The presencegas of primary and secondary phytochemical compounds in petroleum ether, acetone and ethanol extracts of Phyllanthus amarus was detected qualitatively as well as quantitatively. Alkaloids, terpenoids, tannins, polyphenols and quinines were present in the petroleum ether extract of P. amarus. Terpenoids, flavonoids, tannins, saponins, cardiac glycosides and quinines were present in the acetone extract, whereas alkaloids and terpenoids were absent in ethanol extracts of P. amarus. From the different solvent extracts of P. amarus, overall, 20 secondary phytochemicals were identified. Among these, presence of six bioactive compounds {Dodecanoic acid; Propanoic acid, 2-(tricyclo [3.3.1.13, 7] dec-2-ylidene)-; Tetradecanoic acid; Neophytadiene; Hexadecanoic acid, ethyl ester, and 9, 12, 15-Octadecatrienoic acid, (Z, Z, Z)-} have been identified through literature. Among the three solvents, ethanol extract of P. amarus enriched Artemia nauplii was found to produce the best survival, growth, nutritional indices (weight gain and specific growth rate) and concentrations of basic biochemical constituents (total protein, amino acid, carbohydrate, and lipid) in M. rosenbergii early post larvae. This suggests that the general health of the prawn was improved. This study indicates the fact that the primary phytochemical quality and secondary bioactive compounds of P. amarus have the potency to sustainably enhance the survival, growth and nutritional quality of M. rosenbergii. Thus, this herb is recommended as a feed additive.
prawn, growth, artemia, P. amarus, feed additive
Medicinal plants provide an inexhaustible resource of raw materials for the pharmaceutical, cosmetics, food industries and more recently in agriculture for pest control [1]. The herbal extract could be defined as the compounds and /or mixture of compounds obtained from fresh or dried plants, or parts of plants, such as leaves, flowers, seeds, roots and barks. The carry me seed plant, Phyllanthus amarus is a common pantropical weed that grows well in moist, shady and sunny places. It is widely spread throughout tropics and subtropics [2]. It has been used in Ayurvedic medicine for over 2,000 years and has a wide number of traditional uses. It has been reported to be used in treating jaundice by blocking DNA polymerase of hepatitis B virus [3-5]. There are also reports that it is used to treat diabetes, otitis, diarrhoea, gonorrhoea, and frequent menstruation, galactogogue, leucorrhoea, menorrhagia, mammary abscess, skin ulcer, hypertension, pain, inflammation, cough, asthma bronchitis, gastrointestinal disturbances, helminth infestation, nociceptive pain, mutation and cancer. It has a urolithic property, dissolving renal calculi [6].
P. amarus contains moderate amount of protein, rich in carbohydrate and low in fat, ash, crude fiber. It also contains Mg, Ca, K, PO and ascorbic acid, Fe; Zn, thiamine, niacin and riboflavin [7]. It has been reported that P. amarus contains many valuable compounds, such as lignans, flavonoids, hydrolysable tannins (ellagitannins), polyphenols, triterpenes, sterols and alkaloids [8]. The extracts and the compounds isolated from P. amarus reported to have a wide spectrum of pharmacological properties including anti-viral and anti-fungal, anti-bacterial, anti-plasmodial and anti-inflammatory, anti-tumor, anti-cancer, anti-diabetic, anti-oxidative, anti-hyperglycemic, hypolipidemic, immuno-stimulatory, hepatoprotective, nephroprotective and diuretic [7, 9-18].
The research in the use of plant extracts for aquatic animals is increasing with the demand for eco-friendly and sustainable aquaculture [19]. The medicinal plants have phytochemicals, the bioactive substances which are responsible for the biological activities they exhibit. For this reason, herbal extracts were introduced in the pharmacopoeias [20]. The herbal biomedicine active principles in the aquaculture have the characteristics of growth and survival promoting ability, tonic to improve the immune system, anti-microbial capability, anti-stress characteristics and stimulating appetite due to presence of alkaloids, flavonoids, pigments, phenolics, terpenoids, starch, steroids and essential oils. Recent studies showed that the incorporation of medicinal plants (raw material, extracts and phytocompounds) as additives of fish/prawn feeds stimulates the growth and immune responses [21-34].
The freshwater prawn, Macrobrachium rosenbergii is the most popular one for the commercial farming in many countries. It gained momentum after the setback in shrimp farming due to disease outbreaks and other factors. Newly-hatched M. rosenbergii larvae are around 2 to 2.2 mm in length. They normally start feeding about 1 day after hatching and initially require live zooplankton. Their prey should be of a suitable size for ingestion; the optimal size is estimated at 300 to 500 µm.
Live organisms serve as living capsules of nutritive elements such as essential proteins, amino acids, lipids, fatty acids, carbohydrates, vitamins and minerals. Artemia nauplii are the most widely used live food organism for the seed production of marine as well as freshwater fish and crustaceans [35]. Newly-hatched Artemia nauplii are considered the most successful starter diet during the first rearing week but, after that, are usually fed in combination with prepared diets, since this is an omnivorous [36]. Normally, 2, 00,000 to 3, 00,000 nauplii are hatched from each gram of high-quality brine shrimp cysts [37], Prawn larvae and early post-larvae prefer live organisms than formulated feeds.
Artemia has high nutritive value and high conversion efficiency. All the life stages of Artemia, i.e. cysts (after decapsulation), nauplii, juveniles, sub-adults are used as feed. Today, in majority of the commercial aqua hatcheries, Artemia nauplii is virtually used as a sole diet. Frozen adult Artemia, are widely used by aquarists, fish breeders and aqua culturists. Artemia biomass is also used as food additive for domestic livestock or extraction of pharmaceutical products as also in making protein rich food products. It is even used for human consumption in some countries. Owing to its great utility, Artemia trading is a growing business in several parts of the world [38].
Therefore, in the present study, an attempt has been made to check the primary and secondary phytochemical compounds present in petroleum ether, acetone and ethanol extracts of P. amarus, the whole plant. These extracts were subjected to enrichment on Artemia nauplii and fed to M. rosenbergii early post-larvae (PL) for evaluation of its nutritional indices [survival rate (SR), length gain (LG), weight gain (WG) and specific growth rate (SGR)], and quantification of concentrations of basic biochemical constituents (total protein, amino acid, carbohydrate and lipid).
The traditional herb carry me seed plant, Phyllanthus amarus was collected at Bharathiar University campus, Coimbatore, Tamil Nadu, India [Figure 1] and authenticated with Botanical Survey of India, Coimbatore. The herb was thoroughly washed with freshwater, blotted and spread out and dried for 2 weeks at room temperature. Shade dried herb was ground to fine powder. The powered sample was stored in sterile containers for further use.

Figure 1. The carry me seed plant, Phyllanthus amarus Linn. (Schumach & Thonn), Infra Kingdom: Streptophyta; Super Division: Embryophyta; Division: Tracheophyta; Subdivision: Spermatophytina; Class: Magnoliopsida; Superorder: Rosanae; Order: Malpighiales; Family: Phyllanthaceae (Integrated Taxanomic Information System); a). Whole plant; b). Branches of P. amarus with leaves and seeds; c). Root of P. amarus.
P. amarus is a small erect annual herb (10-60 cm tall) with numerous small oblong-elliptic or squarish leaves and glabrous (6-12 mm long). The main stem is simple or branched, terrete smooth or scabridulous in younger parts [39]. Flowers are very small, yellow in colour and hang down in beautiful array hidden below the leaves. The flowers produce very small (2 mm) fruits that burst open and the seeds are hurled away. When the plants are picked, the feathery leaves fold in, completely closing themselves. P. amarus is a common pantropical weed that grows well in moist, shady and sunny places. It is widely spread throughout tropics and subtropics [2].
Preparation of extracts
The powdered whole plant sample of P. amarus (50 g) was taken and packed in Whatmann No. 1 filter paper and put into soxhlet apparatus. The extracts were successively soaked with 300 ml (1:6 w/v) of petroleum ether (99.98% purity, SRL Pvt. Ltd. India), acetone (99.5% purity, SD Fine Chemicals, India) and ethanol (99.9% purity, Changshu Yangyuan Chemicals, China) individually and sequentially extracted for 6-9 h each (30 to 36 cycle) based on their polarity (non-polar to polar). Repeated extraction was done until a clear colorless solution was obtained. The extracts were filtered by using double layer muslin cloth and concentrated at 40-50 °C using rotary vacuum evaporator (ROTAVAP). The extracts obtained were vacuum-dried under 40 °C and used for further investigation. The extracts obtained were appeared as dark green, gummy solid.
Qualitative analysis of primary phytochemical substances
The extracts were subjected to detection of the presence of primary phytochemical bio-molecules, such as alkaloids, terpenoids, flavonoids, tannins, polyphenols, saponins, and cardiac glycosides using the standard qualitative procedures of Trease & Evans [40].
