The periodontal ligament and the root canal system are closely interlinked. This review summarizes the ways of communication between the root canal system (RCS) and the periodontal ligament (PDL). These systems may affect each other in several ways which may result in a perio-endo lesion.
Objective: To help the practitioner arrive at a more accurate diagnosis by reviewing all the anatomical and pathological ways of communication between the RCS and the PDL. A better understanding of the development of a perio-endo lesion can help the general practitioner arrive at a more accurate diagnosis and a better treatment plan.
This literature review summarizes most of our knowledge regarding the anatomical aspect of the perio-endo lesion. This review is based on the English language literature regarding endo-perio lesions. An electronic PubMed bibliographical index search was carried out using the preliminary key words followed by article selection according to relevance and importance to the topic.
periodontal lesion, Endo-Perio Lesions, root canals, endodontic lesion
The periodontal ligament and the root canal system have close developmental and anatomic relationship so that pathologic processes arising in one may affect the other. These two entities are normally separated by dentin, covered by layers of practically impermeable cementum [1]. Under these conditions, no interaction is to be expected between the healthy pulp and the surrounding PDL. Anatomically, the only sites at which the vital pulp can be in contact with the PDL are the apical foramen, or foramina, as well as the outlets of the lateral canals. These may be present at any height of the root, from the apex to the cemento emamel unction (CEJ) as well as the furcation areas in multi-rooted teeth [2].
At these crossroads, irritants (bacteria and their by-products) may attempt to cross and interact with the other anatomic entity involving it in the pathologic process. Some states, such as pathologies related to vertical fractures, cervical resorption, or perforation, affect both systems, directly. Advanced endodontic and periodontal lesions may present by an almost identical loss of alveolar bone, and thus presenting diagnostic and treatment challenges.
When pathology initially sets in the inflamed pulp it is usually still vital and apparently able to recover completely. However, in the absence of adequate therapy, the pulp will die and its cavity will become a sanctuary for microorganisms from which they may spread and cause a local inflammatory reaction at the level of the other compartment [3]. If the initial disease is of periodontal origin, the pulp is not clinically affected until the loss of bone has reached the apex of the root, exposing the neurovascular bundle of the tooth allowing the bacteria to enter the root canal [4-10]. The aim of this article is to review the relationship between the periodontal ligament and the root canal system thus helping the practitioner achieve a correct diagnosis and treatment plan.
A Pubmed search was performed and was limited to English-language papers. The keywords searched on Pubmed were ‘periodontics AND endodontics (1730)’, ‘combined endo-perio lesion (11)’, ‘endo-perio lesions (34)’, ‘perio-endo lesions (21)’, ‘pulp AND PDL (149)’, ‘endodontic pathways of infection (10)’, ‘pulp pathways of infection (32)’, ‘furcation AND endo-perio lesion (3)’, ‘regenerative endodontics (601)’, ‘periodontitis AND endodontics (3220)’, ‘dental anatomy AND pulp infection (539)’, ‘endodontics AND root resorption (1651)’, ‘endodontic periodontal communications (45)’. Then, the reference section of each of those articles was studied to find other suitable sources. The number of retrieved papers was presented in the parentheses. Article selection was according to relevance and importance to the topic of review.
