairment in the recognition or selection of happiness. This result is in line with previous studies showing that happiness is usually better recognized than the other emotions in FTD and AD patients [4]. This finding is normally interpreted in light of the data implying that happiness is easier to recognize compared to negative emotions [30]. In line with our findings, in a group of frontotemporal dementia (FTD) patients, Rosen and colleagues (2004) showed the impairment in emotional perception in temporal variant of FTD patients for negative emotions (sadness, fear, and anger) as opposed to positive emotion (happiness). However, they found that in more severe cases perception of happiness was impaired and this was associated with sever amygdala and orbitofrontal damage.
The perception of happiness has also been the focus of psychophysics studies. For example, using a computational model and psychophysics method Smith and Schyns (2009) showed that happiness and surprise are the two expressions that are best recognized from a distance compared with other expressions [31]. They argue that these two expressions could have had an evolutionary advantage when recognized from a distance, compared to the other emotions. Compared with emotions such as fear and surprise, happiness has been shown to be easily differentiated across different cultures [2]. Moreover, recognition of happiness has been shown to be acquired early during development [32]. Recent studies further revealed that older healthy adults, as well as amnestic MCI participants, show a positive bias in recognition of happy faces [33]. Together these findings to some extent explain why mild AD patients who took part in our study were not impaired on recognition and selection of happiness. Our result regarding intact identification and selection of happiness in mild AD patients shows that perhaps the facial expression of happiness is resilient to the extensive pathology affecting the AD patients' ability to communicate with others around them. It is essential that AD patients in the early stage of their disease can recognize the happiness on other people's faces and perhaps respond with a smile to keep the social interaction going.
In line with previous studies, our AD patients showed impairment in the processing of negative emotions (see for example Rosen et al., 2004) [5]. Several prior imaging studies have shown that amygdala plays a crucial role in recognition of most of the negative emotions in the face [34,35]. Our findings of impaired facial emotional recognition of negative emotions in AD patients is consistent with the results of neuroimaging and brain post-mortem studies which found the neuronal loss in the amygdala in the early stage of the Alzheimer's disease [10,19].
Our finding regarding poor recognition of sadness in faces in AD patients is also consistent with the findings of several previous studies [17]. Sadness has been shown to be one of the problematic emotions concerning recognition even in healthy elderly population [36]. Previous studies also highlighted the role of the amygdala in encoding sadness in faces by recruiting patients with bilateral amygdala damage. In addition to having difficulty in recognizing of fear in faces, these patients show a significant deficit in sadness recognition [37]. Moreover, some experiments have found patients with amygdala lesions to show impairments in rating the intensities of sad, fearful and disgusted faces, but not other expressions such as happiness [38].
Some of the previous studies argue that the visual attention mechanisms underlying worse performance in AD patients might be related the fact that AD patients spend less time exploring the face area compare to the off-face areas [13]. In line with this, previous studies argue that individuals use different visual scanning strategies to detect positive and negative emotions in the face. For example, to identify happiness in the face people spend more time attending to the mouth area whereas for the other emotions such as sadness they spend more time looking at the mouth area [39]. However, in some real-life social situations where people try to regulate or hide their real emotions, we might need to attend to distributed areas in the face and also use the contextual information to understand how the other person feels. This might be particularly difficult for the AD patients as their cognitive resources are limited.
There are limitations and imperfections when it comes to any study, and our study was not an exception. It might seem that the tasks that we used were too easy for the healthy older adults as the performance at the ceiling level suggests. However, we argue that this was not the case as we showed that even healthy older adults did not reach the ceiling performance in recognizing some of the subtler emotions such as sadness. Moreover, considering that we used a variety of tasks even healthy older adults needed different sets of cognitive skills to perform the tasks at the optimal level. Finally, it might be argued that there are potential differences in facial expression of emotions among different cultures. It is worth noting that some prior studies have shown that most basic emotions expressed by western imposers are recognizable above chance by individuals from different cultures [2,39]. However, it is not surprising for individuals to be better at judging the emotions revealed by the member of their group/ culture [39]. Therefore, this shortcoming is not specific to our study.
On the other hand, it might also be argued that the FAB stimuli that we used in our study should have been culturally adapted as the facial expression of emotions are not universal [40]. This issue is still a matter of debate in the field. Some of the previous studies have shown that processing of facial expression of main emotions (anger, disgust, fear, surprise, sadness, and happiness) are universal advocating Darwin's proposal on the universality of facial expression of emotions [41-43]. Indeed, there is still ongoing disagreement on this issue, and it could be argued that there exist both within and between cultural and individual differences in both expression and perception of emotions. Indeed our results regarding the ceiling performance by healthy participants for some emotions confirms that cultural differences per se did not affect the healthy participants’ performance. Rather we argue that the AD led to poor performance by patients in the task.