Gas Chromatography-Mass Spectrum (GC-MS) analysis for secondary phytochemical compounds
Different solvent extracts of P. amarus were subjected to GC-MS analysis (Thermo GC-trace ultra ver-5.0; Thermo MS-DSQ-II; ZB 5-MS capillary standard non-polar column (30 mts, 0.25 mm id, 0.25 µm film) for identification of different phytochemical compounds (South India Textile Research Association (SITRA), Coimbatore, Tamil Nadu, India). The working condition of GC-MS was as follows: Carrier Gas, He; Flow, 1.0ml/min; Temperature Programme, oven temperature raised from 70 °C to 260 °C at 6 °C/min; Injection Volume, 1 µl; Detector, mass selective detector MS-DSQ-II with XCALIBUR software; Injector temperature, 250 °C; Ion source temperature, 200 °C; Interface temperature, 250 °C; Total running time, 40 min; Relative percentage constituents was expressed as percentage with peak area normalization. Different solvent extracts of P. amarus were subjected to GC-MS analysis (Thermo GC-trace ultra ver-5.0; Thermo MS-DSQ-II; ZB 5-MS capillary standard non-polar column (30 mts, 0.25 mm id, 0.25 µm film) for identification of different phytochemical compounds (South India Textile Research Association (SITRA), Coimbatore, Tamil Nadu, India). The working condition of GC-MS was as follows: Carrier Gas, He; Flow, 1.0ml/min; Temperature Programme, oven temperature raised from 70 °C to 260 °C at 6 °C/min; Injection Volume, 1 µl; Detector, mass selective detector MS-DSQ-II with XCALIBUR software; Injector temperature, 250 °C; Ion source temperature, 200 °C; Interface temperature, 250 °C; Total running time, 40 min; Relative percentage constituents was expressed as percentage with peak area normalization.
Peaks resolved with relative abundance of 0-100 were considered as major compounds. To show the minor peaks, the chromatogram was magnified. Identification of various components present in each extract was confirmed based on the peak area, retention time and molecular formula. Identification of various components present in these extracts were assigned by the comparison of their retention indices and mass spectra fragmentation patterns with those stored on the computer library and also with published literatures. National Institute Standard and Technology (NIST4) and WILEY9 [41], on-line library source was also used for matching the identified components.
Proximate composition of P. amarus
The proximate composition of P. amarus whole plant powder, such as contents of moisture, crude protein, crude fibre, crude fat and ash were estimated following AOAC methodology [42], and total nitrogen free extract (carbohydrate) content was calculated by subtracting all the above contents adopting Castell & Tiews method [43].
Artemia cyst hatching and its enrichment
The brine shrimp, Artemia franciscana cysts were purchased from Aqua world, Paris Corner, Chennai, India. The cysts (2 g/ 20 L and 15 g kg-1 body biomass of the prawns for feeding trial) were taken and hydrated in 1 L-1 of purified artificial saltwater (prepared from artificial sea salt powder 35.0 g L-1, pH of 6.5). After 12-15 h, the cysts burst, and the embryo surround by the hatching membrane become visible. After a few hours, the brownish orange colored nauplii came out. The 48-hr old Artemia nauplii were collected on a sieve and enriched with 1% of petroleum ether, acetone and ethanol extracts each of P. amarus separately for 1 h at the rate of 1 g in 100 ml.
Procurement and acclimatization of experimental animal
The early post larvae (PL-10) of the freshwater prawn, M. rosenbergii were procured from Sri Durgai Hatcheries, Chengalpattu, Tamil Nadu, India. They were transported to the laboratory in polythene bags filled with oxygenated water. The prawns were acclimatized to the ambient laboratory condition with ground water in cement tanks (6 × 3 × 3 feet) for a week (temperature, 26.5±1.3 °C; pH, 7.01±0.12; TDS, 0.80±0.05 g/l; DO, 7.30±0.49 mg/l; BOD, 29.0±1.24 mg/l; COD, 124.0±3.2 mg/l; ammonia, 0.026±0.007 mg/l). During acclimatization the PL were fed with un-enriched Artemia nauplii. About half of the tank water was renewed each day and adequately aerated to maintain a healthy environment. This ensures an environment devoid of accumulated metabolic wastes and sufficient oxygen supply to the prawns. The unfed feeds, faeces, moult and dead prawns were removed by siphoning.
Feeding trails with extracts of P. amarus enriched Artemia nauplii
M. rosenbergii early PL ranging from 0.8±0.06 cm in length and 0.06 ± 0.02 g in weight were used in a triplicate experimental set up. The prawns were divided into four groups. One group served as control and fed with un-enriched Artemia nauplii and the other three groups were fed with 1% of petroleum ether, acetone, and ethanol extracts of P. amarus enriched Artemia nauplii at the rate of three times per day (8.00 a.m., 2.00 pm and 10.00 pm) for 30 days. At the end of the feeding trial the final length and weight were measured for calculating nutritional indices and estimating basic biochemical constituents. During the feeding trial the water medium was renewed daily by siphoning method without severe disturbance to the prawn and was aerated adequately.
Determination of nutritional indices
The survival rate (SR), length gain (LG), weight gain (WG), and specific growth rate (SGR) were individually calculated Tekinay & Davis [44].
- Survival (%) = Total No. of live animals/Total No. of initial animals × 100
ii. Length gain (cm) = Final length (cm) – Initial length (cm)
iii. Weight gain (g) = Final weight (g) – Initial weight (g)
iv. Specific growth rate, (%) = log W2 – log W1/ t ×100
Where, W1 & W2 = Initial and Final weight respectively (g), and t = Total number of experimental days.
Estimations of basic biochemical constituents
The initial and final concentrations of basic biochemical constituents, such as total protein, amino acid and carbohydrate were estimated in test prawns adopting the methodology of Lowry et al., [45], Moore & Stein [46], and Roe [47], respectively, and the total lipid was extracted following the method of Folch et al., [48] and estimated gravimetrically following the method of Barnes & Blackstock [49].
Statistical analysis
Data between control versus experiments and between experiments were subjected to statistical analysis through one-way ANOVA and subsequent post hoc multiple comparison with DMRT by adopting SPSS (v20). All the details of statistical analyses were given in respective tables. The P values less than 0.05 were considered statistically (95%) significant.
Primary phytochemicals of P. amarus
The petroleum ether extract of P. amarus showed presence of 5 primary compounds, such as alkaloids, terpenoids, tannins, polyphenols and quinines. Of which, tannins were luxuriantly present, alkaloids and terpenoids were moderately present. The other compound, such as polyphenols and quinones were poorly present [Table 1]. In acetone extract of P. amarus, the presence of 6 compounds, such as terpenoids, flavonoids, tannins, saponins, cardiac gylcosides and quinines were detected. Of which, tannins and cardiac gylcosides were luxuriantly present, flavonoids and saponins were moderately present. The other compounds, such as terpenoids and quinones were poorly present [Table 1]. Similarly, the ethanol extract of P. amarus contains 6 primary compounds, such as, flavonoids, tannins, polyphenols, saponins, cardiac gylcosides and quinines. Of which flavonoids and tannins were luxuriantly present, polyphenols and quinones were moderately present, and other compounds, such as saponins and cardiac gylcosides were poorly present [Table 1]. Therefore, overall, P. amarus contains 8 primary phytochemical compounds. In this study, ethanol has served as the best solvent for extraction of flavonoids. For the extraction of tannins, all three solvents can be used. Ethanol can be used for the extraction of saponins, while, acetone can be used for the extraction of cardiac glycosides. For extractions of alkaloids and terpenoids, petroleum ether can be used. Ethanol can also be used for extractions of polyphenols and quinines.
Table 1: The primary phytochemicals present in P. amarus extracted with different solvents.
Phytochemicals |
Solvents |
Petroleum ether
(non-polar) |
Acetone
(middle-polar) |
Ethanol
(Polar) |
Alkaloids |
++ |
-- |
-- |
Terpenoids |
++ |
+ |
-- |
Flavonoids |
-- |
++ |
+++ |
Tannins |
+++ |
+++ |
+++ |
Polyphenols |
+ |
-- |
++ |
Saponins |
-- |
++ |
+++ |
Cardiac gylcosides |
-- |
+++ |
+ |
Quinones |
+ |
+ |
++ |
+, Poorly present; ++, Moderately present; +++, Luxuriantly present; --, Absent
Secondary phytochemicals of P. amarus
Overall, different solvent extracts of P. amarus revealed presence of 20 secondary compounds, of these, 7 are in petroleum ether, 6 are by acetone and 7 are from ethanol extracted samples [Table 2]. The details of compounds present in the individual extract, the active principles with their retention time, molecular formula, molecular weight, peak area, similar index and reverse similar index in percentage are presented [Tables 3-5]. About 30% of active principal compounds possessed biological properties, which have been identified from known sources.
Table 2: Overall secondary phytochemical compounds present in different solvent extracts of P. amarus.