Root anatomy
Lateral canals: Tagger, et al. showed that when the pulp becomes necrotic in monkeys’, dogs’ and rats’ teeth an inflammatory response occurs in the periodontal ligament, apical foramen or in the furcation areas in 21% of the teeth that were examined (Figure 1) [3,11]. In human specimens, under a microscope, canals were found in the furcation area in 74% of the teeth [12]. Using Basic Fuchsin stain accessory canals were shown in 57% and with the fluid filtration method, in 1% [13,14]. The variability in the number of canals in human teeth is probably dependent on the material (origin of the teeth) and the methods used for detection of the canals [15]. Accessory canals could be found anywhere along the root, mostly in the apical third (Figure 2) [16]. Seltzer et al. reported that pulpal inflammation may cause an inflammatory reaction in the interradicular periodontal tissues [17]. Histologically, inflammatory response can be present in front of the openings of accessory canals, but its clinical impact is negligible [18-20] (Figure 3). However, it should be noted that when a lesion exists in the furcation area, both treatment and prognosis change according to the presence of accessory canals [21]. Lin et al 2008 suggest that teeth with furcation involvement should be treated with bonding after the root canal treatment [22].
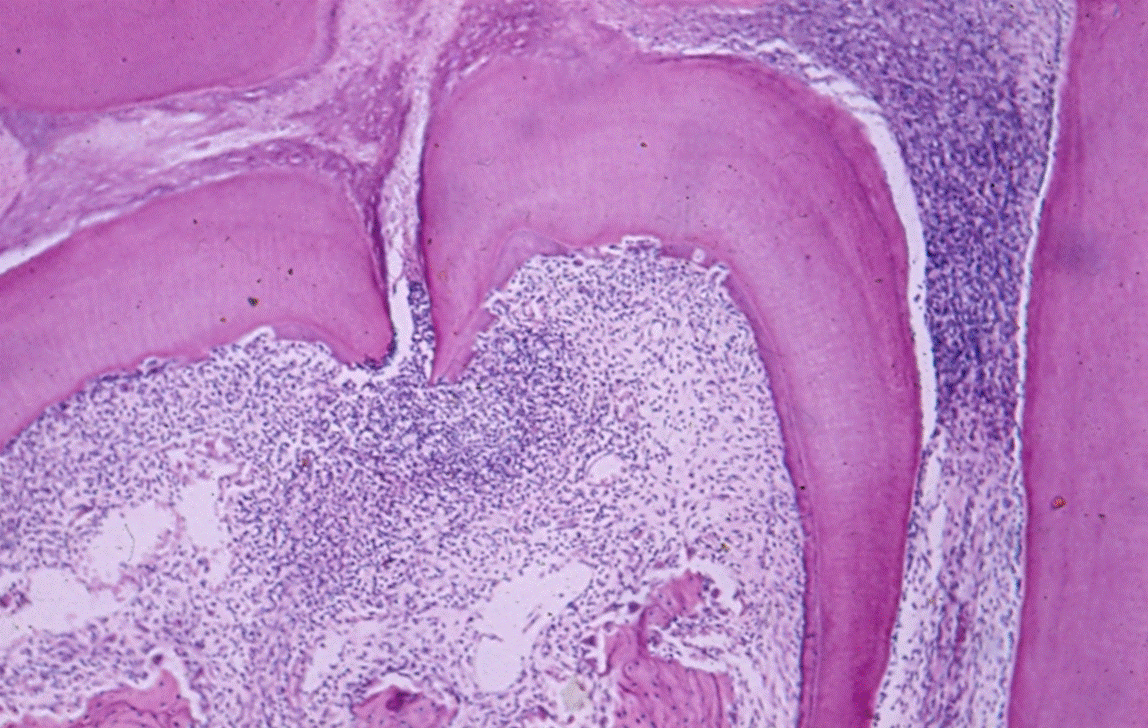
Figure 1. Infection in furcation. H&E histologic image showing infiltration in the furcation area

Figure 2. Lateral Canals at the apex of a root
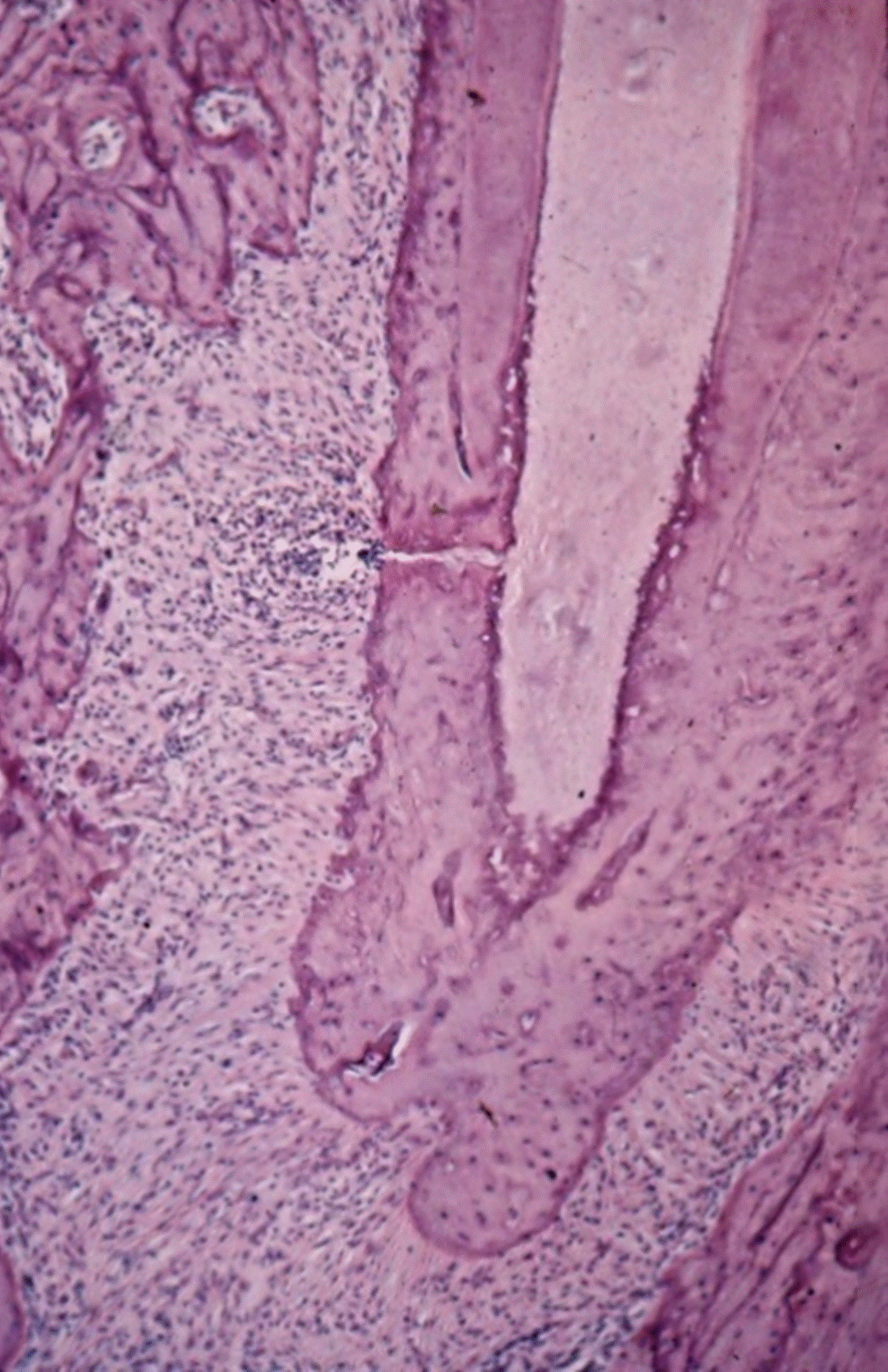
Figure 3. Root apex of rat molar. The necrotic pulp and the inflamed PDL are in direct connection via a lateral canal. Opposite the inflammation the bone is being resorbed - see numerous osteoclasts
Along the root surface, lateral canals were found in 17% of the teeth in the apical third, 9% in the central third and less than 2% in the coronal third. In 1,000 extracted teeth examined due to periodontal disease, only 2% of the lateral canals opened to the periodontal pocket [23]. In addition, not all lateral canals can be penetrated by micro-organisms or their products [24].
Dentinal tubules
The dentinal tubules have a slightly conical shape, with a diameter of 1-3 µm (smaller in the cementum and larger in the pulp), and are filled with interstitial fluid [25]. With age, as sclerosis occurs, the tubules become narrower. Along the pulp cavity the tubule density is greater in the crown area than that in the root dentine.
When the cementum and enamel do not meet at the cemento–enamel junction, the tubules remain exposed, thus creating pathways of communication between the pulp and the periodontal ligament. The cemento-enamel junction becomes important when assessing the progression of endodontic pathogens, as well as the effect of root scaling and planing, on cementum integrity, trauma and bleaching and one of the responses to inflammatory cervical root resorption (ICRR).
In one study, 18% of premolars and molars, and 25% of incisors showed dentin exposure in the CEJ area [26]. Another study had found that in almost one third of 67 examined premolars there was a gap between enamel and cementum with dentin exposure. This exposure is not uniform around the tooth and may results in infection of the tubules [27-29] (Figure 4).
