Particularly in the field of smart drug delivery, polymer played a significant role because it can deliver therapeutic agents directly into the intended site of action, with superior efficacy. The perfect requirements for designing nano-particulate delivery system are to effectively be controlled particle size, surface character; enhance permeation, flexibility, solubility and release of therapeutically active agents in order to attain the target and specific activity at a predetermined rate and time. The smart drug delivery systems have been successfully made by the advances in polymer science in the bio-nanotechnology field. Recently, these advances have been found in various medical applications for nano-scale structures in smart drug delivery. The smart drug delivery systems should possess some important feature such as pre-scheduled rate, self controlled, targeted, predetermined time and monitor the delivery. This article comments on the important efficacy of polymer nanoparticles for drug delivery which is has enormous benefits to medical science and polymer industry.
Polymer nano particles, drug delivery, applications
In the field of smart delivery of drug into human system polymer nano particles have been playing significant role in medical field. In order to treat the effected area. Drug-targeting aims at reducing the obstacles caused by extra-target effects, the narrow therapeutic index and the inactivation of the drug before reaching its target. Unfortunately, most of the pharmaceutics used nowadays lack a selective delivery into their target tissues. The selective delivery or selective activation in the targeted tissue minimizes toxic side effects [1]. The improvement of the pharmacokinetic profile should be accomplished by drug targeting strategies [2,3]. However, diverse drug-targeting concepts have been pinpointed in order to fulfill those principles [4,5].
A variety of drug delivery systems carriers such as viral vectors [6], colloidal particles or macromolecular carriers (such as liposomes [7], nanoparticles [8], microspheres [9], lipid particles and polymeric micelles [10-12], modified-plasma proteins, polysaccharides [13], biodegradable carriers [14], dendrimers [15], antibodies [16], and peptide carriers have been developed [17].
A huge diversity of drug delivery systems has been developed to boost the therapeutic effect in the target tissue. The exclusive transfer of a drug to the targeted site of action that has been accomplished with minimal toxic side effects, and the usage of a pharmacologically inactive vector are main features of an ideal carrier for drug-targeting delivery system [18]. A specifically targeting drug delivery system can serve not only as a therapeutic vehicle but also as a research tool. Generally, the drug-targeting approach depends on the drug to be delivered, the target tissue, and of course the proper delivery system [17].
Carriers might be classified into three major types; the particle-type, the soluble and the cellular carriers. Liposomes, nanoparticles, microspheres, lipid particles, and polymeric micelles are all included in the particle-type carriers. Whereas peptides, modified-plasma proteins, polysaccharides, and biodegradable carriers are classified under soluble carriers. However, viral vectors belong to the cellular carriers. Despite the advantages provided by the cellular carriers depending upon their natural biocompatibility and their possibility to cause an immunological response is still a obstacle [18].
In recent decades, polymers are widely used as biomaterials due to their favorable properties such as good biocompatibility, easy design and preparation, a variety of structures and interesting bio-mimetic character. Particularly in the field of smart drug delivery, polymer played a significant role since it can deliver therapeutic agents directly into the intended site of action, with superior efficacy. The ideal requirements for designing nano-particulate delivery system are to effectively be controlled particle size, surface character; enhance permeation, flexibility, solubility and release of therapeutically active agents in order to attain the target and specific activity at a predetermined rate and time [18]. The smart drug delivery systems have been profitably made by the advances in polymer science in the bio-nanotechnology field. Recently, these advances have been found in various medical applications for nano-scale structures in smart drug delivery.
Polymers can be classified according to their natural or synthetic origin, stability (whether biodegradable or not), backbone, and upon their chemical nature (vinyl and acrylic polymers, polyethylene glycol (PEG), polysaccharides, polyamino acids, etc.) [19]. The natural polymer carriers comprises several polysaccharides (dextrans, inulin, or chitosan), proteins (albumin) or glycoproteins (transferrin), as well as cationic polymeric carriers such as PLL (poly-l-lysine) backbones [20-25].