Sl. No. |
Peak
RT |
Solvent |
Name of the compound |
Molecular
formula |
Chemical structure
|
1. |
3.98
|
Acetone |
2-Butanol, 3-methoxy-
|
C5H12O2 |
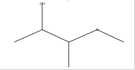
|
2 |
4.71 |
Ethanol |
Cholest-5-ene-1á,3á,16á,26-tetrol
|
C27H46O4 |
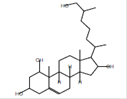
|
3 |
5.18 |
Petroleum ether |
(R)-2-(1-Methylethylidene)-cyclohexane-drt |
C9H15D
|
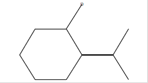
|
4 |
8.13 |
Ethanol |
Cholestane-3á,5à,6á,26-tetrol - 26-Acetate |
C29H50O5 |
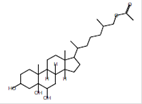
|
5 |
9.78
|
Acetone |
2-Nitrocyclohexanone (CAS)
Neophytadiene |
C6H9NO3 |
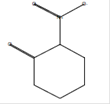
|
6 |
12.35 |
Ethanol |
3,10-Dioxa-2,11-disiladodecane, 2,2,11,11-tetramethyl- |
C12H30O2Si2
|
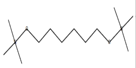
|
7. |
14.25 |
Petroleum ether |
d-Xylose
|
C5H10O5 |
|
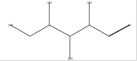
8. |
18.52 |
Petroleum ether |
Dodecanoic acid*
(lauric acid) |
C12H24O2 |
|
9. |
20.00
|
Acetone |
Propanoic acid, 2-(tricyclo[3.3.1.13,7]dec-2-ylidene)-*
|
C13H18O2
|
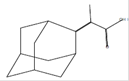
|
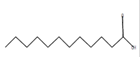
10. |
20.68 |
Ethanol |
3-(4-Acetyl-3- hydroxyphenyl)-2-propenoicacid |
C11H10O4 |
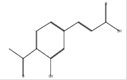
|
11. |
22.86 |
Petroleum ether |
Tetradecanoic acid*
(myristic acid) |
C14H28O2 |
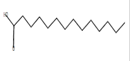
|
12. |
24.40
|
Acetone |
Neophytadiene*
|
C20H38
|
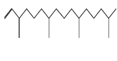
|
13. |
27.19 |
Petroleum ether |
(E)-5,10-secocholest-1(10)-en-3,5-dione |
C27H44O2
|
|
14. |
28.16 |
Ethanol |
Hexadecanoic acid, ethyl ester*
(palmitic acid) |
C18H36O2 |
|
15. |
29.99
|
Acetone |
9,12,15-Octadecatrienoic acid, (Z,Z,Z)-*
(linolenic acid) |
C18H30O2
|
|
16. |
30.26 |
Petroleum ether |
2,4-Dihydroxy-2-(2'-hydroxyethyl)cyclohex-5-en-1-one |
C8H12O4 |
|
17. |
32.61 |
Ethanol |
(Sax)-(-)-2,2'-Bis(di-2-furylphosphino)-1,1'-binaphthalene |
C36H24O6P2
|
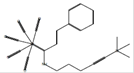
|
18. |
35.71
|
Acetone |
Methyl
(3R)-3-(tert-butyldimethylsilyloxy)-3-[3-((1S)-1-(tert-but
yldimethylsilyloxy)-3-butynyl)phenyl] propanoate |
C26H44O4Si2
|
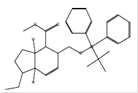
|
19. |
36.28 |
Ethanol |
5-Methoxy-8,8-dimethyl-6-(2-methylbutanoyl)-4-phenyl-2
H,8H-benzo[1,2-b:3,4-b']dipyran-2-one
|
C26H26O5 |
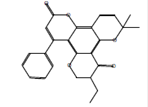
|
20. |
38.42 |
Petroleum ether |
3,3'-Dibromo-2,2'-diquinolinyl Disulfide |
C18H10Br2N2S2 |
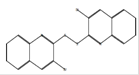
|
RT- Retention Time; *Compounds having bioactive properties
The petroleum ether extract of P. amarus revealed the presence of 7 different secondary compounds. They are, (R)-2-(1-Methylethylidene)-cyclohexane-drt; d-Xylose; Dodecanoic acid; Tetradecanoic acid; (E)-5, 10-secocholest-1(10)-en-3, 5-dione; 2, 4-Dihydroxy-2-(2'-hydroxyethyl) cyclohex-5-en-1-one; and 3, 3’-Dibromo-2, 2’-diquinolinyl Disulfide. Of which, 2 compounds, Dodecanoic acid, and Tetradecanoic acid possessed biological properties [Figure 2 & 2a; Table 3].
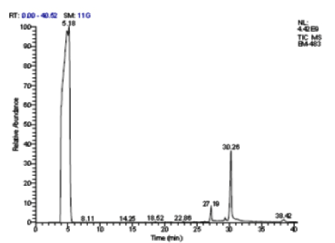
Figure 2. GC- MS chromatogram of petroleum ether extracted P. amarus (Relative abundance, 0-100).
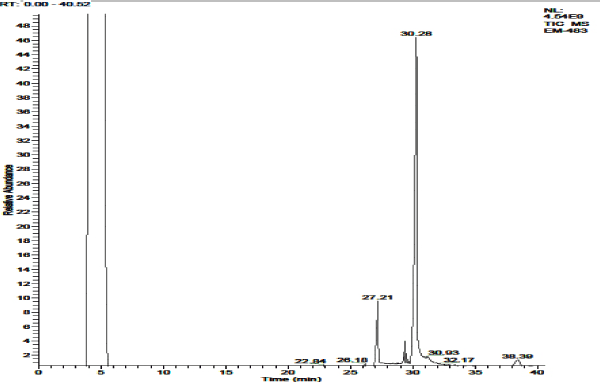
Figure 2a. GC-MS chromatogram of petroleum ether extracted P. amarus (with relative abundance, 0-40.52).
Table 3: GC-MS profiles of secondary phytochemicals of P. amarus in petroleum ether extract.
RT |
Name of the active principle
compound |
P
|
MF |
MW |
Area
(%) |
SI |
RSI |
Compound having biological properties
(by literature only) |
5.18 |
(R)-2-(1-Methylethylidene)-cyclohexane-drt |
32.33 |
C9H15D |
124 |
18.71 |
851 |
902 |
-- |
8.11 |
NV |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
14.25 |
d-Xylose |
11.87 |
C5H10O5 |
150 |
0.01 |
421 |
486 |
-- |
18.52 |
Dodecanoic acid* |
69.79 |
C12H24O2 |
200 |
0.04 |
820 |
863 |
Antimicrobial activity [50] |
22.86 |
Tetradecanoic acid* |
75.87 |
C14H28O2 |
228 |
0.07 |
858 |
888 |
Antioxidant, Anti-cancer, Hyper cholesterolemic, Larvicidal,
Repellent activity [51] |
27.19 |
(E)-5,10-secocholest-1(10)-en-3,5-dione |
59.40 |
C27H44O2 |
400 |
1.25 |
438 |
594 |
--- |
30.26 |
2,4-Dihydroxy-2-(2'-hydroxyethyl)cyclohex-5-en-1-one |
35.10 |
C8H12O4 |
172 |
6.76 |
648 |
990 |
-- |
38.42 |
3,3'-Dibromo-2,2'-diquinolinyl Disulfide
|
53.83 |
C18H10Br2N2S2
|
476 |
0.33 |
674 |
898 |
-- |
RT, Retention time; NV, Not validated; P, Probability; MF, Molecular formula; MW, Molecular weight; SI, Similar index; RSI, Reverse similar index; *Compounds having bioactive properties.
The acetone extract of P. amarus showed presence of 6 different secondary compounds. They are, 2-Butanol, 3-methoxy-; 2-Nitrocyclohexanone (CAS); Propanoic acid, 2-(tricyclo[3.3.1.13,7]dec-2-ylidene)-; Neophytadiene; 9,12,15-Octadecatrienoic acid, (Z,Z,Z)-; and Methyl (3R)-3-(tert-butyldimethylsilyloxy)-3-[3-((1S)-1-(tert-butyldimethylsilyloxy)-3-butynyl) phenyl]propanoate. Of which, 3 compounds, Propanoic acid, 2-(tricycle [3.3.1.13,7] dec-2-ylidene)-; Neophytadiene; and 9,12,15-Octadecatrienoic acid, (Z,Z,Z)-} possessed biological properties [Figure 3 & 3a; Table 4].
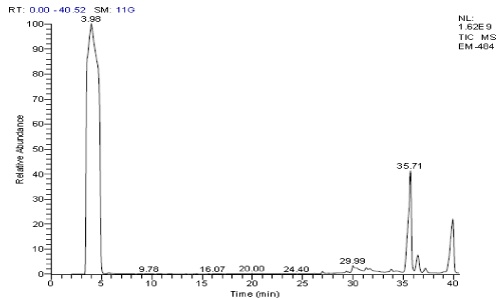
Figure 3. GC- MS chromatogram of acetone extracted P. amarus (Relative abundance, 0-100).
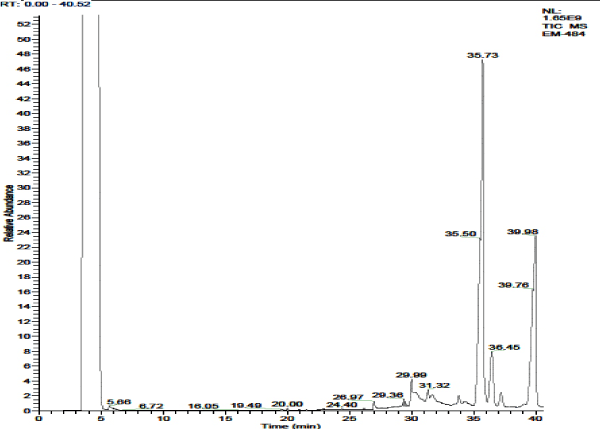
Figure 3a. GC- MS chromatogram of acetone extracted P. amarus (Relative abundance, 0-40.52).
Table 4: GC-MS profiles of secondary phytochemicals of P. amarus in acetone extract.
RT |
Name of the active principle
compound |
P
|
MF |
MW |
Area
(%) |
SI |
RSI |
Compound having biological properties (by literature only) |
3.98 |
2-Butanol, 3-methoxy- |
4.18 |
C5H12O2 |
104 |
84.04 |
993 |
999 |
-- |
9.78 |
2-Nitrocyclohexanone (CAS) |
6.82 |
C6H9NO3 |
143 |
0.00 |
369 |
868 |
-- |
16.07 |
NV |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
20.00 |
Propanoic acid, 2-(tricyclo[3.3.1.13,7]dec-2-ylidene)-* |
29.76 |
C13H18O2 |
206 |
0.02 |
641 |
678 |
Antioxidant [52] |
24.40 |
Neophytadiene* |
24.32 |
C20H38 |
278 |
0.01 |
702 |
809 |
Antipyretic, Analgesic, Anti‑inflammatory,
Antimicrobial, Antioxidant [53] |
29.99 |
9,12,15-Octadecatrienoic acid, (Z,Z,Z)-* |
9.57 |
C18H30O2 |
278 |
0.71 |
740 |
783 |
Antimicrobial agent [53] |
35.71 |
Methyl (3R)-3-(tert-butyldimethylsilyloxy)-3-[3-((1S)-1-(tert-but
yldimethylsilyloxy)-3-butynyl) phenyl] propanoate |
51.59 |
C26H44O4Si2
|
476 |
7.74 |
955 |
996 |
-- |
RT, Retention time; NV, Not validated; P, Probability; MF, Molecular formula; MW, Molecular weight; SI, Similar index; RSI, Reverse similar index; *Compounds having bioactive properties.
