Sarcocystis is one of the most important and common protozoan parasites in the world. Various species of Sarcocystis reported in groups of mammals, birds and reptiles. In the life cycle of these parasite there are 2 hosts including hunted and hunter. Usually, omnivores and herbivores, as intermediate hosts (hunted) and carnivores, are considered as the definitive host (hunter) of this parasite. This research for the first time examines the contamination of Sarcocystis (microcyst) in native birds of Mazandaran province (Amol city). For this purpose, randomly, 57 native bird’s breast muscles which include 18 pieces of native ducks and 39 native chickens were tested by digestion method. The results of the experiment showed that 55 cases (96.5%) were infected with Sarcocystis bradyzoite that contributed 100% to the local duck and 94.78% to the native species. Based on age groups, the percentage of infection in the group age under 6 months was 80%, in the age between 6 months and one year was 97.91% and in the age group over one year, was 100%. The Chi-square test did not show a significant difference in the percentage of infection between two types of birds (duck-chicken) and age groups (P <0.05).
sarcocystis, poultry carcasses, Mazandaran
The parasitic members of the genus Sarcocystis are coccidia protozoa belonging to the Sarcocystidae family that cause intracellular cysts. This family currently contains more than 220 species [1]. These parasites have 2 obligatory hosts in their life cycle, including intermediate and definitive. Vegetarians and omnivores are commonly referred to as intermediate hosts (hunted) and carnivores as the definitive hosts (hunter) of this parasite. Asymptomatic proliferation of the parasites is mediated by hosts, followed by division of the merogenic cysts in the muscles. The parasite Sexual stage, which is associated with the formation of oocysts or sporocysts, occurs in the definitive host intestine [2], and reported in a variety of Sarcocystis species in mammalian, avian and reptile groups. Sarcocystis are able to carry out sexual and asexual reproduction in a host [3]. DNA analysis and parasitological morphological studies indicate that some of the species are present in at least two different intermediate host [4,5]. Some of sarcocyte species are pathogenic for humans and domestic animals and cause Sarcocystisosis. The parasitic pathogen is mainly caused by intermediate hosts and is mild in the definitive host. The rate of complications of this parasite depends on factors such as the species, the severity of the infection and the location of the parasite in the body. Pregnancy, lactation, stress and lack of nutrients can increase the severity of the parasitic pathogenesis [6,7]. So far, about 30 species of Sarcocystis have identified in birds that produce cysts in at least thirteen orders of the bird (14) [5]. The definitive host of two species, Sarcocystis Wenzley and Sarcocystis Horwath in chickens, are dogs and cats (18) [8]. For other bird species, the Sarcocystis species did not mentioned. In North America, large Sarcocystis cysts have identified in goose and duck [9]. These macrocysts attributed to Riley's Sarcocystis, which resemble rice grains [10,11]. The wild duck has also been introduced as an intermediate host for this protozoan. It seems in the protozoan life cycle, there are more intermediate hosts [12]. Because of Sarcocystis’s mild pathological complication, contaminated bird’s meat is unsuitable for food consumption [13,14]. In wildlife, Sarcocystis contamination occurs frequently. Sarcocystis falcachula, which is the ultimate host of the eposome and the intermediate host of sparrows and native poultry, can cause disease in domestic birds living in an open and caged environment [15]. However, strains of Sarcocystis recognized as infectious agents in domestic poultry around the world but they are usually less pathologically important. Cysts caused by this protozoan in intermediate hosts are large (macrocystic) or small (microcystic) depending on the species and definitive host of the parasite. If the cysts are large, they can easily diagnose but if the cyst is small, the diagnosis is impossible, and the parasite easily enters the human food cycle or other carnivorous organisms. In Iran, research on Sarcocystis contamination in poultry, unlike ruminants, is infrequent. Similarly, in a randomized study of pigeons infected with the nematode Hagyla Trankata, the Sarcocystis was first isolated and identified from the muscular layer of its gizzard [16]. This study for the first time investigates the contamination of Sarcocystis (Microcyst) in native birds of Mazandaran province (Amol city).