Nevertheless, our find2021 Copyright OAT. All rights reservr improving the social well being of the AD patients. It is essential for the future studies to conduct more in-depth investigations of the effects of the AD on the processing of facial expression. This field of research can help neuroscientists to extend their understanding of social functioning in neurodegenerative diseases. Future studies require more ecologically valid facial displays of emotion and a reference situation that more closely approximates an actual social interaction. Future studies might also start investigating emotion recognition in other modalities. Future research needs to adopt a more systematic approach. It will be so beneficial if future studies to utilize both behavioral and structural and functional MRI scanning to provide more robust evidence to support the previous findings.
This project was funded by Neurology Research Center of Iranmehr Hospital, Tehran, Iran.
- Haxby JV, Hoffman EA, Gobbini MI (2000) The distributed human neural system for face perception. Trends Cogn Sci 4: 223-233. [Crossref]
- Ekman P, Friesen WV, O'Sullivan M, Chan A, Diacoyanni-Tarlatzis I, et.al (1987) Universals and cultural differences in the judgments of facial expressions of emotion. J Pers Soc Psychol 53: 712-717 [Crossref]
- Gray HM, Tickle-Degnen L (2010) A meta-analysis of performance on emotion recognition tasks in Parkinson’s disease. Neuropsychology 24: 176–191. [Crossref]
- Keane J, Calder AJ, Hodges JR, Young AW (2002). Face and emotion processing in frontal variant frontotemporal dementia. Neuropsychologia 40: 655–665. [Crossref]
- Rosen HJ, Pace-Savitsky K, Perry RJ, Kramer JH, Miller BL, et.al (2004) Recognition of emotion in the frontal and temporal variants of frontotemporal dementia. Dement Geriatr Cogn Disord 17: 277–281. [Crossref]
- Wenk GL (2003) Neuropathologic changes in Alzheimer's disease. J Clin Psychiatry 64 Suppl 9: 7-10. [Crossref]
- Carton JS, Kessler EA, Pape CL (1999) Nonverbal decoding skills and relationship well-being in adults. J Nonverbal Behav 23: 91-100.
- Shimokawa A, Yatomi N, Anamizu S, Torii S, Isono H, et.al (2001) Influence of deteriorating ability of emotional comprehension on interpersonal behavior in Alzheimer-type dementia. Brain Cogn 47: 423–33. [Crossref]
- Bucks RS, Radford SA (2004) Emotion processing in Alzheimer's disease. Aging Ment Health 8: 222-232. [Crossref]
- Burnham H, Hogervorst E (2004) Recognition of facial expressions of emotion by patients with dementia of the Alzheimer type. Dement Geriatr Cogn Disord 18: 75-79. [Crossref]
- Fernandez-Duque D, Black SE (2005) Impaired recognition of negative facial emotions in patients with frontotemporal dementia. Neuropsychologia 43: 1673-1687. [Crossref]
- Lavenu I, Pasquier F, Lebert F, Petit H, Van der Linden M (1999) Perception of emotion in frontotemporal dementia and Alzheimer disease. Alzheimer Dis Assoc Disord 13: 96-101. [Crossref]
- Ogrocki PK, Hills AC, Strauss ME (2000). Visual exploration of facial emotion by healthy older adults and patients with Alzheimer disease. Neuropsychiatry Neuropsychol Behav Neurol 13: 271-278. [Crossref]
- Hargrave R, Maddock RJ, Stone V (2002) Impaired recognition of facial expressions of emotion in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 14: 64–71. [Crossref]
- Henry JD, Ruffman T, McDonald S, O'Leary MA, Phillips LH, et al. (2008) Recognition of disgust is selectively preserved in Alzheimer's disease. Neuropsychologia 46: 1363-1370. [Crossref]
- Roudier M, Marcie P, Grancher AS, Tzortzis C, Starkstein S, et al. (1998) Discrimination of facial identity and of emotions in Alzheimer's disease. J Neurol Sci 154: 151-158. [crossref]
- McLellan T, Johnston L, Dalrymple-Alford J, Porter R (2008) The recognition of facial expressions of emotion in Alzheimer's disease: a review of findings. Acta Neuropsychiatr 20: 236-250. [Crossref]
- Weiss EM, Kohler CG, Vonbank J, Stadelmann E, Kemmler G, et.al (2008) Impairment in emotion recognition abilities in patients with mild cognitive impairment, early and moderate Alzheimer disease compared with healthy comparison subjects. Am J Geriatr Psychiatry 16: 974-980. [Crossref]
- Najlerahim A, Bowen DM (1988) Regional weight loss of the cerebral cortex and some subcortical nuclei in senile dementia of the Alzheimer type. Acta Neuropathol 75: 509-512. [Crossref]
- Spoletini I, Marra C, Di Iulio F, Gianni W, Sancesario G, et.al (2008) Facial emotion recognition deficit in amnestic mild cognitive impairment and Alzheimer disease. Am J Geriatr Psychiatry 16: 389-398. [Crossref]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (IV-TR), 4th edn—text r2007vised. Washington, DC: 2000.
