Abstract
The aim of targeted therapies is to inhibit the driving set of molecules, or pathways, in cancer. New research regarding tumor drivers and escape pathways has led researchers to investigate combinational therapies in cancer, either through simultaneous or sequential application. In this article, we discuss a strategy to inhibit the actionable targets while blocking feedback loops, by epigenetic therapies and recognizing and understanding the role these treatments play in controlling escape pathways. We present a case of a patient with a neuroendocrine tumor of the liver who was treated with this strategy. Our results showed a positive response and led us to conclude that further studies are needed to structure an enhanced analysis of tumor escape pathways. This would enable us to formulate more effective therapies in neuroendocrine tumor as well as other solid tumors.
Key words
sequential targeted therapy, neuroendocrine tumor, epigenetic therapy, trametinib
Background
In recent years, the research and literature has explored adaptive and acquired resistance to molecular targets that were initially actionable early during disease course. Resistance is thought to be mediated by activation of downstream targets. For example, Ras and MAPK target activation have been identified in cases of EGFR drug resistance. By an understanding of the complex networks of molecular targets and their feedback loops, scientists may be able to apply this knowledge to navigate a treatment strategy that works through the sequencing of targeted therapies [1].
In relation to alterations that can be identified, the number of actionable targets in the treatment of cancer is relatively low [2]. Therefore, well thought-out and educated decisions should be implored in the treatment of actionable targets, as it becomes nearly impossible to treat the consequential activation of targets through escape pathways [3]. For example, inhibition of the PI3k pathway through mTOR inhibitors may lead to activation of the EGFR/KRAS/RAF/MEK/MAPK pathway, creating resistant, and difficult to treat disease. In many studies, dual inhibitions have failed to improve a patients’ survival, which is thought to be due to activation of feedback loops, or mutations in downstream molecules [4]. In other hand, we have recently recognized the role of epigenetic therapies in treatment of wide range of tumors, including liver and neuroendocrine tumors. Changes in the levels of epigenetic-modifying enzymes are an important factor in altering expression of genes involved in cancer-related pathways [5-8].
Genetic and epigenetic dysregulation of genes involved in pathways such as the hypoxia-inducible pathway, the mTOR pathway, and the cMET-RAF-MEK-ERK pathway contribute to the progression of different types of cancers [9].
In this paper, we discuss a strategy by which with application of epigenetic therapies in combination with Mek inhibitors, we are able to improve outcome. It is hypothesized here that such improved outcome is due to inhibition of the actionable target (Mek), as well as block the feedback loops. The main pitfall of this type of treatment is that there are not a wide range of genomic alterations that can be identified by molecular profiling to distinguish feedback loops or their relationship. The fingerprint of these feedback loops is only recognized after secondary activation of targets are identified on liquid biopsy, once available.
Methods and materials
Multitargeted epigenetic therapy consisting Polyphenol and butyrate intravenous administration per protocol, Trametinib oral drug at 2 mg per day
Case study
A 40-year-old female, living in Mexico, had an abdominal mass found during routine physical exam in 2012. She underwent abdominal ultrasound, which showed a mass in her liver. At the time of diagnosis, the patient denied having any other health conditions. She had no complaints and did not display any signs and symptoms of disease. She was eating well, her weight was stable, and she had no pain. In March of 2012, the patient underwent a liver resection, and tolerated procedure well. The pathology report indicated the following of the mass: negative angiotensin-converting enzyme (ACE), negative CA-125, negative CA19-9, negative CD45, negative CK20, negative HMB-45, negative Hepar, negative glypican, and negative Thyroid transcription factor-1 (TTF-1). These findings ruled out metastases to the liver from other types of adenocarcinoma, as well as other causes of liver dysfunction. In addition, the pathology was positive for Chromogranin A (CgA), CD56, CK7, and calcitonin. The positive findings of CgA along with the other findings indicated that the tumor removed with consistent with a neuroendocrine malignant neoplasm (carcinoid type).
The patient was followed with imaging, and a year later in April 2013, a recurrent liver lesion was identified. The patient was experiencing abdominal distention and discomfort, along with lower extremity edema. Chemo-embolization of the liver lesion was performed. In 2014, the patient went on to receive Octreotide injections, and was clinically stable. In April 2016, the patient began to experience severe headaches, which prompted MRI evaluation. Brain MRI was performed, and identified two lesions, which were subsequently resected in May 2016. The patient did not receive any radiation therapy. Following resection, the patient completed three cycles of oral temozolomide and capecitabine. She was followed by CT scan, and imaging from October 2016 done after chemotherapy indicated progression of disease. All chemotherapies were discontinued, and patient was referred to palliative care/hospice, as well as to our clinic for evaluation.
Upon her presentation to our clinic in November 2016, the patient reported that she was in pain and did not have an appetite. She was evaluated and was found to have a poor function and ECOG score. Her labs were drawn and showed significantly elevated markers of disease. These included elevated lactate dehydrogenase (LDH) of 508 IU/L (normal 119-226 IU/L), Human epididymis protein 4 (HE4), CA- 125, Transforming growth factor beta 1 (TGF-b1), and Chromogranin A. She also had signs of liver failure by laboratory evaluation, including increased INR, increased ALT and AST, and elevated alkaline phosphatase of 294 IU/L (normal 39-117 IU/L). Liquid biopsy for circulating tumor DNA (ctDNA) was performed by Guardant 360 laboratories and showed positive for PI3k (with a mutation allele fraction of 16.4%), as well as positive APC, ESR1, TP53, and cKIT. Here circulating tumor cells (CTCs) were evaluated by BioFocus laboratories on 11/10/16, and were positive for the markers PDGFR-b, and negative for chromogranin.