In the ethanol extract of P. amarus, 7 different secondary compounds were detected. They are, Cholest-5-ene-1á,3á,16á,26-tetrol; Cholestane-3á,5à,6á,26-tetrol - 26-Acetate; 3,10-Dioxa-2,11-disiladodecane, 2,2,11,11-tetramethyl-; 3-(4-Acetyl-3- hydroxyphenyl)-2-propenoic acid; Hexadecanoic acid, ethyl ester; (Sax)-(-)-2,2'-Bis(di-2-furylphosphino)-1,1'-binaphthalene; and 5-Methoxy-8,8-dimethyl-6-(2-methylbutanoyl)-4-phenyl-2H, 8H-benzo [1, 2-b: 3, 4-b'] dipyran-2-one. Of which, only one compound, Hexadecanoic acid, ethyl ester possessed bioactive properties [Figure 4 & 4a; Table 5].
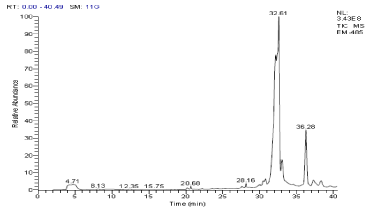
Figure 4. GC- MS chromatogram of ethanol extracted P. amarus (Relative abundance, 0-100).
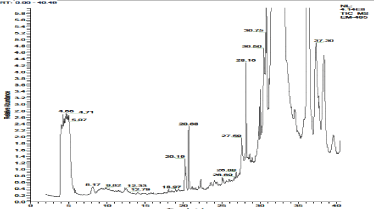
Figure 4a. GC- MS chromatogram of ethanol extracted P. amarus (Relative abundance, 0-40.49).
Table 5: GC-MS profiles of secondary phytochemicals of P. amarus in ethanol extract.
RT |
Name of the active principle compound |
P |
MF |
MW |
Area (%) |
SI |
RSI |
Compound having biological properties (by literature only) |
4.71 |
Cholest-5-ene-1á,3á,16á,26-tetrol |
10.63 |
C27H46O4 |
434 |
2.43 |
338 |
524 |
-- |
8.13 |
Cholestane-3á,5à,6á,26-tetrol - 26-Acetate |
88.65 |
C29H50O5 |
478 |
0.12 |
521 |
541 |
-- |
12.35 |
3,10-Dioxa-2,11-disiladodecane, 2,2,11,11-tetramethyl- |
13.28 |
C12H30O2Si2
|
262 |
0.06 |
439 |
554 |
-- |
15.75 |
NV |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
20.68 |
3-(4-Acetyl-3- hydroxyphenyl)-2-propenoic acid |
37.27 |
C11H10O4 |
206 |
0.37 |
646 |
663 |
-- |
28.16 |
Hexadecanoic acid, ethyl ester* |
83.88 |
C18H36O2 |
284 |
0.46 |
824 |
857 |
Antioxidant, Hypocholesterolemic, Nematicide,
Pesticide, Lubricant, Anti-androgenic, Flavour, Haemolytic,
5-Alpha reductase inhibitor [54] |
32.61 |
(Sax)-(-)-2,2'-Bis(di-2-furylphosphino)-1,1'-binaphthalene |
45.31 |
C36H24O6P2
|
614 |
71.70 |
897 |
990 |
-- |
36.28 |
5-Methoxy-8,8-dimethyl-6-(2-methylbutanoyl)-4-phenyl-2
H,8H-benzo[1,2-b:3,4-b'] dipyran-2-one |
57.04 |
C26H26O5 |
418 |
12.64 |
670 |
750 |
-- |
Proximate composition of P. amarus
The proximate composition of P. amarus was found as follows, crude protein (6.13%), crude fat (6.3%), crude fibre (24.66%), total ash (6.78%) and total nitrogen free extract (43.38%). It has 2567 k.cal/kg of gross energy.
Nutritional indices and basic biochemical constituents in M. rosenbergii PL fed with P. amarus extracts enriched Artemia nauplii
The morphometric data, length and weight, and nutritional indices, such as SR, WG, and SGR were found to be significantly increased (P<0.05) in petroleum ether, acetone and ethanol extracts of P. amarus enriched Artemia nauplii fed PL groups when compared with control (Table 6). Similarly, the contents of biochemical constituents, such as total protein, total amino acid, total carbohydrate and total lipid were found to be significantly higher (P<0.05) in petroleum ether, acetone and ethanol extracts enriched Artemia nauplii fed PL when compared with control (Table 6). Among these, ethanol extracts enriched Artemia nauplii produced the best results followed by acetone and petroleum ether extracts.
Table 6. Nutritional indices and concentrations of biochemical constituents in M. rosenbergii PL fed with different solvent extracts of P. amarus enriched Artemia nauplii.
Parameter |
Artemia nauplii |
Artemia nauplii enriched with extracts of P. amarus (1.0%) |
Petroleum ether |
Acetone |
Ethanol |
Nutritional
Indices |
SR (%) |
82.22±5.09c |
93.33±3.33ab |
94.44±3.09ab |
96.66±3.33a |
Length (cm) |
1.62±0.11d |
1.95±0.14c |
2.17±0.12b |
2.34±0.15a |
Weight (g) |
0.68±0.03cd |
0.86±0.04c |
1.02±0.04b |
1.07±0.05a |
LG (cm) |
1.55±0.03d |
1.88±0.04c |
2.01±0.03b |
2.27±0.05a |
WG (g) |
0.62±0.02cd |
0.80±0.02c |
0.96±0.01b |
1.01±0.02a |
SGR (%) |
3.90±0.02cd |
4.01±0.03c |
4.08±0.03ab |
4.10±0.04a |
Biochemical constituents
(mg/g wet wt.) |
Total protein |
62.63±1.60d |
97.96±3.21c |
106.52±3.34b |
112.41±2.78a |
Total amino acid |
22.03±1.41d |
41.94±3.55c |
48.54±2.15b |
55.61±2.15a |
Total carbohydrate |
11.66±1.13d |
25.56±1.13b |
27.04±1.13b |
31.76±1.54a |
Total lipid |
7.18±0.75d |
13.36±0.21c |
16.14±0.78b |
18.03±0.57a |
Each value is mean ± standard deviation of three individual observations.
Initial length and weight were 0.80±0.06 cm and 0.06±0.02 g respectively
Mean values within the same row sharing different alphabetical letter superscripts are statistically significant at P < 0.05 (one-way ANOVA and subsequent post hoc multiple comparison with DMRT).
SR, survival rate; LG, length gain; WG, weight gain, SGR, specific growth rate
Primary phytochemicals of P. amarus
Similar to that of the present study, the presence of alkaloids, tannins, terpenoids, saponins, phenolics, flavonoids and steroids have been reported in the hexane extract of P. amarus [55]. Alkaloids in ethyl acetate extracts of leaf and root, saponins in aqueous and methanol extracts of the stem, and flavonoids in petroleum ether extract of all parts of P. amarus have been reported [56]. Phenols and flavonoids from the root, stem and leaf of P. amarus have also been reported using different solvents, ethyl acetate, dimethylformamide, chloroform, dichloromethane and n-Hexane [57]. Dimethylformamide was reported to be the best solvent for extraction of phenols and flavonoids from the root, stem and leaf of P. amarus [58]. Qualitative phytochemical studies have also been reported in different species of Phyllanthus [59, 60].
It has been reported that alkaloids, such as morphine, atropine and quinine are biologically and therapeutically active [61]. In the present study, alkaloids are moderately present in petroleum ether extract of P. amarus.
Terpenoids are known to possess antimicrobial, antifungal, anti-parasitic, anti-viral, antiallergenic, anti-spasmodic, anti-hyperglycaemic, anti-inflammatory, immunomodulatory and anticancer properties, and they have inhibition of cholesterol synthesizing property as well [62, 63]. In the present study, terpenoids are moderately present in petroleum ether extract of P. amarus.
The flavonoids and other polyphenols are potentially beneficial to human health. They are known to contain a broad spectrum of chemical and biological activities including antioxidant, free radical scavenging, anti-ageing, anti-allergic, anti-inflammatory, anti-microbial, anti-leukemic, vasodilator, anticancer and antibacterial properties and are reported to be useful for improving blood circulation in brain of Alzheimeric patients [64 - 68]. The flavonoids, anthrax quinines and terpenes stimulate glucose uptake in cells [69]. Certain flavonoids exhibited hypoglycemic activity [70] and also beta cell regeneration in pancreas [69]. The leaf was reported to have the highest activity of phenols and flavonoids compared to root and stem. The antioxidant activity of flavonoids is efficient in trapping superoxide anion (O2), hydroxyl (OH), peroxyl (ROO-) and alcohoxyl (RO) radicals [71]. In the present study, ethanol extract of P. amarus contains luxuriant presence of flavonoids.
Tannins are used as the astringent substance in treatment of burns. They precipitate the proteins of exposed tissues to form a protective covering. They are used as mild antiseptics in treatment of diarrhoea, and to check small haemorrhages [72]. They are also used as healing agents in inflammation, leucorrhoea, gonorrhoea and piles. Tannins have been found to have antiviral, antibacterial, anti-parasitic, anti-inflammatory, anti-ulcer and antioxidant properties [73]. In the present study, all the three solvent extracts of P. amarus contain luxuriant presence of tannins.