The method used in this study is observational and analytical sectional. For this purpose, 57 native bird species (native duck and native chickens) were selected at random. Table 1 shows the number and age of each bird studied. After slaughter, samples were taken from each bird's breast muscle for testing. Samples were analyzed by the digestive method of Dobby et al. [17]. For this purpose, first select 20 g of each sample and after grinding, with 100 ml of digestive solution including: 10 ml of 32% sulfuric acid plus 2.5 g of pepsin powder (Merck 7185 and 0.7 PIP-u / g) Mixed in one liter of distilled water and place in a hot water bath at 37 ° C for 30 minutes. After this time and tissue digested, the samples were refined using a two-layer filter. The obtained solutions were centrifuged at 1500 rpm for 10 min and the precipitates were prepared on slides of monotonic spreads and fixed with methanol after drying. At last, the slides were stained with 10 percent Giemsa and examined by light microscope. SAS 9/4 software and chi-square test with 95% confidence level (P <0.05) were used to compare the frequency of infection in the studied bird species and to compare the percentage of infection in different age groups.
Table 1. Bird species categorized by different ages
total |
> 1 year |
6 months to 1 year |
˂ 6 months |
Bird type |
18 |
3 |
13 |
2 |
Native duck |
39 |
1 |
35 |
3 |
Native chicken |
57 |
4 |
48 |
5 |
total |
In this study, a total of 57 native bird species including 18 native ducks and 39 native chickens were studied (Table 1). The results of digestion experiments on the samples showed that 55 (96.5%) were infected with Sarcocystis bradyzoite (Figure 1), and the percentage of contamination in native ducks, was 100% and in native chickens, 94.78% (Table 2). The studied birds were categorized as under 6 months, 6 months to one year and over one year in Table 1. Accordingly, the infection rate in the age group under 6 months was 80%, in the age group of 6 months to one year, 97.91% and in the age group above one year was 100% (Table 3).
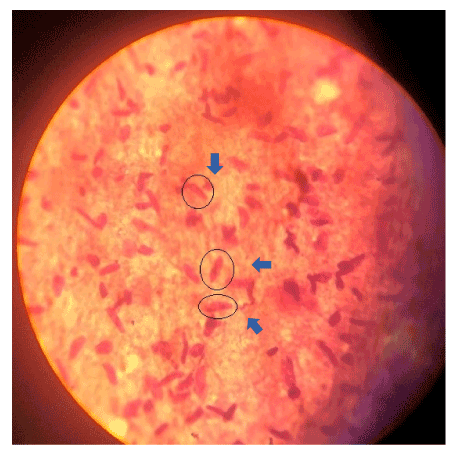
Figure 1. Sarcocystisic bradyzoites stained by Giemsa with 1000X magnification
Sarcocystisosis is one of the most common protozoan parasitic diseases in the world. This study for the first time examines the microcysts in native poultry muscles of Mazandaran province (Amol city). For this purpose, 57 native poultry muscles including 18 native ducks and 39 native chickens were tested. Although 11 rural birds infected with Sarcocystis have been studied in three cases with acute pulmonary symptoms, in five cases with musculoskeletal disease and in three others with neurological symptoms [18], in our study no clinical signs was recorded and didn’t observed in the studied birds. Based on the results, 96.5% of all studied samples infected with Sarcocystis (Table 2). The results of 191 chickens, 514 ducks and 9 pigeons showed that only 17 (9.8%) of the studied chickens had Sarcocystis isolated from their nervous system and identified but in other species (ducks, pigeons) no parasites observed. Results of poultry survey in central Nigeria showed that 3 out of 40 poultry infected with Sarcocystis [19]. Surveys of native birds in New Zealand have shown 11% of Sarcocystis infection [20]. Lithuania's results showed that only one of the 97 poultry (21 turkeys and 76 poultry) were infected by Sarcocystis [21]. Comparison of the results of this study with the results of other researchers in different parts of the world proves that the infection of this protozoan in native poultry of Mazandaran province is at high rate. Since the identification of parasite’s species and their definitive hosts were not considered in this study, therefore, irrespective of the type of parasitic species and their definitive hosts, the main reasons for this may be due to the presence of suitable parasitic species and the diversity of the definitive hosts. Our study area, together with other environmental factors, has provided the appropriate conditions for this protozoan activity. However, this requires substantial research in this area.