Her CT scan done October 2016 was reviewed, and showed
Partial hepatectomy with marked enlargement of the residual liver, which was replaced by several large masses. Largest liver mass measured 16.4 x 9.3cm, with extensive tumor throughout remaining liver, as well as porta hepatis region with moderate scattered abdominal ascites. Tumor thrombus partially occluded the portal vein. There was extensive confluent lymphadenopathy in the region of the pancreatic head, porta hepatis and celiac region of the upper abdomen. Prominent small bowel without definite obstruction, and moderate ascites.
Several pulmonary lesions consistent with metastatic disease. The largest nodes involved the lower lobes and measured 5.4 x 4.8 cm on the left and 4.8 x 3.7 cm of the right. There was extensive mediastinal lymphadenopathy.
The patient began treatment with us in November and was treated five days a week for three weeks. She received IV epigenetic therapies in a protocol called multitargeted epigenetic therapy (MTET). This included sequential targeted therapy consisting of a multi-phase MAPK and MEK inhibition, using a polyphenol, (known to inhibit the MAPK and Erk), and trametinib, (a known drug to inhibit MEK 1 and 2). The patient was reassessed following three weeks of sequential therapy. The restaging scan dated 12/13/16 showed reduction of tumor size from 16 cm to 12 cm, compared that with her prior scan on 11/10/16, based on the report the tumor was stabilized in its size, as well as its pulmonary mets, improved along with the porta hepatis. Tumor involving pancreas was seen in prior scan, not visualized. Her laboratory studied including tumor markers improved. LDH dropped to 255 from 508, Interleukin-8 dropped to 50 from 98, and HE4 dropped to 163 from 198. Her amylase came down to 800 from 2000. Her liver enzymes improved including AST/ ALT normalized, and her INR of 1.6 dropped to 1.2 post-therapy. BioFocus laboratory repeated testing on her circulating tumor cells, which were not detected after treatment (Figures 1 and 2).

Figure 1. Circulating tumor cells
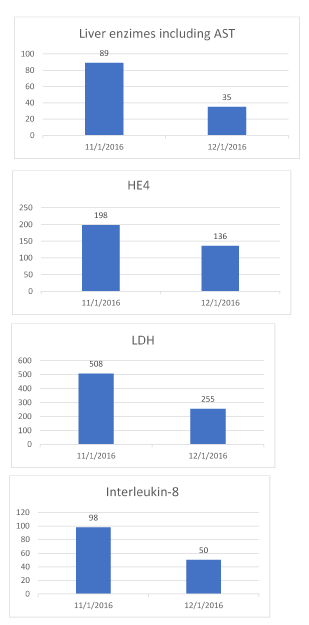
Figure 2. Labs
The patient had experienced improved quality of life and exceeded her expected survival.
Conclusion
Sequential and biphasic Inhibition of downstream targets/pathways of MAPK and MEK may significantly, and quickly translate in better outcome in advanced solid tumors, where the liquid biopsy suggests overactive driver mutated genes. Such approach by implementing epigenetic therapies is novel as it addresses existing therapeutic challenges (for first time to our knowledge) in neuroendocrine tumor, where the clinical advantage of sequential targeted therapy has been investigated with good results. We recommend further analysis and trials in investigating this therapeutic approach in wide range of tumors.
References
2021 Copyright OAT. All rights reserv
- Hopper-Borge EA, Nasto RE, Ratushny V, Weiner LM, Golemis EA, et al. (2009) Mechanisms of tumor resistance to EGFR-targeted therapies. Expert opinion on therapeutic targets 13: 339-362. [Crossref]
- von Manstein V, Yang CM, Richter D, Delis N, Vafaizadeh V, et al. (2013) Resistance of cancer cells to targeted therapies through the activation of compensating signaling loops. Curr Signal Transduct Ther 8: 193-202. [Crossref]
- Cassier P, Trédan O, Seigne C, Lavergne E, Fayette J (2014) Françoise desseigne. Developmental therapeutics-clinical pharmacology and experimental therapeutics - identifying actionable targets in advanced cancer patients: preliminary results from the profiler program. J Clin Oncol.
- Mendoza MC, Er EE, Blenis J (2011) The Ras-ERK and PI3K-mTOR Pathways: Cross-talk and compensation. Trends biochem sci 36: 320-328. [Crossref]
- Yiji Liao, Kexin Xu (2018) Epigenetic regulation of prostate cancer: the theories and the clinical implications Asian J Andrology. [Crossref]
- Li Ma, Mei-Sze Chua, Ourania Andrisani, Samuel So (2014) Epigenetics in hepatocellular carcinoma: An update and future therapy perspectives. World J Gastroenterol 20: 333-345. [Crossref]
- Karpathakis A, Dibra H, Thirlwell C (2013) Neuroendocrine tumours: Cracking the epigenetic code. Endocr Relat Cancer 20: 65-82. [Crossref]
- Di Domenico A, Wiedmer T, Marinoni I, Perren A (2017) Genetic and epigenetic drivers of neuroendocrine tumours (NET). Endocr Relat Cancer 24: 315-334. [Crossref]
- Banumathy G, Cairns P (2010) Signaling pathways in renal cell carcinoma. Cancer Biol Ther 10: 658-664. [Crossref]