Phenols are structural and allelopathic components which are associated with diverse functions, like nutrient uptake, protein synthesis, enzyme activity and photosynthesis [74]. Phenolic compounds have biological and pharmacological properties, such as anti-microbial, anti-viral, anti-inflammatory, cytotoxic, anti-mutagenic and anti-carcinogenic activities [67, 75]. In the present study, moderate presence of polyphenols was detected in the ethanol extract of P. amarus.
Saponins have hypo-cholesterolemic, anti-carcinogenic, anti-inflammatory, antimicrobial, antioxidant activities, anti-haemolytic and antibacterial activities [76, 77]. In the present study, ethanol extract of P. amarus contains luxuriant presence of saponins.
The cardiac glycosides are one of the most naturally occurring plant phytoconstituents and are basically steroid drug with an inherent ability to afford a very specific and powerful action mainly on the cardiac muscle when administered through injection. Cardiac glycosides and catecholamines are agent of choice in treatment of congestive cardiac failure [78]. In the present study, luxuriant presence of cardiac glycosides was detected in the acetone extract of P. amarus.
Quinonines have anti-inflammatory, anti-bacterial and immunomodulatory potentials, and are very much used in the treatment of malaria and more recently of tumors [79, 80]. In the present study, moderate presence of quinones was detected in the ethanol extract of P. amarus. The primary phytochemicals serve as health tonic in aquaculture nutrition [31, 81, 82].
Secondary phytochemicals of P. amarus
In the present study, dodecanoic acid (C12H24O2), propanoic acid, 2-(tricyclo[3.3.1.13,7]dec-2-ylidene)-(C13H18O2), tetradecanoic acid (C14H28O2), neophytadiene (C20H38), hexadecanoic acid, ethyl ester (C18H36O2) and 9,12,15-Octadecatrienoic acid, (Z,Z,Z)- were the active principle compounds of secondary phytochemicals/ bioactive substances identified from P. amarus. The dodecanoic acid (lauric acid: N-dodecanoic acid) is a common saturated fatty acid with a 12-carbon atom chain. Lauric acid is found in coconut and palm kernel oils. It is also found in human breast milk, cow’s milk and goat’s milk [83, 84]. It is used for treating viral infections including influenza (the flu), swine flu, avian flu, the common cold, fever blisters, cold sores, genital herpes caused by herpes simplex virus (HSV), genital warts caused by human papilloma virus (HPV) and HIV/AIDS. It is also used for preventing the transmission of HIV from mothers to children. Further, it is also used in treatment of bronchitis, gonorrhoea, yeast infections, chlamydia, intestinal infections caused by a parasite called Giardia lamblia, and ringworm. Some research suggests lauric acid might be a safer fat than trans-fats in food preparations. It is safe for pregnant and breast-feeding women in food amounts (Lauric acid Wikipedia).
The propanoic acid is a naturally occurring carboxylic, short chain fatty acid. Its anion CH3CH2CO2 as well as the salts and esters are known as propionates/ propanoates. Propionic acid inhibits the growth of mould and some bacteria, as a result, used as a food preservative. It is also used to make pesticides and pharmaceuticals. The esters of propionic acid are used as solvents or artificial flavorings [85]. In biogas plants, propionic acid is a common intermediate product, which is formed by fermentation with propionic acid bacteria. Its degradation in anaerobic environments requires the activity of complex microbial communities [86]. In propionic acidemia, a rare inherited genetic disorder, propionate acts as a metabolic toxin in liver cells by accumulating in mitochondria as propionyl-CoA (the initial metabolic product of propanoic acid) and its derivative, methylcitrate, two tricarboxylic acid cycle inhibitors [87, 88]. In a study, Al-Lahham et al., [89] demonstrated that propionic acid lowers fatty acids content in liver and plasma, reduces food intake, exerts immunosuppressive actions and probably improves tissue insulin sensitivity. Thus, increased propionic acid in the body might be considered beneficial in the context of prevention of obesity and diabetes.
The tetradecanoic acid (myristic acid: 1-tetradecanoic acid) is also a common saturated fatty acid. Its salts and esters are commonly referred to as myristates. It was first isolated from nutmeg (Myristica fragrans) by Playfair Lyon [90]. Both lauric acid and myristic acid have positive effects on HDL (good cholesterol) level [91-93]. They have high hydrophobicity and act as lipid anchors in bio membranes of eukaryotic cells.
Terpenes and terpenoids are the primary constituents of the essential oils of many types of medicinal plants and flowers. The neophytadiene is a naturally occurring diterpenoid branched hydrocarbon belongs to the group of compounds known as phytanes. It is a member of the class of compounds known as sesquiterpenoids, which are terpenes with three consecutive isoprene unit makes neophytadiene a potential biomarker for the consumption of plant food product. Its presence can be estimated in the subcutaneous fat of animals [94]. These hydrocarbons are derived mainly from plant origin, being important components of vegetable wax [95]. They often have a strong odour and may protect the plants that produce them by deterring herbivores and by attracting predators and parasites of herbivores [96, 97]. Terpenes have desirable properties for use in food, cosmetics and pharmaceutical products [98, 99]. They are usually active ingredients of natural agricultural pesticides [100].
The hexadecanoic acid (palmitic acid) is the most common saturated fatty acid found in animals, plants and microorganisms [101]. Palmitates are the salts and esters of palmitic acid. The palmitate anion is the observed form of palmitic acid at physiologic pH (7.4). The excess carbohydrates in the body of human and animals are converted to palmitic acid. It is the first fatty acid produced during fatty acid synthesis and is the precursor to longer fatty acids. Palmitate negatively feeds back on acetyl-CoA carboxylase, which is responsible for converting acetyl-CoA to malonyl-CoA, which in turn is used to add to the growing acyl chain, thus preventing further palmitate generation. According to the WHO excess consumption of palmitic acid increases the risk of developing cardiovascular disease due to increase in LDL levels in the blood [102]. Among all fatty acids, palmitic acid has the strongest effect in boosting the metastatic potential of CD36+ metastasis-initiating cells in mouse models [103].
9,12,15-Octadecatrienoic acid, (Z,Z,Z)- (C18H30O2), Linolenic acid; α-Linolenic acid (ALA); (Z,Z,Z)-9,12,15-Octadecatrienoic acid, methyl ester (C19H32O2); Methyl linolenate) is an n-3 (ω-3) polyunsaturated fatty acid with 18-carbon chain and three cis double bonds found mostly in plant foods such as hempseed, chiaseed, walnuts, tofu, and vegetable oils, including flaxseed (linseed oil), rapeseed (canola oil), soybean oils and Perilla seed oil (mint family, Lamiaceae), pumpkin seed oil. It is precursor for the other long-chain n-3 fatty acids, 20:5, all-cis-5,8,11,14,17-eicosapentaenoic acid (EPA) and 22:6, all-cis-4,7,10,13,16,19 docosahexaenoic acid (DHA) [104]. It is associated with cardiovascular (by helping to maintain normal heart rhythm, heart pumping and reduces blood clots) and neuropathologic diseases, and type 2 diabetes [105]. It is a potential nutraceutical to protect the brain from stroke, characterized by its pleiotropic effects in neuroprotection, vasodilation of brain arteries, and neuroplasticity [106]. It is also used to reduce high cholesterol, high blood pressure, asthma (decrease inflammation and improve lung function). ALA is used to treat rheumatoid arthritis, multiple sclerosis, lupus, diabetes, renal disease, ulcerative colitis, and Crohn's disease. It is also used to prevent pneumonia, chronic obstructive pulmonary disease, migraine headache, skin cancer, depression, and allergic and inflammatory conditions such as psoriasis and eczema. Ironically, ALA may raise some men's risk of getting prostate cancer. Its isomer is γ-linolenic acid (GLA, 18:3, n-6).
According to Arun et al., [107], acetone and ethanol extracts of P. amarus leaf contain the following secondary phytochemicals: The predominant compounds present in acetone extract of P. amarus were 3, 4-Dimethoxy-di-phenylanine; Phenethylamine, 2-methoxy-alpha-methyl-4, 5- (Methlenedioxy); and 5, 8, 11, 14- Eicosa tetraynoic acid. The compounds at least level presence were Benzhydrazide,4-methoxy-N2-(2-bromo-5-(2-prophnyloxy) benzylideno; 2(3H)-Cyclopent(e)-1,3-oxaxin-2-one, hexahydro, 2H-Pyan-2,6(3H)-dione, dihydro-4,4-dimethyl, Hex-5-encylamine, Cycloheptylamine; and Cyclooctanamine. In the case of ethanol extract, the major compounds reported were, benzene, 1, 2–dimethoxy–4-[[(4-methylphenyl) sulfonyl] methyl]; Phenethylamine, 2-methoxy-alpha-methyl-4,5-(methylenedioxy); and phenanthylamine, 2-methoxy. The minor compounds were, cyclopentane, phentyl; 3-(3-(1-Axirdinyl) propoxy)-2, 5-dimethylpyrazine; 3-(Cycloprophylamino) propioitrile; and3, 5-di-t-butyl phenol. According to Mamza et al., [108], the ethanol extract of P. amarus contains, methyl 14–methyl pentadecanoate; palmitic acid (hexadecanoic acids; 10–octadecanoate; 9–hexadecenal; glycerol 1, 3-dipalmitate; 2, 13- octadecadiene-1-ol; Dioctyl ester; and Heptanoic acid (9-dece-1-yl ester). The reported hexadecanoic acid was matched with the detected bioactive compound in the present study. Therefore, detection of phytochemical compounds are based on various factors like the quantity of their presence, soil type, soil nutrients and climatic conditions under which the plant was grown, and age of the plant.