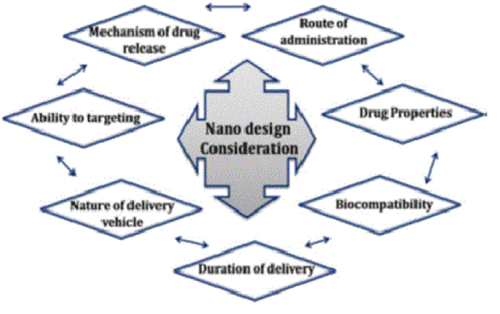
Figure 1. Requirements of several factors for simultaneous consideration to design a polymeric nanoparticle for the smart drug delivery system [5].
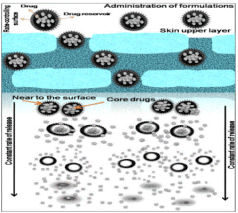
Figure 2. Schematic diagrams represent the rate controlled drug delivery systems of topical pplications.
Table 1. Examples of various nanoparticles with different functionalization and therapeutic uses based on the targetPolymer Nanoparticles for Smart Drug Delivery [5].
Nanoparticles |
Functionalization |
Drug |
Use |
Reference |
Human serum |
albumin Amino/acid group |
Doxorubicin |
Antineoplastic |
260 |
Trimyristin |
Sterically stabilized |
Paclitaxel |
Ovarian, lung, breastcancer
|
261 |
PLLA-b-PEG |
Folate targeted |
Doxorubicin |
Solid tumors |
262 |
PEG-PE |
Lipid conjugated |
Paclitaxel |
Various cancers |
263 |
PEG |
Lipid conjugated |
Tamoxifen |
Lung carcinoma |
264 |
Polymer-lipid hybrid |
Lipid conjugated |
Doxorubicin |
Solid cancer |
265 |
PCL-b-trimethylene carbonate-PEG
|
Serum protein |
Ellipticin |
Anticancer |
266 |
PAMAM |
dendrimers |
Folic acid ethotrexate |
Epithelial cancer |
267 |
PEG |
Albumin bound |
Doxorubicin |
Various cancers |
268 |
Micelles |
Biotin-antibodyconjugated |
Daunomycin |
Brain tumor |
269 |
Poly(DEAP-Lys)-b-PEG -b-PLLA)
|
Poly(lysine |
Doxorubicin |
pH sensitive tumor |
271 |
PLGA-b-PEG-COOH |
PSMA |
Anti cancer |
Prostate Cancer |
272 |
PEG or PE particles |
Transferrin |
Oligonucleotide |
Brain- gene |
273 |
PLLA-PEG |
Biotin |
Anti cancer |
Cancer therapy |
274 |
Polystyrol |
Sc-TNF |
Anti cancer |
Cancer therapy |
275 |
PLA |
Aptamer |
Anti cancer |
Prostate cancer |
276 |
PE |
RGD peptides |
siRNA |
Vasculature cancer |
277 |
mPEG/PLGA |
Peptidomimetics |
Anti cancer |
Brain cells cancer |
278 |
PLA |
Galactose |
Retinoic acid |
Hepatocytes |
279 |
PLGA |
MP lipid A |
Anti cancer |
Dentritic cells |
280 |
Nanoparticles and microspheres can be assign either to the soluble or to the particle type carriers. The major backbone of these carriers is based on range of polymers such as dextran, ficoll, sepharose, or PLL. Nowadays, poly(D,L-lactic-co-glycolic-acid) (PLGA) microspheres have been expansively studied to gain a wide acceptance for application as nanoparticles and microspheres following to their approval for use in humans by the American Food and Drug Administration (FDA) [26]. Parenteral application of microspheres and nanoparticles for the cell selective delivery of drugs is not the only administration route since they have been studied more recently for their application in oral and pulmonary delivery of peptides and peptidomimetics [20].