Table 2. Percentage and Number of different infected species of birds with Sarcocystis
P-Value |
df |
χ2 |
Non- Infected |
Infected |
Bird type |
˂0.05 |
1 |
0.02 |
Percentage |
Number |
Percentage |
Number |
|
0 |
0 |
100 |
18 |
Native duck |
2 |
5.21 |
2 |
94.78 |
37 |
Native chicken |
|
3.5 |
2 |
96.5 |
55 |
total |
Based on the results all of the studied ducks (100%) were infected with the Sarcocystis protozoa, which is higher than the percentage of indigenous chicken (94.78%) Table 2. Chi-square test showed no significant difference between infection rates between the two groups (native duck - native chickens) (P <0.05). However, the reason for this difference may depends on the environment and the way the ducks live. Basically, ducks live in humid and abundant water. This makes it easy for the definitive host to excrete the stool and spread the parasite. Therefore, the contamination is higher than other native chickens. Research shows that ducks are more likely to be infected than other birds due to direct and permanent contact with muddy and sludge fields along with the excretion of definitive hosts or contaminated meats containing adult cysts [9].
According to the results of this study, the percentage of infection in different age groups in native ducks was 100% and there was no difference between them (Table 3). Whereas in the studied poultry, the percentage of infection was different in different age groups and the percentage of contamination increased with increasing age of the poultry (Table 3). Chi-square test showed no significant difference between infection rates among different age groups (P <0.05). Also, this difference was not significant in the studied poultry (native duck - native poultry) (P <0.05). In one study of poultry, Sarcocystis infection in under eight weeks’s old group was zero and in over eight weeks’s group was 7.5% [19]. Although with age, the likelihood of getting involved with infectious agents increases but due to the short life span of the parasite [22], this difference is not significant in our age groups with a range of six months.
Table 3. The relationship between the age of the birds and the prevalence of Sarcocystis
Bird type |
Age |
Infected |
Non-Infected |
χ2 |
df |
P-Value |
|
|
Number |
Percentage |
Number |
Percentage |
0 |
2 |
˂0.05
|
Native duck |
˂ 6 months |
12 |
100 |
0 |
0 |
6 months to 1 year |
13 |
100 |
0 |
0 |
> 1 year |
3 |
100 |
0 |
0 |
Native chicken
|
˂ 6 months |
2 |
66.33 |
1 |
33.33 |
2.703 |
6 months to 1 year |
34 |
97.22 |
1 |
2.77 |
> 1 year |
1 |
100 |
0 |
0 |
total |
˂ 6 months |
4 |
80 |
1 |
20 |
1.689 |
6 months to 1 year |
47 |
97.91 |
1 |
2.09 |
> 1 year |
4 |
100 |
0 |
0 |
Infectious Sarcocystis is an opportunistic infection that can be easily manifested in people with AIDS or immunocompromised patients [23]. We hope that the results of this study In the future, in addition to better understanding the epidemiology of this parasite in poultry population, help to identification of common species in the province and examining its possible relationship with human populations in the province of Mazandaran should be a step in improving community health.
We would like to thank the laboratory staffs of the Veterinary Research Center who provided the necessary assistance to cooperate with our project.
- Tenter AM, Johnson AM (1997) Phylogeny of the tissue cyst-forming coccidia. Adv Parasitol 39: 69-139. [Crossref]
- Mehlhorn H, Heydorn AO (1978) The Sarcosporidia (Protozoa, Sporozoa): life cycle and fine structure. Advan Parasitol 16: 43-91.