Survival, growth and biochemical constituents of M. rosenbergii PL fed with P. amarus extracts enriched Artemia nauplii
In the present study, ethanol extract of P. amarus enriched Artemia nauplii produced the best SR, WG and SGR (96.6%, 1.01g, and 4.1%, respectively against the control 82%, 0.6g and 3.9%, respectively) in M. rosenbergii PL than that of other two solvent extracts (Table 6). This is because of the bioactive principle compounds like dodecanoic acid (lauric acid), tetradecanoic acid (myristic acid), hexadecanoic acid (palmitic acid) and 9,12,15-Octadecatrienoic acid, (Z,Z,Z)- (linolenic acid) present in P. amarus might have been taken up by Artemia nauplii and transferred to M. rosenbergii PL. In addition to polyunsaturated fatty acid (PUFA), the saturated fatty acids (SFA) also required for better survival and growth of prawns. This in turn reflected on significant (P<0.05) elevations of total protein, amino acid carbohydrate and lipid levels (112, 55, 31 and 18 mg/g wet tissue, respectively against the control 62, 22, 11 and 7 mg/g wet tissue, respectively) in M. rosenbergii PL fed with ethanol extract of P. amarus enriched Artemia nauplii than that of other two solvent extracts (Table 6). The antimicrobial, immunomodulatory and protective effects of other bioactive compounds, like propionic acid and neophytadiene present in P. amarus might have helped in maintenance of general health of M. rosenbergii PL. Artemia nauplii itself a very good live feed, it serves digestive enzymes and PUFA for larval growth of fishes and prawns. Herbal enriched Artemia has additional advantage that it carries bioactive substances.
The herbal products are eco-friendly, and have the characteristics ability of growth promoting, immune boosting, and appetizing effects in aquaculture animals. They increase consumption, induce maturation, and have antimicrobial as well as anti-stress capacities in aquaculture of shrimps and fin fishes [109]. It has been reported that in P. monodon PL fed with A. franciscana nauplii enriched with diets prepared by using Hygrophila spinosa, Withania somnifera, Zingiber officinalis, Solanum trilobatum, Andrographis paniculata, Psoralea corylifolia and cod-liver oil has significantly been improved the survival, growth and resistance to environmental stresses due to salinity [110]. In fish, Heros severus larvae, canola oil enriched A. nauplii produced better growth and stress resistance to temperature and oxygen deficiency and has converted n-3 fatty acids to EPA and DHA [111]. In rainbow trout, Oncorhynchus mykiss, the Artemia urmiana enriched with fish and plant oils (sunflower and canola oils) have produced significantly higher survival rate, specific growth rate and lower food conversion ratio. Further, they have improved the eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) levels in Artemia nauplii, and the fish fed with canola oil enriched Artemia showed significantly higher stress resistance against thermal, salinity and hypoxia [112]. In Penaeus monodon, the methanol extract of Mucuna pruriens enriched Artemia had improved larval survival, whereas methanol extract of Withania somnifera showed improved total protein and lipid levels [113]. The survival, growth, and disease tolerance have been reported to increase in shrimp, P. indicus fed with individual herbal extract (Ricinus communis, Phyllanthus niruri, Leucus aspera, and Manihot esculenta) and seaweed (Ulva lactuca, and Sargassum wightii) enriched Artemia [114]. The enriched Artemia nauplii with sunflower oil, Spirulina and cod liver oil have been produced better survival, growth and concentrations of biochemical constituents in M. rosenbergii PL also [25, 115, 116]. No report available so far for P. amarus in Macrobrachium.
In the present study, petroleum ether extract, acetone extract and particularly ethanol extract of P. amarus enriched Artemia nauplii produced significant elevations in survival, growth, and concentrations of total protein, amino acid, carbohydrate and lipid levels in M. rosenbergii PL when compared with un-enriched Artemia nauplii fed PL. This clearly indicates the fact that the primary phytochemical constituents and bioactive substances present in P. amarus should have been taken to prawn through Artemia. Thus this herb sustainably exerted growth promotion in M. rosenbergii. Hence, P. amarus can be incorporated or taken as a feed additive in low cost on-farm feed formulations. This study can be extended further for isolation, purification and characterization of different bioactive substances of P. amarus for various biological applications.
The Botanical Survey of India (BSI), Coimbatore, India, is acknowledged for authentication of Phyllanthus amarus. The South India Textile Research Association (SITRA), Coimbatore, India, is acknowledged for providing GC-MS outsourcing service.
- Rice EL (1995) Biological control of weeds and plant disease. Advances in applied allelopathy, University of Oklahoma Press, Norman, Oklahoma 439.
- Cabieses F (1993) Apuntes de medicina traditional la racionalizacion de lo irracional. Notes of traditional medicine. Consejo nacional de ciencia y technologia CONCYTEC Lima-Peru: 414.
- Thyagarajan SP, Subramanian S, Thirunalasundari T, Venkateswaran PS, Blumberg BS (1998) Effects of Phyllanthus amarus on the chronic carriers of hepatitis B virus. Lancet 2: 764-766. [Crossref]
- Wang MH, Cheng Y, Li L, Meng G, Zhao K (1995) Herbs of the genus Phyllanthus amarus in the treatment of chronic hepatitis B: Observations with three preparations from different geographic sites. J Lab Clin Med 126: 350-352. [Crossref]
- Oluwafemi F, Debiri F (2008) Antimicrobial effect of Phyllantus amarus and Parquetinani grescens on Salmonella typhi. Afr J Biomed Res 11: 215-219.
- Joseph B, Raj SJ (2011) An Overview: Pharmacognostic properties of Phyllanthus amarus Linn. Int J Pharmacol 7: 40-45.
- Okiki PA, Olatunji BP, Adebimpe ASE, Comfort O (2015) A comparative study of nutritional and phytochemical composition of Phyllanthus amarus leaf and seed. Am Eurasian J Toxicol Sci 7: 321-327.
- Negri G, Tabach R (2013) Saponins, tannins and flavonols found in hydroethanolic extract from Periandra dulcis roots. Rev Bras Farmacogn 23: 851-860.
- Rajeshkumar NV, Joy KL, Kuttan G, Ramsewak RS, Nair MG, Kuttan R (2002) Antitumor and anti carcinogen activity of Phyllanthus amarus extract. J Ethnopharmacol 81: 17-22.
- Kassuya CA, Silerstre AA, Rehder V, Calixto JB (2003) Antiallodynic and antioedematogeni properties of the lignan from Phyllanthus amarus in models of persistent inflammatory and neuropathic pain. Eur J Pharm 478: 145-153. [Crossref]
- Lim YY, Murtijaya J (2007) Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT-Food Sci Technol 40: 1664-1669.
- Islam A, Mazumder UK, Gupta M, Ghosal S (2008) Phyto-Pharmacology of Phyllanthus amarus, an overview. Pharmacol 3: 202-209.
- Taiwo IA, Oboh BO, Francis-Garuba PN (2009) A haematological property of aqueous extracts of Phyllantus amarus (Schum and Thonn.) and Xylopia aethiopica (Dunal) a rich in albino rats. Stud Ethno Med 3: 99-103.
- Akinjogunla OJ, Eghafona NO, Enabulele IO, Mboto CI, Ogbemudia FO (2010) Antibacterial activity of ethanolic extracts of Phyllanthus amarus against extended spectrum β-lactamase producing Escherichia coli isolated from stool samples of HIV sero-positive patients with or without diarrhea. Afr J Pharm Pharmacol 4: 402-407.
- Herbert OCM, Clement J, Idongesit J, Godwin E, Udeme E, et al. (2011) Evaluation of the hypoglycemic effect of aqueous extract of Phyllanthus amarus in alloxan-induced diabetic albino rats. Int J Pharm Biomed Res 2: 158-160.
- Patel JR, Tripathi P, Sharma V, Chauhan NS, Dixita VK (2011) Phyllanthus amarus: ethno medicinal uses, phytochemistry and pharmacology, a review. J Ethnopharmacol 138: 286-313. [Crossref]
- Hakim Md, Kamruzzaman O, bydulHoq (2016) A review on ethno medicinal, phytochemical and pharmacological properties of Phyllanthus niruri. J Med Plants Stud 4: 173-180.
- Devi S, Rashid R, Kumar M (2017) Phytochemistry and pharmacological properties of Phyllanthus amarus Schum: A review. Pharma Inno J 6: 169-172.
- Lewis K, Ausubel FM (2006) Prospects for plant-derived antibacterials. Nat Biotechnol 24: 1504-1507. [Crossref]
- Kamboj A (2012) Analytical evaluation of herbal drugs, In: Vallisuta, O. (Ed.), Drug Discovery Research in Pharmacognosy.
- Chakrabarti R, Rao YV (2012) Achyranthes aspera enhances immunity and antigen clearance in common carp, Cyprinus carpio L. J Fish Dis 35: 389-392. [Crossref]
- Bhavan PS, Jayanthi S, Rabecca AA (2011) Growth performance of the freshwater prawn Macrobrachium rosenbergii post larvae fed with Ocimun sanctum (Tulsi) and Withania somnifera (Ashwagandha) incorporated feeds. Int J Biol Res Devp 1: 34-53.
- Bhavan PS, Manickam N, Radhakrishnan S (2012) Influence of herbal greens, Murraya koenigii, Coriandrum sativum and Menthe arvensis on growth performance of the freshwater prawn Macrobrachium rosenbergii post larvae. Res J Biotechnol 7: 149-157.
- Bhavan PS, Saranya C, Manickam N, Muralisankar T, Radhakrishnan S, et al. (2013a) Effects of Piper longum, Piper nigram and Zingibero fficinale on survival, growth, activities of digestive enzymes and contents of total protein, vitamins and minerals in the freshwater prawn Macrobrachium rosenbergii. Elixir Bio Tech 58: 14824-14828.