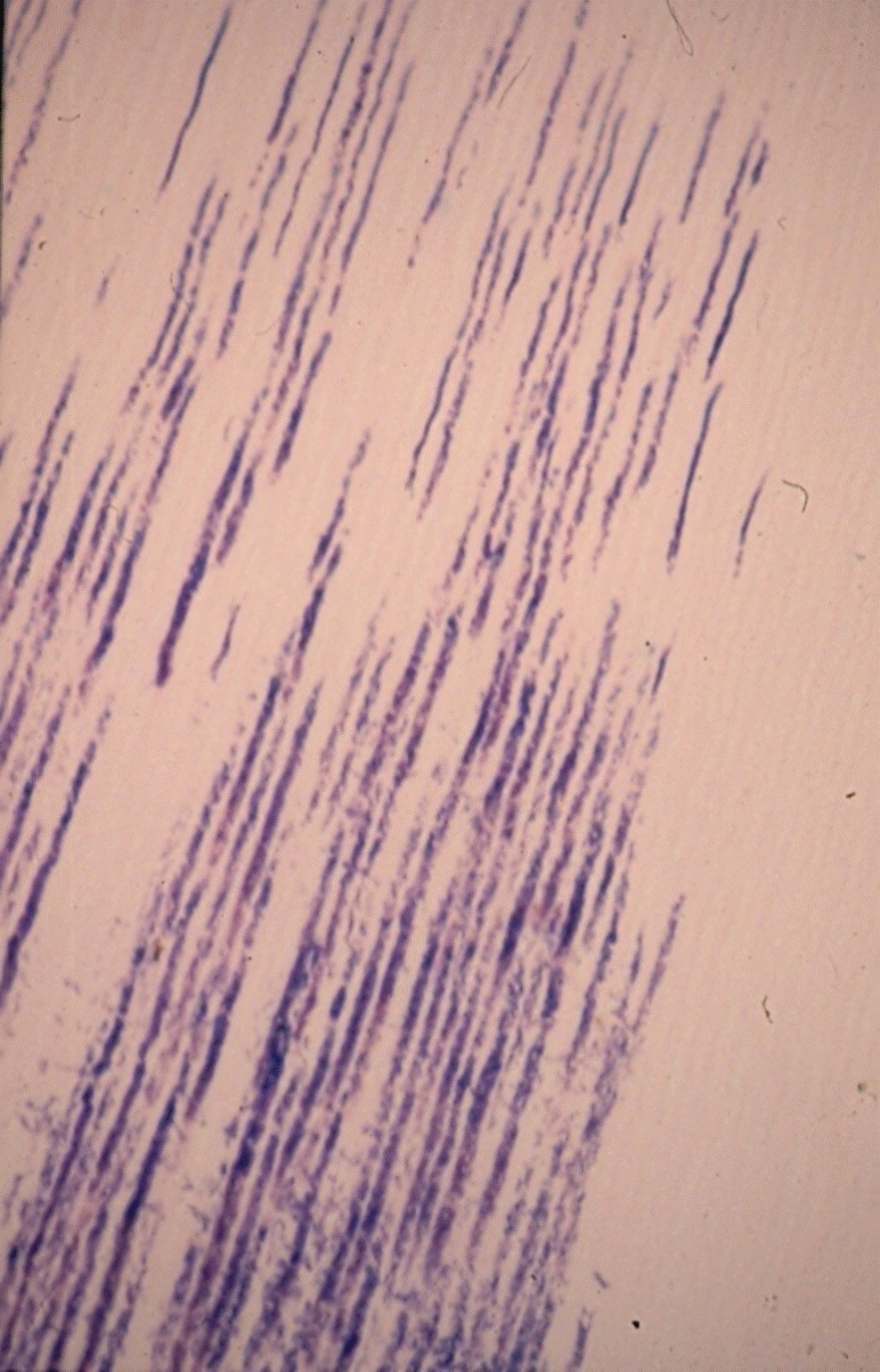
Figure 4. Bacteria in tubules
Apical foramen
The apical foramen is the principal route of communication between the pulp and the periodontium. Microbial and inflammatory by-products may exit readily through the apical foramen to cause periradicular pathoses. The apex is also a potential portal of entry of inflammatory by-products from deep periodontal pockets to the pulp. Pulp inflammation or pulp necrosis extends into the periradicular tissues causing a local inflammatory response often associated with bone and root resorption [17]. The endodontic treatment aims at eliminating the intraradicular etiologic factors, thereby leading to healing of the affected periradicular tissues.
Anatomic developmental malformations
The radicular groove is a developmental anomaly predominantly found on the palatal surface of maxillary lateral incisors [30-32]. In rare cases it may appear on the buccal aspect of maxillary incisors [33]. The prevalence of radicular grooves is reported to be 0.79% to 21% in both maxillary incisors, and 1.9% to 14% in lateral incisors alone [25,34-38].
The grooves usually begin in the central fossa of maxillary central and lateral incisors crossing over the cingulum and continuing apically down the root for varying distances. As long as the epithelial attachment remains intact, the periodontium remains healthy. Once the epithelial attachment is breached and the groove becomes contaminated, severe localized periodontal attachment loss including pocket formation and alveolar bone loss can occure along entire length of the root [39,40].
Radiographically, the area of bone destruction follows the course of the groove [41]. This condition must be differentiated from a vertical fracture, which may give a similar radiographic appearance.
Enamel pearl is an ectopic globule of enamel that is firmly adherent to the tooth root and varies in size [42]. Moscow and Canut reviewed the studies on enamel pearls and reported a prevalence of 1.1% to 9.7%, with distinct racial differences observed. They are usually found on the root surface of molar teeth. Enamel pearls have been shown to facilitate the progression of periodontal breakdown [43,44].
Disease relationships
When the pulp becomes infected, it elicits an inflammatory response in the periodontal ligament at the apical foramen and/or adjacent to openings of the small portals of exit [11]. Inflammatory by-products of pulpal origin may permeate through the apex or through smaller canals in the apical third of the root canal system and exposed dentinal tubules, and trigger an inflammatory vascular response in the periodontium. Among those are living pathogens such as certain strains of bacteria, fungi and viruses, as well as nonliving pathogens [45-57].
It has been suggested that periodontal disease has no effect on the pulp, at least until it involves the apex [4]. On the other hand, several studies suggest that the effect of periodontal disease on the pulp is degenerative in nature and includes an increase in calcification, fibrosis and collagen resorption, as well as a direct inflammatory affect [10,58]. It appears that the pulp is usually not severely affected by periodontal disease until recession has opened a lateral or accessory canal to the oral environment. At this stage, pathogens leaking from the oral cavity through the lateral or accessory canal into the pulp may cause a chronic inflammatory reaction followed by pulp necrosis. However, as long as the lateral or accessory canals are protected by sound cementum, necrosis usually does not occur.