- Hu JJ, Liao JY, Meng Y, Guo YM, Chen XW, et al. (2011) Identification of Sarcocystis cymruensis in wild Rattus flavipectus and Rattus norvegicus from Peoples Republic of China and its transmission to rats and cats. J Parasitol 97: 421-424. [Crossref]
- Gjerde B (2012) Morphological and molecular characterization and phylogenetic placement of Sarcocystis capreolicanis and Sarcocystisilvan. Sp. from roe deer (Capreoluscapreolus) in Norway. Parasitol Res 110: 1225-1237. [Crossref]
- Kutkiene L, Prakas P, Sruoga A, Butkauskas D (2010) The mallard duck (Anas platyrhynchos) as intermediate host for Sarcocystis wobeseri sp. nov. from the barnacle goose (Branta leucopsis). Parasitol Res 107: 879-888. [Crossref]
- Dubey JP, Speer CA, Fayer R (1989) Sarcocystisosis of animals and man. Boca Raton, Florida: CRC Press.
- Fayer R (2004) Sarcocystis spp. In human infections. Clini Microbiol Reviews 17: 894-902.
- Odening K (1997) Relations between occurrences of Sarcocystisinfection in wild, domestic and zoo animals. Der Zoologische Garten 67: 317-340 (In German with English summary).
- Erickson AB (1940) Sarcocystis in birds. The Auk, lvii. pp. 514-519.
- Cawthorn RJ, Rainnie D, Wobeser G (1981) Experimental transmission of Sarcocystis p. (Protozoa: Sarcocystidae) between the shoveler (Anasclypeata) duck and striped skunk (Mephitis mephitis). J Wildl Dis 17: 389-394.
- Wicht RJ (1981) Transmission of Sarcocystisrileyito the striped skunk (Mephitis mephitis). J Wildl Dis 17: 387-288.
- Dubey JP, Rosenthal BM, Felix TA (2010) Morphologic and molecular characterization of the Sarcocystis of Sarcocystis rileyi (Apicomplexa: Sarcocystidae) from the mallard duck (Anas platyrhynchos). J Parasitol 96: 765-770.
- Costanzo GR (1990) Sarcocystis in American black ducks wintering in New Jersey. J Wildl Dis 26: 387-389. [Crossref]
- Fedynich AM, Pence DB (1992) Sarcocystis in mallards on the Southern High Plains of Texas. Avian Dis 36: 1067-1069. [Crossref]
- Box ED, Smith JH (1982) The intermediate host spectrum in a Sarcocystis species of birds. J Parasitol 68: 668-673. [Crossref]
- Khordadmehr M, Ranjbar VR, Shabazi P, Zeinoddin M (2018) CO-INFECTION OF SARCOCYSTIS SP. AND HADJELIA TRUNCATA IN FANTAIL PIGEONS (COLUMBA LIVIA DOMESTICA). Bulg J Veter Med 21: 115-121.
- Nourollahi Fard SR, Asghari M, Nouri F (2009) Survey of Sarcocystis infection in slaughtered cattle in Kerman, Iran. Trop Anim Health Prod 41: 1633-1636. [Crossref]
- Villar D, Kramer M, Howard L, Hammond, Cray C, et al. (2008). Clinical Presentation and Pathology of Sarcocystisosis in Psittaciform Birds: 11 Cases. Avian Dis 52: 187-194.
- Peter AM, Tukur MI, Deborah MB, Philomena EA, Stephen A (2018) Prevalence of Sarcocystis Species in Meat of Cattle, Pigs and Birds slaughtered in North Central Nigeria. J Veter Sci Med Diagn 7:1-6.
- Moré G, Basso W, Bacigalupe D, Venturini MC, Venturini L (2008) Diagnosis of Sarcocystis cruzi, Neospora caninum, and Toxoplasma gondii infections in cattle. Parasitol Res 102: 671-675.
- Petras P (2012) Protozoan parasites from genus Sarcocystis and their investigations in Lithuania. EKOLOGIJA 58: 45-58.
- Franz RM (1981) Life Cycle of Sarcocystis between Poikilothermie Hosts. Lizards are Intermediate Hosts for S. podarcicolubris sp. Nov, Snakes Function as Definitive Hosts. Z. Naturforsch. 36 c, 1093-1095.
- Adejinmi JO, Osayomi JO (2010) Prevalence of intestinal protozoan parasites of dogs in Ibadan, south western Nigeria. J Anim Plant Sci 7: 783-788.