- Bhavan PS, Kavithamani N, Radhakrishnan S, Muralisankar T, Srinevasan V, et al. (2013b) Comparison of nutritional quality of sunflower oil and cod liver oil enriched Artemia nauplii for assessing their efficacies on growth of the prawn Macrobrachium rosenbergii post larvae. Int J Curr Sci 7: 11-17.
- Bhavan PS, Anisha TC, Srinivasan V, Muralisankar T, Manickam N (2014a) Effects of spices, Papaver somniferum, Elettaria cardamomum, Foeniculum vulgare and Syzygium aromaticum on growth promotion in Macrobrachium malcolmsonii early juveniles. Int J Pure Appl Biosci 2: 120-131.
- Bhavan PS, Mohammedsiddiq S, Srinivasan V, Muralisankar T, Manickam N (2014b) Effects of seeds of medicinal plants, Syzygium cumini, Phylanthus emblica, Azadirachta indica and Ricinus communis on growth promotion in Macrobrachium malcolmsonii early juveniles. Int J Res Stud Biosci 2: 95-106.
- Shanthi R, Bhavan PS, Radhakrishnan S (2012) Influence of medicinal herbs, (Andrographis pani culata, Cissus qua drangularis and Eclipta alba) on growth, digestive enzymes, biochemical constituents and protein profile of the freshwater prawn Macrobrachium rosenbergii. Elixir Bio Tech 42: 6478-6484.
- Poongodi R, Bhavan PS, Muralisankar T, Radhakrishnan S (2012) Growth promoting potential of garlic, ginger, turmeric and fenugreek on the freshwater prawn Macrobrachium rosenbergii. Int J Pharma Bio Sci 3: 916-926.
- Radhakrishnan S, Bhavan PS, Seenivasan C, Shanthi R, Poongodi R (2013) Influence of medicinal herbs (Alteranthera sessilis, Eclipta alba and Cissus quadrangularis) on growth and biochemical parameters of the freshwater prawn Macrobrachium rosenbergii. Aquacult Int 22: 551-572.
- Pourmoghim H, Haghighi M, Rohani MS (2015) Effect of dietary inclusion of Origanum vulgare extract on nonspecific immune responses and hematological parameters of rainbow trout (Oncorhynchus mykiss). Bull Env Pharmacol Life Sci 4: 33-39.
- Dhanalakshmi K, Bhavan PS, Rajkumar G, Nathiya V, Srinivasan V, et al. (2016) Phytochemical characterization of couch grass (Cynodon dactylon) and its growth promoting potential on the freshwater prawn Macrobrachium rosenbergii Post-Larvae. Br Biotechnol J 14: 1-24.
- Kanagarasu R, Bhavan PS, Rajkumar G, Nathiya V, Satgurunathan T, et al. (2017) Phytochemical characterization of Alternanthera sessilis and assessment of its growth promoting potential on the freshwater prawn Macrobrachium rosenbergii. Int J Res Stud Zoo 3: 25-38.
- Manjula T, Saravana BP, Rajkumar G, Muralisankar T, Udayasuriyan R et al. (2018) Phytochemical characterization of Eichhornia crassipes and Sargassum cristaefolium, and their effects on the growth of the prawn Macrobrachium rosenbergii. Sch Acad J Biosci 6: 71-83.
- Van Stappen G (1996) Introduction, Biology and Ecology of Artemia. In: Manual on the production and use of live food for aquaculture. FAO Fisheries Technical Paper (Ed. By P. Lavens & P. Sorgeloos). FAO 79-106.
- Dhont J, Lavens P, Sorgeloos P (2010) Preparation and use of Artemia as food for shrimp and prawn larvae. In: J.P. McVey (ed.), CRC Handbook of mariculture, 2nd Edn, Crustacean aquaculture. CRC Press, Boca Raton, FL. USA 1: 61-93.
- Treece GD (2000) Artemia production for marine larval fish culture, SRAC Publication No. 702, Texas A&M University, Sea Grant College Program.
- Lim CL, Dhert P, Soregloos P (2003) Recent developments in the application of live feeds in the fresh water ornamental fish culture. Aquaculture 227: 319 -331.
- Mitra RL, Jain SK (1985) Concept of Phyllanthus niruri (Euphorbiaceae) in Indian floras. Bull Bot Surv Ind 27: 161-176.
- Trease GE, Evans WC (1989) Pharmacology 11th Edn. Bailliere Tindall Ltd. London 60-75.
- Vandendool H, Kratz PD (1963) A generalization of the retention index system including liner temperature programmed gas-liquid partition chromatography. J Chromatogr 11: 463-467. [Crossref]
- AOAC (1995) Official Methods of Analysis of AOAC International. 2 vol. 16th edn. Arlington, VA. Association of Official Analytical Chemists USA.
- Castell JD, Tiews K (1980) Report on the EIFAC, IUNS and ICES working group on the standardization of methodology in fish nutrition research. Hamburg, Federal republic of Germany, EIFAC Technical paper No: 36. FAO 1-24.
- Tekinay AA, Davies SJ (2001) Dietary carbohydrate level influencing feed intake, nutrient utilization and plasma glucose concentration in the rainbow trout, Oncorhynchus mykiss. Turk J Vet Anim Sci 25: 657-666.
- Lowry OH, Rosenbrough WJ, Fair AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
- Moore S, Stein WH (1948) Photometris ninhydrin method for use in the chromatography of amino acids. J Biol Chem 176: 367-388.
- Roe SH (1955) The determination of sugar in blood and spinal fluid with anthrone reagent. J BioI Chem 212: 334-343. [Crossref]
- Folch J, Lees M, Bloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 266: 497-509. [Crossref]
- Barnes H, Blackstock J (1973) Estimation of lipids in marine animals and tissues. Detail investigation of the sulpho-phosphovanillin method for total lipids. J Exp Mar Bio Ecol 12: 103-118.
- Nakatsuji T, Kao MC, Fang J, Zouboulis C, Zhang L, et al. CM (2009) Antimicrobial property of lauric acid against Propionibacterium acnes: Its therapeutic potential for inflammatory acne vulgaris. J Invest Dermatol 129: 2480-2488.
- Sivakumar R, Jebanesan A, Govindarajan M, Rajasekar P (2011) Larvicidal and repellent activity of tetradecanoic acid against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say.) (Diptera Culicidae). Asian Pac J Trop Med 4: 706-710.
- Merlani M, Barbakadze V, Amiranashvili L, Gogilashvili L, Mulkijanyan K (2011) Synthesis of some caffeic and 2,3-Dihydroxy-3-(3,4- Dihydroxyphenyl)-propanoic acids amides. Bull Georg Natl Acad Sci 5: 107-111.
- Patel J, Reddy V, Kumar GS (2016) Preliminary phytochemical screening and hepatoprotective activity of methanol extract of Artocarpus hirsutus leaves. Int J Phytomed 83: 79 -383.
- Parthiban S, Gopalasatheeskumar K, Boopathi T, Sangeetha G, Thanga Kokila M, et al (2017) In-vitro anti inflammatory activity of stem of Zanthoxylum rhetsa (Roxb.) Dc. W J Pharmacal 6: 591-600.
- Zubair MF, Olubunmi A, Ibrahim SO, Adebisi OO, Hamid AA, et al. (2017). Chemical constituents and antimicrobial properties of Phyllanthus amarus. BAJOPAS 10: 238 -246.
- Awasthi P, Mahajan V, Rather IA, Gupta AP, Rasool S, et al. (2015) Plant Omics: Isolation, identification, and expression analysis of cytochrome P450 gene sequences from Coleus forskohlii. Omics 19: 782-792. [Crossref]
- Shah RK, Yadav RNS (2015) Qualitative phytochemical analysis and estimation of total phenols and flavonoids in leaf extract of Sarcochlamys pulcherrima Wedd. Glob J Biosci Bio-Technol 4: 81-84.
- Sravanthi PS, Padmavathi S, Archana Giri TVS (2016) Metabolic fingerprinting of root, stem and leaf extracts of Phyllanthus amarus. J Phytol 8: 17-21.
- Gopinath SM, Rakesh CK, Murthy TP, Dayananda KS (2012) Preliminary phytochemical evaluation of leaf extracts of Gymnema sylvestre, Phyllanthus amarus, Phyllanthus reticulatus of siddarabetta, Tumkur district, Karnataka. Int J Pharm Phyto Res 3: 109-111.
- Kavit M, Patel BN, Jain BK (2013) Phytochemical analysis of leaf extracts of Phyllanthus fraternus. Res J Recent Sci 2: 12-5.
- Shulka YM, Dhruve JJ, Patel NJ, Bhatnagar R, Talati JG, et al. (2009) Plant secondary meatabolites. New India Publishing Agency, New Delhi, India.
- Rabi T, Bishayee A (2009) Terpenoids and breast cancer chemoprevention, Breast Cancer Res Treat 155: 223-239. [Crossref]
- Wagner KH, Elmadfa I (2003) Biological relevance of terpenoids. Overview focusing on mono-di and tetraterpenes. Ann Nutr Metab 47: 95-106. [Crossref]
- Sharma DK (2006) Pharmacological properties of flavonoids including flavonolignans-integration of petrocrops with drug development from plants. J Sci Ind Res 65: 477-484
- Kahkonen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, et al. (1999). Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem 47: 3954-3962. [Crossref]
- Prior RL, Wu H, Gu L (2006) Flavonoid metabolism and challenges to understanding mechanisms of health effects. J Sci Food Agri 86: 2487-2491.
- Mungole AJ, Awati R, Chaturvedi A, Zanwar P (2012) Preliminary phytochemical screening of Ipomoea obscura (l) -a hepatoprotective medicinal plant. Int J Pharm Res 2: 2307-2312.