Kakehashi, et al. [59]. Moller, et al. [60]. & Blomlof, et al. [61]. demonstrated the relationship between the presence of bacteria and diseases in pulp and periradicular tissues.
Jansson, et al. [62], in a retrospective radiographic 3-year study, evaluated 175 endodontically treated single-rooted teeth of 133 patients. Patients who were more susceptible to periodontitis and exhibited evidence of endodontic treatment failures showed an increase, of approximately threefold, in marginal bone loss compared with patients without endodontic infection.
Perforation
Perforation is a pathologic or artificial communication between the pulp cavity and the tissue surrounding the tooth [63]. Kvinnsland et al [64]. reported that 47% of all iatrogenic perforations, occurred during cleaning and shaping of the root canal and 53% during canal preparation for a post.
In 1996, Fuss and Trope [65] classified the prognosis of perforations, with emphasis on the location of the perforation in relation to the crestal bone, its size, and the time elapsed since its formation until treatment, with location as the most important factor. A perforation at the height of the crestal bone has the poorest prognosis. Bacterial infection or irritation by an irrigation solution may cause periodontal pockets.
Trauma
Trauma to teeth and alveolar bone may involve the pulp and the periodontal ligament. Both tissues can be affected either directly or indirectly. Dental injuries may take many shapes but generally can be classified as enamel fractures, crown fractures without pulp involvement, crown fractures with pulp involvement, crown–root fracture, root fracture, luxation and avulsion [66-71]. Treatment of traumatic dental injuries varies depending on the type of injury and this will determine pulpal and periodontal ligament healing prognosis [68].
Vertical root fractures (VRF)
Vertical fractures are complications observed mostly after root canal treatment and placement of a post. The preferred treatment is tooth extraction or removal of the fractured roots (Fig. 5). A prevalence rate of 2-5% has been found when radiographs were used to diagnose the fractures [72]. However, Fuss et al. [73] found a 10.9% prevalence rate in teeth extracted for various reasons. This shows the problem of using radiographs as the only diagnostic tool of this phenomenon. "Halo" lesion, perilateral radiolucency, and angular resorption of the alveolar bone, combined with diffuse or defined lesions devoid of corticated borders, indicated a high probability of vertical root fracture in maxillary premolars [74]. Typical signs and symptoms of vertical fractures have been examined in 22 patients with VRF [75]. Sixty five percent had only mild pain or a dull discomfort and a radiolucent lesion was noted in 75% of the population. Fractures most frequently involve premolars and the mesial roots of the first mandibular molar.
Increased mastication forces such as clenching and grinding are the main cause of vertical fracture in teeth without root canal treatment. The term “fatigue root fracture” was first used in 1997 [76]. Vertical fractures were more commonly found in teeth with root fillings than without root fillings.
Cervical root resorption
Infrequently, external root resorption can occur after injury of the pre-cementum apical to the epithelial attachment followed by bacterial irritation originating from the periodontal sulcus [77]. Dental trauma, chemical irritation, such as use of harsh bleaching agents (e.g., 30% hydrogen peroxide) may cause injury [78]. Bacteria from the periodontal sulcus can penetrate patent dentinal tubules, coronal to the epithelial attachment and exit apical to the epithelial attachment without penetrating the pulpal space [77]. Consequently, hard-tissue is resorbed, penetrating into dentin through a small denuded area. This results in the spread of resorption inside the root. With time, the process usually penetrates the root canal. Furthermore, periodontal infection resorption may include the alveolar bone adjacent to the resorption lacuna in the tooth. When the resorptive process reaches a supragingival area, the vascularized granulation tissue of the resorption lacuna may be visible through the enamel, with pink discoloration at the crown. The etiologic factors of this resorption are not fully understood. In 1985, Cvek and Lindvall79 suggested that the pathological factor is bacteria originally from the gingival sulcus, and in 2004, Fuss, et al. [79] called this resorption a periodontal infection root resorption due to the origin of the causative factors (Figure 6). More research is required to configure the cause and effect relationship of these suggested aetiological factors [80].
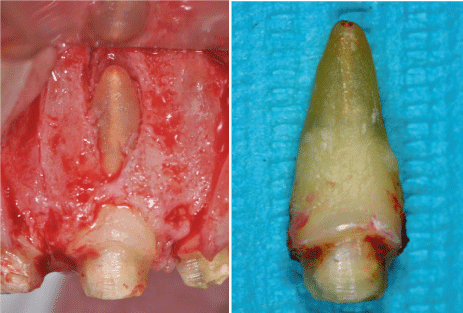
Figure 5. Vertical root fracture
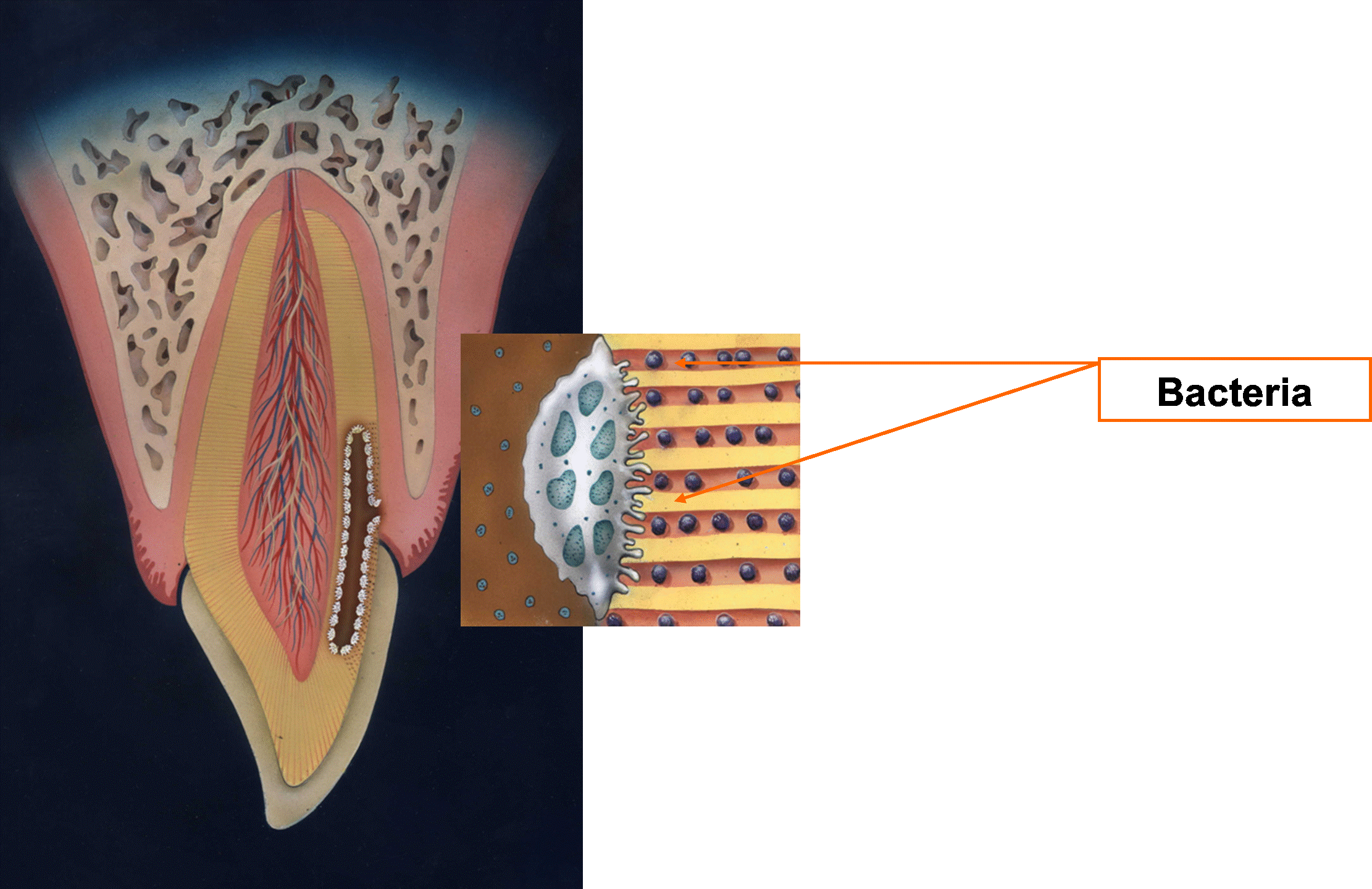
Figure 6. Invasive cervical root resorption seen as a single resorption lacuna in the dentin usually at the crestal bone level extending to the coronal and apical direction. With progression of the process, radiolucency can be observed at the alveolar bone adjacent to the resorption lacuna of the dentin