Antibodies are aimed at targeting tumor-associated antigens with the aim of over-expressed by tumor cells. Therefore, the birth of innovative techniques such as recombinant DNA and protein engineering has led to the development of optimal tailored-antibodies [22]. The stretch of amino acids or peptides, contained by a biomolecule which is responsible for specific receptor binding, is called the homing-ligand. Their covalent attachment to a carrier backbone can result in targeted DDS [23]. Polymeric micelles are small (10-100nm) in size and they have a core-shell structure. In contrast to their hydrophobic core, the shell represents their hydrophilic part. Micelles provide a penetration property within the tissue for targeted DDS. Usually, their efficacy is still demanding since they disintegrate rapidly in vivo [23-26]. Usually, the application of peptide carriers requires an over-expressed receptor and adequatein vivo stability. Additionally, the conjugated drug should not hamper with the binding region of the peptide. Peptides were developed in different ways, for example for targeted transport using carrier peptides for tumor and tissue targeting [25-26].
Kidney diseases require, usually, long-term administration of therapeutics; therefore, they are always accompanied with systemic toxicities and adverse side effects. The uses of bio-nanotechnology in therapeutics a number of unexpected inventions have been done recently on polymer based nano meters, which have great attention in the field of smart drug delivery applications. Several attempts have been performed aiming to target the kidneys, and the majority of kidney-specific delivery systems targets the proximal tubular cells and may contribute to the treatment of renal diseases. Additionally, the overall charge of the carrier seems to play a key role in kidney-specific drug delivery. The biomaterials including protein based nano polymers, polysaccharide based polymers, natural or synthetic or semi-synthetic nano polymers, various biomaterials and combination of polymer have utilized to prepare various kinds of nano-formulations towards the smart drug delivery applications. Such strategies involve LMWPs, PVP, PVD, LMWC, G3-C12 peptide,ε PLL derivatives and the carrier peptide (KKEEE)3K. The specific uptake by renal proximal tubular cells is confirmed in many cases to bemegalin-mediated endocytosis. Several nanoparticle based drug delivery systems have been approved in clinical trials, some of them in under pre-clinical trial levels.
The authors acknowledge management of University of Namibia for encouragement in research.
- Ding S, Bierbach U (2015) Target-selective delivery and activation of platinum-based anticancer agents. Future Med Chem 7: 911-927. [Crossref]
- Mickan A, Sarko D, Haberkorn U, Mier W (2014) Rational design of CPP-based drug delivery systems: considerations from pharmacokinetics. Curr Pharm Biotechnol 15: 200-209. [Crossref]
- Sarko D, Beijer B, Garcia RB, Nothelfer EM, Leotta K, et al. (2010) The pharmacokinetics of cell-penetrating peptides. Mol Pharm 7: 2224-2231.
- Mier W, Hoffend J, Haberkorn U, Eisenhut M (2005) Current Strategies in Tumor-Targeting. In: Marek Los & Spencer B Gibson (Eds.), Springer US, New York, USA, pp: 343-355.