- Raja RDA, Jeeva S, Prakash JW, Antonisamy JM, Irudayaraj V (2011). Antibacterial activity of selected ethnomedicinal plants from south India. Asian Pacif J Trop Biomed 4: 375-378. [Crossref]
- Latha M, Pari L (2003) Antihyperglycaemic effect of Cassia auriculata in experimental diabetes and its effects on key metabolic enzymes involved in carbohydrate metabolism. Clin Exp Pharmacol Physiol 30: 38-43. [Crossref]
- Ahmad M, Akhtar MS, Malik T, Gilani AH (2000) Hypoglycaemic action of the flavonoid fraction of Cuminum nigrum seeds. Phytother Res 14: 103-106. [Crossref]
- Verma R, Mishra V, Sasmal D, Raghubir R (2010) Pharmacological evaluation of glutamate transporter 1 (GLT-1) mediated neuroprotection following cerebral ischemia/reperfusion injury. Eur J Pharm 638: 65-71. [Crossref]
- Kokate KK, Purohit AP, Gokhale SB (2008) Pharmacognosy, 42nd ed. Vallabh Prakashan, India 13-44.
- Ndhlala AR, Kasiyamhuru A, Mupure K, Chitindingu M, Benhura A, et al. (2007) Phenolic composition of Flacourtia indica, Opuntia megacantha and Sclerocarya birrea. Food Chem 103: 82-87.
- Wu H, Haig T, Pratley J, Lemerie D, An M (2000). Allelochemicals in wheat (Triticumaestivum L): variation of phenolic acids in root tissue. J Agric Food Chem 48: 5321-5325 [Crossref]
- Anpin RRD, Jeeva S, Prakash JW, Johnson M, Irudayaraj V (2011) Antibacterial activity of selected ethnomedicinal plants from South India. Asian Pacific J Trop Biomed 4: 375-378. [Crossref]
- Sparg SG, Light ME, Van Staden J (2004) Biological activities and distribution of plant saponins. J Ethnopharm 94: 219-243. [Crossref]
- Rao A, Gurfinkel D (2000) The bioactivity of saponins: Triterpenoid and steroidal glycosides. Drug Metabol Drug Interact 17: 211-235. [Crossref]
- Firn R (2010) Nature’s Chemicals. Oxford University Press, Oxford 74-75.
- AI-khalil S, Atkofahi A, EI-Eisawi D, AI-shibib A (1998). Transtorine, a new quinoline alkaloid from Ephedra fransitoria. Nat Prod Res 61: 262-283.
- Bergh JC, Lazovios A, Somogyi G, Lengyel L, Feher J (2006) The first clinical pilot of Roquinimax (Linomide) fl cancer patients with special focus on immunological effects. Cancer Invest 15: 204-211.
- Citarasu T (2010) Herbal biomedicines: a new opportunity for aquaculture industry. Aquac Int 18: 403-414.
- Tripathi SD (2003) Inland fisheries in India. In Fish for all national launches, Kolkata. India 33-57.
- Beare-Rogers J, Dieffenbacher A, Holm JV (2001) Lexicon of lipid nutrition (IUPAC Technical Report). Pure Appl Chem 73: 685-744.
- Anneken DJ, Both S, Christoph R, Fieg G, Steinberner U, et al (2006) "Fatty Acids" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim.
- Bertleff W, Roeper M, Sava X (2005) Carbonylation, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim.
- Ahlert S, Zimmermann R, Ebling J, Konig H (2016) Analysis of propionate-degrading consortia from agricultural biogas plants. Microbiol 5: 1027-1037. [Crossref]
- MacFabe DF, Cain DP, Rodriguez-Capote K, Franklin AE, Hoffman JE, et al. (2007) Neurobiological effects of intraventricular propionic acid in rats: Possible role of short-chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res 176: 149-169. [Crossref]
- Nguyen NH, Morland TC, Villa Gonzalez S, Rise F, Storm-Mathisen J, et al. (2007). Propionate increases neuronal histone acetylation, but is metabolized oxidatively by glia. Relevance for propionic acidemia. J Neurochem 101: 806-814. [Crossref]
- Al-Lahham SH, Maikel P, Peppelenbosch, Han Roelofsen, Roel J, et al. (2010) Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta 1801: 1175-1183. [Crossref]
- Lyon P (2009) On a new fat acid in the butter of nutmegs. Philosophical Magazine Series 3, 18(115): 102-113.
- Kromhout D, Yasuda S, Geleijnse JM, Shimokawa H (1995) Dietary saturated and trans fatty acids and cholesterol and 25-Year mortality from coronary heart disease: The seven countries study. Prev Med 24: 308-315. [Crossref]
- Mensink RP, Zock PL, Kester AD, Katan MB (2003) Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a metaanalysis of 60 controlled trials. Am J Clin Nutr 77: 1146-1155. [Crossref]
- Thijssen MA, Mensink RP (2005) Fatty acids and atherosclerotic risk. Handb Exp Pharmacol 170: 165-194. [Crossref]
- Hernández-Matamorosa A, Gonzálezb E, García-Cascoc JM, Tejedaa JF (2013) Determination of neophytadiene in the subcutaneous fat of Iberian pigs from different feeding systems. Grasas y Aceites 64: 173-180.
- Post-Beittenmiller D (1996) Biochemistry and molecular biology of wax production in plants. Ann Rev Plant Physiol Plant Mol Biol 47: 405-430. [Crossref]
- Martin DM, Gershenzon J, Bohlmann J (2003) Induction of volatile terpene biosynthesis and diurnal emission by Methyl jasmonate in foliage of Norway spruce. Plant Physiol 132: 1586-1599. [Crossref]
- Pichersky E (2006) Biosynthesis of plant volatiles: Nature's diversity and ingenuity. Science 311: 808-811. [Crossreef]
- Thimmappa R, Geisler K, Louveau T, O’Maille P, Osbourn A (2014) Triterpene biosynthesis in plants. Annu Rev Plant Biol 65: 225-257. [Crossref]
- Augustin JM, Kuzina V, Andersen SB, Bak S (2011) Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 72: 435-457. [Crossref]
- Isman MB (2000) Plant essential oils for pest and disease management. Crop protection 19: 603-608.
- Gunstone FD, Harwood JL, Dijkstra AJ (2007) The lipid handbook, 3rd ed. Boca Raton: CRC Press.
- WHO (2003) Technical Report Series 916, Diet, Nutrition and the prevention of chronic diseases. Report of a joint WHO/FAO Expert consultation, Geneva, 88.
- Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, et al. (2016). Targeting metastasis initiating cells through the fatty acid receptor CD36. Nature News 480, 437–445. [Crossref]
- Burdge GC, Calder PC (2005) Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev 45: 581-97. [Crossref]
- Sujatha R (2014) Health benefits of plant-derived α-linolenic acid. Am J Clin Nutr 100: 443-448. [Crossref]
- Blondeau N, Lipsky RH, Bourourou M, Duncan MW, Gore lick PB, et al. (2015) Alpha-linolenic Acid: An Omega-3 fatty acid with neuroprotective properties - Ready for use in the stroke clinic? Bio Med Res Int 8.
- Arun T, Senthilkumar B, Purushothaman K, Aarthy A (2012). GC-MS Determination of bioactive components of Phyllanthus amarus (L.) and its antibacterial activity. J Pharma Res 5: 4767-4771.
- Mamza UT, Sodipo OA, Khan IZ (2012) Gas chromatography-mass spectrometry (GC-MS) analysis of bioactive components of Phyllanthus amarus leaves. Int Res J Plant Sci 3: 208-215.
- Citarasu T (2010). Herbal biomedicines: a new opportunity for aquaculture industry. Aquacult Int 18: 403-414.
- Citarasu T, Micheal Babu M, Sekar RJR, Peter Marian M (2002) Developing Artemia enriched herbal diet for producing quality larvae in Penaeus monodon fabricius. Asian Fish Sci 15: 21-32.
- Motamedi TJ, Ebrahimi DE, Goli SAH, Akbary P (2014) Enrichment of Artemia (Leach) nauplii with canola oil: Effect on Heros severus (Heckel) larvae performance and environmental stress. Adv Microbiol 4: 1242-1249.
- Kazemi E, Agh N, Malekzadeh Viayeh R (2016) Potential of plant oils as alternative to fish oil for live food enrichment: effects on growth, survival, body compositions and resistance against environmental stresses in rainbow trout, Oncorhynchus mykiss. Iran J Fish Sci 15: 1-15.
- Michael BM, Sivaram V, Immanuel G, Citarasu T, Punitha SMJ (2008) Effects of herbal enriched artemia supplementation over the reproductive performance and larval quality in spent spawners of the tiger shrimp (Penaeus monodon). Turk J Fish Aquat Sci 8: 301-307.
- Immanuel G, Vincybai VC, Sivaram V, Palavesam A, Marian MP (2004). Effect of butanolic extracts from terrestrial herbs and seaweeds on the survival, growth and pathogen (Vibrio parahaemolyticus) load on shrimp Penaeus indicus juveniles. Aquaculture 236: 53-65.
- Bhavan PS, Devi VG, Shanthi R, Radhakrishnan S, Poongodi R (2010) Basic biochemical constituents and profiles of amino acids in the post larvae of Macrobrachium rosenbergii fed with Spirulina and Yeast enriched Artemia. J Sci Res 2: 539-549.
- Babitha Rani AM, Reddy AK, Sahu NP (2006) Growth enhancement and survival of Macrobrachium rosenbergii larvae fed Artemia nauplii enriched with cod liver oil and/or Lactobacillus. J Aquacult 58: 183-190.


