Radiographically, periodontal infection resorption is seen as a single resorption lacuna in the dentin usually at the crestal bone level, extending to the coronal and apical direction. With progression of the process, a radiolucent area can be observed at the alveolar bone adjacent to the resorption lacuna of the dentin.
The influence of endodontic state on the periodontal tooth ligament
The spread of infection from the root canal into the periodontal ligament is due to the combined anatomic routes and bacterial resemblance. A relationship has been shown between the periapical lesion and the periodontal ligament [50,62,81-83]. Periapical lesions in teeth induce formation of periodontal pockets and delay healing [50,62]. In teeth with an identical alveolar bone level the pocket depth is usually increased by 0.2 mm when a pathologic process is present at in the root apex [81]. In molars, the level of furcation involvement is higher [84, 85]. Teeth with periapical pathology typically present a three-fold increase in proximal alveolar bone loss, 0.19 mm/year in teeth with periapical lesions compared with 0.06 mm/year without periapical lesions [86]. This indicates that infection in the root apex might be the source of damage to the periodontal tooth ligament. Thus, endodontic treatment should be completed before periodontal treatment to promote healing.
Others have shown that tooth condition has no influence on the periodontal system. Nyman, et al. examined patients with bone loss of 50% over 5 to 8 years. The bone height remained constant in vital teeth and in teeth with root canal treatment. The level of alveolar bone has been examined comparing teeth with and without root canal filling but with a periapical lesion [87]. In patients with periodontitis teeth after root canal treatment have significantly more bone loss than untreated contralaterals [84]. In a pilot study of 9 periodontal-endodontic lesions, root canal treatment carried out before periodontal treatment was effective in decreasing probing depth and attachment gain [88].
The influence of the state of the pulp on healing after periodontal surgery in dogs was examined in four groups: intact tooth, tooth open to the oral space, tooth with a root canal filled with ZOE, and tooth with a root canal filled with calcium hydroxide [89]. Histologically, teeth were examined 6 months post-operatively. The state of the pulp affected healing. Calcium hydroxide showed the best healing result. The slowest healing was in the group with the pulp exposed to the mouth space. The only difference indicated was the rate of healing.
Cortellini &Tonetti studied the effect of tooth vitality on periodontal regeneration procedures. They concluded that tooth vitality had no influence on the outcome of surgery neither did the surgical procedure influence tooth vitality [90].
However it is reasonable to propose that in severe loss of periodontal tissue the possibility of an endodontic infection is greater.
Influence of the periodontal state on the pulp
The relationship between periodontal disease and periodontal treatment to the state of the pulp is not fully understood. Some consider periodontal disease and its treatment significant factors in pulp inflammation and necrosis, even more than dental caries while others claim the opposite [91,92].
On the other hand no inflammatory changes in the pulp were found in teeth extracted after amputation due to progressive bone loss [8]. In another study, intact teeth with and without periodontal disease were compared [4]. The pulp state was the same for both groups. Furthermore, inflammatory changes in the pulp were seen in teeth with extensive caries centers or major restorations.
The influence of periodontal treatment on the pulp has been examined [8,9]. Initial preparation includes scaling and root planing, resulting in calculus and bacteria removal from the root surface areas. During initial preparation, cementum and superficial dentin is sometimes removed exposing dentinal tubules to the oral cavity. Frequently, dentinal sensitivity develops after scaling and root planning due to the open dentinal tubules. Pain is caused by external stimuli such as acidic food, traumatic abrasion, neuronal changes, caries, inadequate restoration, and occlusal trauma. After several weeks, this sensation, usually, subsides with the natural obliteration of the tubule openings. Adequate oral hygiene and treatment of the sensitivity with high concentrations of fluorides or oxalate salts may be helpful. Sensitivity ultimately subsides with no degenerative changes in the pulp [93].
Periodontal maintenance includes scaling as well as oral hygiene instruction and patient motivation every 3-4 months. In a follow-up of patients, 4 to 13 years after periodontal maintenance treatment, pulp necrosis was rare (3%), occurring only in carious teeth [9].
In another study, the influence of periodontal maintenance treatment on the dentition was examined for 5-14 years [94]. Only 1 tooth out of 571 required root canal treatment. Previously, bone loss progression in the maxillary molars with furcation involvement was followed for 5-24 years and included regular periodontal maintenance [7]. Root canal filling was required in only 4% of the teeth after periodontal treatment, including those resulting from deep caries or pulp necrosis due to major restorations. The endodontic status of the teeth did not influence the periodontal prognosis.