- Melisi D, Rimoli MG (2011) Recent developments in prodrug design: drug targeting, pharmacological and pharmacokinetic improvements related to a reduction of adverse effects. Curr Top Med Chem 11: 2264. [Crossref]
- Ewer KJ, Lambe T, Rollier CS, Spencer AJ, Hill AV, et al. (2016) Viral vectors as vaccine platforms: from immunogenicity to impact. Curr Opin Immunol 41: 47-54. [Crossref]
- Nguyen TX, Huang L, Gauthier M, Yang G, Wang Q, et al. (2016) Recent advances in liposome surface modification for oral drug delivery. Nanomedicine 11: 1169-1185. [Crossref]
- Majzoub RN, Ewert KK, Safinya CR (2016) Cationic liposome-nucleic acid nanoparticle assemblies with applications in gene delivery and gene silencing. Philos Trans A Math Phys Eng Sci 374: 20150129. [Crossref]
- Andhariya JV, Burgess DJ (2016) Recent advances in testing of microsphere drug delivery systems. Expert Opin Drug Deliv 13: 593-608. [Crossref]
- Tanbour R, Martins AM, Pitt WG, Husseini GA (2016) Drug Delivery Systems Based on Polymeric Micelles and Ultrasound: A Review. Curr Pharm Des 22: 2796-2807. [Crossref]
- Gothwal A, Khan I, Gupta U (2016) Polymeric Micelles: Recent Advancements in the Delivery of Anticancer Drugs. Pharm Res 33: 18-39. [Crossref]
- Biswas S, Kumari P, Lakhani PM, Ghosh B (2016) Recent advances in polymeric micelles for anti-cancer drug delivery. Eur J Pharm Sci 83: 184-202. [Crossref]
- Maruyama A, Ishihara T, Kim JS, Kim SW, Akaike T (1997) Nanoparticle DNA Carrier with Poly(l-lysine) Grafted Polysaccharide Copolymer and Poly (d,l-lactic acid). Bioconjugate Chem 8: 735-742. [Crossref]
- Farahidah M, van der WCF (2008) Engineering biodegradable polyester particles with specific drug targeting and drug release properties. J Pharm Sci 97: 71-87. [Crossref]
- Kaminskas LM, Kelly BD, McLeod VM, Boyd BJ, Krippner GY, et al. (2009) Pharmacokinetics and Tumor Disposition of PEGylated, Methotrexate Conjugated Poly-l-lysine Dendrimers. Mol Pharm 6: 1190-1204. [Crossref]
- Schaedel O, Reiter Y (2006) Antibodies and their fragments as anti-cancer agents. Curr Pharm Des 12: 363-378. [Crossref]
2021 Copyright OAT. All rights reserv
- Akhtar S (2006) Non-viral cancer gene therapy: beyond delivery. Gene Ther 13: 739-740. [Crossref]
- Aiache JM (1991) The ideal drug delivery system: a look into the future. J Aerosol Med 4: 323-334. [Crossref]
- Cao X, Lai S, Lee LJ (2001) Design of a Self-Regulated Drug Delivery Device. Biomedical Micro devices 3:109-118.
- Leroux J-C, Allemann E, Doelker E, Gurny R (1995) New Approach for the Preparation of Nanoparticles by an Emulsification-Diffusion Method. European Journal of Pharmaceutics and Biopharmaceutics 41: 14-18.
- Schroeder U, Sommerfeld P, Ulrich S, Sabel BA (1998) Nanoparticle Technology for Delivery of Drugs across the Blood-Brain Barrier. J Pharm Sci 87: 1305-1307. [Crossref]
- Arshady R (1991) Preparation of Biodegradable Microspheres and Microcapsules: 2. Polylactides and Related Polyesters. Journal of Controlled Release 17: 1-22.
- Rodrigues Jr JM, Fessi H, Bories C, Puisieux F, Devissaguet JP, et al. (1995) Primaquine-Loaded Poly(Lactide) Nanoparticles: Physicochemical Study and Acute Tolerance in Mice. Int J Pharmaceut 126: 253-260.
- Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S, et al. (1989) Nanocapsule Formation by Interfacial Polymer Deposition Following Solvent Displacement. Int J Pharmaceut 55: R1-R4.
- Peltonen L, Koistinen P, Karjalainen M, Hakkinen A, Hirvonen J, et al. (2002) The Effect of Cosolvents on the Formulation of Nanoparticles from Low-Molecular-Weight Poly(L)Lactide. AAPS Pharm Sci Tech 3: E32. [Crossref]
- Niwa T, Takeuchi H, Hino T, Nohara M, Kawashima Y, et al. (1995) Biodegradable Submicron Carriers for Peptide Drugs: Preparation of Dl-Lactide/Glycolide Copolymer (PLGA) Nanospheres with Nafarelin Acetate by a Novel Emulsion-Phase Separation Method in an Oil System. Int J Pharmaceut 121: 45-54.