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, et al. (2001) Current concepts in mild cognitive impairment. Arch Neurol 58: 1985-1992. [Crossref]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, et.al (2004) Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256: 240-246. [Crossref]
- Folstein MF, Folstein SE, McHugh PR (1975) "Mini-mental state": A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198. [Crossref]
- O’Bryant SE, Humphreys JD, Smith GE, Ivnik RJ, Graff-Radford NR (2008) Detecting dementia with the mini-mental state examination in highly educated individuals. Arch Neurol 65: 963-967. [Crossref]
- Ghassemzadeh H, Mojtabai R, Karamghadiri N, Ebrahimkhani N (2005) Psychometric properties of a Persian-language version of the Beck Depression Inventory-Second edition: BDI-II-PERSIAN. Depress Anxiety 21: 185-192. [Crossref]
- Steer RA, Beck AT (1997) Beck Anxiety Inventory.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, et.al (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: 695-699. [Crossref]
- Bowers D, Blonder LX, Heilman KM (1998) Florida affect battery. Centre for Neuropsychological Studies, Department of Neurology.
- Hager JC, Ekman P (1979) Long-distance of transmission of facial affect signals. Ethology and Sociobiology 1: 77-82.
- Smith FW, Schyns PG (2009) Smile through your fear and sadness: transmitting and identifying facial expression signals over a range of viewing distances. Psychol Sci 20: 1202-1208. [Crossref]
- Hiatt SW, Campos JJ, Emde RN (1979) Facial patterning and infant emotional expression: Happiness, surprise, and fear. Child Dev 50: 1020-1035. [Crossref]
- Werheid K, Gruno M, Kathmann N, Fischer H, Almkvist O, et.al (2010) Biased recognition of positive faces in aging and amnestic mild cognitive impairment. Psychol Aging 25: 1-15. [Crossref]
- Adolphs, R, Tranel D (2004) Impaired Judgments of Sadness But Not Happiness Following Bilateral Amygdala Damage. J cog neuroscience 16: 453-462. [Crossref]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL (2006) Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage 30: 1441-1448. [Crossref]
- Sullivan S, Ruffman T (2004) Emotion recognition deficits in the elderly. Int J Neurosci 114: 403-432. [Crossref]
- Adolphs, R, Tranel D (2004) Impaired Judgments of Sadness But Not Happiness Following Bilateral Amygdala Damage. J cog neuroscience 16: 453-462. [Crossref]
- Anderson AK, Phelps EA (2002) Is the human amygdala critical for the subjective experience of emotion? Evidence of intact dispositional affect in patients with amygdala lesions. J Cogn Neurosci 14: 709-720. [Crossref]
- Elfenbein HA, Ambady N (2002) On the universality and cultural specificity of emotion recognition: a meta-analysis. Psychol Bull 128: 203-235. [Crossref]
- Jack RE, Garrod OG, Yu H, Caldara R, Schyns PG (2012) Facial expressions of emotion are not culturally universal. Proceedings of the National Academy of Sciences, 109: 7241-7244.
- Susskind JM, Lee DH, Cusi A, Feiman R, Grabski W, et al. (2008) Expressing fear enhances sensory acquisition. Nat Neurosci 11: 843-850. [Crossref]
- Darwin C (1998) The expression of the emotions in man and animals. Oxford University Press, USA.
- Elovainio M, Kivimäki M, Puttonen S, Heponiemi T, Pulkki L, et al. (2004) Temperament and depressive symptoms: a population-based longitudinal study on Cloninger's psychobiological temperament model. J Affect Disord 83: 227-232. [Crossref]