The objective of periodontal surgery is to reduce periodontal pockets remove bacterial deposits and regenerate lost periodontal tissues
Regenerative endodontic procedures
In recent years a new treatment modality utilizing stem cells from the periodontal tissues surrounding the tooth has emerged [95]. This treatment is commonly termed “revascularization”, “revitalization” or “regenerative endodontic procedures”, and is directed towards treating necrotic immature permanent teeth. Its aims, in addition to the resolution of periapical pathosis, are to achieve revitalization and continued root development, thereby strengthening the tooth and decreasing the risk of root fracture. Briefly, this technique takes advantage of periodontal ligament stem cells or stem cells of the developing root apical papilla (SCAPs). Cells derived from these cells can differentiate into odontoblasts, cementoblastlike cells, adipocytes and connective tissue, both in vitro and in vivo [96].
To date, more than 65 case reports and case series with over 320 teeth treated following a regenerative treatment protocol have been published, and in 2013 the American Association of Endodontists (AAE) published a position statement in which regenerative endodontics was nominated as part of the endodontic treatment spectrum [97].
Migration of junctional epithelium after conservative surgical periodontal treatment prevents root resorption [98]. Concern for root resorption increases as epithelial migration of the junctional epithelium is prevented, e.g., in regenerative procedures. Severe cervical resorption of the root 6 months after guided bone formation has been described [99].
Endodontic- periodontal lesions
Recently the American Academy of Periodontology (AAP) and the European Federation of Periodontology (EFP) published a new Classification of Periodontal and Peri-Implant Diseases and Conditions including Endodontic-Periodontal Lesions (EPL) under the category "Other periodontal conditions" with periodontal abscesses [100,101].
Previously EPL were classified according to the primary lesion (e.g., primary endodontic with secondary periodontal). However recent research presented the similarities in the bacterial profile and difficulty identifying the primary lesion led to the new classification based on the clinical presentation of the lesion.
EPL involve the pulp and the periodontal tissues surrounding the root. The most common feature is pain. These lesions can manifest either as an acute or chronic lesion. Clinically a deep periodontal pocket can be probed with a non-vital pulp. Other signs such as bone resorption in the apical or furcation area and purulent exudate with a sinus tract may be present.
Two major etiologies can be present (1) infections of the pulp or periodontium (2) trauma or iatrogenic factors. EPL should be classified according to the signs and symptoms present. The presence of a deep periodontal pocket reaching, with no periodontal disease present, accompanied by a non-vital pulp should lead to the diagnosis of an EPL.
The authors declare that there are no conflicts of interest in this study
Pathologic changes in the pulp and in periodontal tissues can affect both ways. The pulp is highly resistant and possesses a high ability to servive disease and treatment. However, pulp necrosis is a risk factor that damages periodontal structures. Periodontal disease and its treatment exhibit a slight affect on the pulp. Larger periodontal defects present higher risk for endodontic infections
- 1. Orban BJ, Sicher H (1962) Orban's Oral Histology and Embryology, Fifth Edition.
- 2. De Deus QD (1975) Frequency, location, and direction of the lateral, secondary, and accessory canals. J Endod 1: 361-366. [Crossref]
- 3. Tagger M, Massler M (1975) Periapical tissue reactions after pulp exposure in rat molars. Oral Surg Oral Med Oral Pathol 39: 304-317. [Crossref]
- 4. Czarnecki RT, Schilder H (1979) A histological evaluation of the human pulp in teeth with varying degrees of periodontal disease. J Endod 5: 242-253. [Crossref]
- 5. Torabinejad M, Kiger RD (1985) A histologic evaluation of dental pulp tissue of a patient with periodontal disease. Oral Surg Oral Med Oral Pathol 59: 198-200. [Crossref]
- 6. Tagger M, Smukler H (1977) Microscopic study of the pulps of human teeth following vital root resection. Oral Surg Oral Med Oral Pathol 44: 96-105.
- 7. Ross IF, Thompson RH, Jr. (1978) A long term study of root retention in the treatment of maxillary molars with furcation involvement. J Periodontol 49: 238-244. [Crossref]
- 8. Tagger M, Perlmutter S, Tagger E (1988) Histological study of untreated pulps in hemisected teeth in baboons. J Endod 14: 288-292. [Crossref]
- 9. Bergenholtz G, Nyman S (1984) Endodontic complications following periodontal and prosthetic treatment of patients with advanced periodontal disease. J Periodontol 55: 63-68. [Crossref]
- 10. Langeland K, Rodrigues H, Dowden W (1974) Periodontal disease, bacteria, and pulpal histopathology. Oral Surg Oral Med Oral Pathol 37: 257-270. [Crossref]
- 11. Seltzer S, Bender IB, Nazimov H, et al (1967) Pulpitis-induced interradicular periodontal changes in experimental animals. J Periodontol 38: 124-129. [Crossref]
- 12. Burch JG, Hulen S (1974) A study of the presence of accessory foramina and the topography of molar furcations. Oral Surg Oral Med Oral Pathol 38: 451-455. [Crossref]
- 13. Niemann RW, Dickinson GL, Jackson CR, Wearden S, Skidmore AE (1993) Dye ingress in molars: furcation to chamber floor. J Endod 19: 293-296. [Crossref]
- 14. Welch JD, Anderson RW, Pashley DH, Weller RN, Kimbrough WF (1996) An assessment of the ability of various materials to seal furcation canals in molar teeth. J Endod 22: 608-611. [Crossref]
- 15. Strobel S, Lenhart E, Woelber JP, Fleiner J, Hannig C, et al. (2017) Comparison of two cone-beam computed tomography systems in the visualization of endodontic structures. Swiss Dent J 127: 221-229. [Crossref]
- 16. Ahmed HM, Hashem AA (2016) Accessory roots and root canals in human anterior teeth: a review and clinical considerations. Int Endod J 49: 724-736. [Crossref]
- 17. Seltzer S, Bender IB, Ziontz M (1963) THE INTERRELATIONSHIP OF PULP AND PERIODONTAL DISEASE. Oral Surg Oral Med Oral Pathol 16: 1474-1490. [Crossref]
- 18. Ricucci D, Siqueira JF Jr (2008) Apical actinomycosis as a continuum of intraradicular and extraradicular infection: case report and critical review on its involvement with treatment failure. J Endod 34: 1124-1129. [Crossref]
- 19. Ricucci D, Siqueira JF Jr (2008) Anatomic and microbiologic challenges to achieving success with endodontic treatment: a case report. J Endod 34: 1249-1254. [Crossref]
- 20. Barthel CR, Zimmer S, Trope M (2004) Relationship of radiologic and histologic signs of inflammation in human root-filled teeth. J Endod 30: 75-79. [Crossref]
- 21. Lim JH, Lee JH, Shin SJ (2014) Diagnosis and treatment of teeth with primary endodontic lesions mimicking periodontal disease: three cases with long-term follow ups. Restor Dent Endod 39: 56-62. [Crossref]
- 22. Lin S, Tillinger G, Zuckerman O (2008) Endodontic-periodontic bifurcation lesions: a novel treatment option. J Contemp Dent Pract 9: 107-114. [Crossref]
- 23. Kirkham DB (1975) The location and incidence of accessory pulpal canals in periodontal pockets. J Am Dent Assoc 91: 353-356. [Crossref]
- 24. Dammaschke T, Witt M, Ott K, et al (2004) Scanning electron microscopic investigation of incidence, location, and size of accessory foramina in primary and permanent molars. Quintessence Int 35: 699-705. [Crossref]
- 25. Mjör IA, Nordahl I (1996) The density and branching of dentinal tubules in human teeth. Arch Oral Biol 41: 401-412. [Crossref]
- 26. Muller CJ, van Wyk CW (1984) The amelo-cemental junction. J Dent Assoc S Afr 39: 799-803. [Crossref]
- 27. Arambawatta K, Peiris R, Nanayakkara D (2009) Morphology of the cemento-enamel junction in premolar teeth. J Oral Sci 51: 623-627. [Crossref]
- 28. Schroeder HE, Scherle WF (1988) Cemento-enamel junction--revisited. J Periodontal Res 23:53-59. [Crossref]
- 29. Haapasalo M, Orstavik D (1987) In vitro infection and disinfection of dentinal tubules. J Dent Res 66: 1375-1379. [Crossref]
- 30. August DS (1978) The radicular lingual groove: an overlooked differential diagnosis. J Am Dent Assoc 96: 1037-1039. [Crossref]
- 31. Withers JA, Brunsvold MA, Killoy WJ, Rahe AJ (1981) The relationship of palato-gingival grooves to localized periodontal disease. J Periodontol 52: 41-44. [Crossref]
- 32. Gu YC (2011) A micro-computed tomographic analysis of maxillary lateral incisors with radicular grooves. J Endod 37: 789-792. [Crossref]
- 33. Kerezoudis NP, Siskos GJ, Tsatsas V (2003) Bilateral buccal radicular groove in maxillary incisors: case report. Int Endod J 36: 898-906. [Crossref]
- 34. Pécora JD, da Cruz Filho AM (1992) Study of the incidence of radicular grooves in maxillary incisors. Braz Dent J 3: 11-16. [Crossref]
- 35. TC-C HG-L (1988) A Study of the Palato-Radicular Groove in Chinese Adults I. Prevalence, Location, Conformation and Symmetry. J Formosa Dent Assoc 11: 349-354.
- 36. Kogon SL (1986) The prevalence, location and conformation of palato-radicular grooves in maxillary incisors. J Periodontol 57: 231-234. [Crossref]
- 37. Everett FG, Kramer GM (1972) The disto-lingual groove in the maxillary lateral incisor; a periodontal hazard. J Periodontol 43: 352-361. [Crossref]
- 38. Gher ME, Vernino AR (1980) Root morphology--clinical significance in pathogenesis and treatment of periodontal disease. J Am Dent Assoc 101: 627-633. [Crossref]
- 39. Baci� M, Karakas Z, Kai� Z (1990) The association between palatal grooves in upper incisors and periodontal complications. J Periodontol 61: 197-199. [Crossref]
- 40. Corr AFP, Alcântara CE, Santos CR (2011) Palatal radicular groove: clinical implications of early diagnosis and surgical sealing. J Indian Soc Pedod Prev Dent 29: S92-94. [Crossref]
- 41. Al-Hezaimi K, Naghshbandi J, Simon JH, Rotstein I (2009) Successful treatment of a radicular groove by intentional replantation and Emdogain therapy: four years follow-up. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107: e82-85 [Crossref]
- 42. Risnes S (1974) The prevalence, location, and size of enamel pearls on human molars. Scand J Dent Res 82: 403-412. [Crossref]
- 43. Moskow BS, Canut PM (1990) Studies on root enamel (2). Enamel pearls. A review of their morphology, localization, nomenclature, occurrence, classification, histogenesis and incidence. J Clin Periodontol 17: 275-281. [Crossref]
- 44. Skinner MA, Shiloah J (1989) The role of enamel pearls in localized severe periodontitis. Quintessence Int 20: 181-183. [Crossref]
- 45. Baumgartner JC (2004) Microbiologic aspects of endodontic infections. J Calif Dent Assoc 32: 459-468. [Crossref]
- 46. Baumgartner JC, Falkler WA Jr (1991) Bacteria in the apical 5 mm of infected root canals. J Endod 17: 380-383. [Crossref]
- 47. Dahle UR, Tronstad L, Olsen I (1996) Characterization of new periodontal and endodontic isolates of spirochetes. Eur J Oral Sci 104: 41-47. [Crossref]
- 48. Egan MW, Spratt DA, Ng YL, Lam JM, Moles DR, et al. (2002) Prevalence of yeasts in saliva and root canals of teeth associated with apical periodontitis. Int Endod J 35: 321-329. [Crossref]
- 49. Haapasalo M, Ranta H, Ranta K, Shah H (1986) Black-pigmented Bacteroides spp. in human apical periodontitis. Infect Immun 53:149-153. [Crossref]
- 50. Jansson L, Ehnevid H, Blomlöf L, Weintraub A, Lindskog S (1995) Endodontic pathogens in periodontal disease augmentation. J Clin Periodontol 22: 598-602. [Crossref]
- 51. Jung IY, Choi BK, Kum KY, Roh BD, Lee SJ, et al. (2000) Molecular epidemiology and association of putative pathogens in root canal infection. J Endod 26: 599-604. [Crossref]
- 52. Nair PN (2004) Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med 15: 348-381. [Crossref]
- 53. Ozok AR, Persoon IF, Huse SM, Keijser BJF, Wesselink PR (2012) Ecology of the microbiome of the infected root canal system: a comparison between apical and coronal root segments. Int Endod J 45: 530-541. [Crossref]
- 54. Siqueira JF, Jr Rôças IN, Souto R, Uzeda M, Colombo AP (2000) Checkerboard DNA-DNA hybridization analysis of endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 89:744-748. [Crossref]
- 55. El-Labban NG (1979) Electron microscopic investigation of hyaline bodies in odontogenic cysts. J Oral Pathol 8: 81-93. [Crossref]
- 56. Nair PN (1999) Cholesterol as an aetiological agent in endodontic failures--a review. Aust Endod J 25:19-26. [Crossref]
- 57. Silver GK, Simon JH (2000) Charcot-Leyden crystals within a periapical lesion. J Endod 26: 679-681. [Crossref]
- 58. MANDI FA (1972) Histological Study of the Pulp Changes caused by Periodontal Disease. International Endodontic Journal 6: 80-83.
- 59. Kakehashi S, Stanley HR, Fitzgerald RJ (1965) The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol 20: 340-349. [Crossref]
- 60. Möller AJ, Fabricius L, Dahlén G, Ohman AE, Heyden G (1981) Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Scand J Dent Res 89: 475-484. [Crossref]
- 61. Blomlöf L, Lengheden A, Lindskog S (1992) Endodontic infection and calcium hydroxide-treatment. Effects on periodontal healing in mature and immature replanted monkey teeth. J Clin Periodontol 19: 652-658. [Crossref]
- 62. Jansson L, Ehnevid H, Lindskog S, L Blomlöf (1995) The influence of endodontic infection on progression of marginal bone loss in periodontitis. J Clin Periodontol 22: 729-734. [Crossref]
- 63. Weine FS (1996) Endodontic therapy Mosby, St. Louis.
- 64. Kvinnsland I, Oswald RJ, Halse A, Grønningsaeter AG (1989) A clinical and roentgenological study of 55 cases of root perforation. Int Endod J 22: 75-84. [Crossref]
- 65. Fuss Z, Trope M (1996) Root perforations: classification and treatment choices based on prognostic factors. Endod Dent Traumatol 12: 255-264. [Crossref]
- 66. Andreasen FM (1989) Pulpal healing after luxation injuries and root fracture in the permanent dentition. Endod Dent Traumatol 5: 111-131. [Crossref]
- 67. Andreasen FM, Flugge E, Daugaard-Jensen J, Munksgaard EC (1992) Treatment of crown fractured incisors with laminate veneer restorations. An experimental study. Endod Dent Traumatol 8: 30-35. [Crossref]
- 68. Andreasen JO, Andreasen FM, Skeie A, Hjørting-Hansen E, Schwartz O (2002) Effect of treatment delay upon pulp and periodontal healing of traumatic dental injuries -- a review article. Dent Traumatol 18: 116-128. [Crossref]
- 69. Bechara B, McMahan CA, Nasseh I, Geha H, Hayek E (2013) Number of basis images effect on detection of root fractures in endodontically treated teeth using a cone beam computed tomography machine: an in vitro study. Oral Surg Oral Med Oral Pathol Oral Radiol 115: 676-681. [Crossref]
- 70. Edlund M, Nair MK, Nair UP (2011) Detection of vertical root fractures by using cone-beam computed tomography: a clinical study. J Endod 37: 768-772. [Crossref]
- 71. Nair MK, Nair UDP, Gröndahl HG (2001) Detection of artificially induced vertical radicular fractures using tuned aperture computed tomography. Eur J Oral Sci 109: 375-379. [Crossref]
- 72. Testori T, Badino M, Castagnola M (1993) Vertical root fractures in endodontically treated teeth: a clinical survey of 36 cases. J Endod 19: 87-91. [Crossref]
- 73. Fuss Z, Lustig J, Tamse A (1999) Prevalence of vertical root fractures in extracted endodontically treated teeth. Int Endod J 32: 283-286. [Crossref]
- 74. Tamse A, Fuss Z, Lustig J, Ganor Y, Kaffe I (1999) Radiographic features of vertically fractured, endodontically treated maxillary premolars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 88: 348-352. [Crossref]
- 75. McGuire MK, Nunn ME (1996) Prognosis versus actual outcome. III. The effectiveness of clinical parameters in accurately predicting tooth survival. J Periodontol 67: 666-674. [Crossref]
- 76. Yeh CJ (1997) Fatigue root fracture: a spontaneous root fracture in non-endodontically treated teeth. Br Dent J 182: 261-266. [Crossref]
- 77. Tronstad L (1988) Root resorption-etiology, terminology and clinical manifestations. Dental Traumatology 4: 241-252. [Crossref]
- 78. Cvek M, Lindvall AM (1985) External root resorption following bleaching of pulpless teeth with oxygen peroxide. Endod Dent Traumatol 1: 56-60. [Crossref]
- 79. Fuss Z, Tsesis I, Lin S (2003) Root resorption--diagnosis, classification and treatment choices based on stimulation factors. Dent Traumatol 19: 175-182. [Crossref]
- 80. Patel S, Mavridou AM, Lambrechts P, Saberi N (2018) External cervical resorption-part 1: histopathology, distribution and presentation. Int Endod J 51:1205-1223. [Crossref]
- 81. Sabeti M, Simon JH, Nowzari H, J Slots (2003) Cytomegalovirus and Epstein-Barr virus active infection in periapical lesions of teeth with intact crowns. J Endod 29: 321-323. [Crossref]
- 82. Jansson L, Ehnevid H, Lindskog S, Blomlöf L (1993) Relationship between periapical and periodontal status. A clinical retrospective study. J Clin Periodontol 20: 117-123. [Crossref]
- 83. Ehnevid H, Jansson L, Lindskog S, Blomlöf L (1993) Periodontal healing in teeth with periapical lesions. A clinical retrospective study. J Clin Periodontol 20: 254-258. [Crossref]
- 84. Timmerman MF, Van der Weijden GA (2006) Bone level around endodontically treated teeth in periodontitis patients. J Clin Periodontol 33: 620-625. [Crossref]
- 85. Narang S, Narang A, Gupta R (2011) A sequential approach in treatment of perio-endo lesion. J Indian Soc Periodontol 15:177-180. [Crossref]
- 86. Nyman S, Lindhe J (1979) A longitudinal study of combined periodontal and prosthetic treatment of patients with advanced periodontal disease. J Periodontol 50: 163-169. [Crossref]
- 87. Miyashita H, Bergenholtz G, Gröndahl K, Wennström JL (1998) Impact of endodontic conditions on marginal bone loss. J Periodontol 69: 158-164. [Crossref]
- 88. Vakalis SV, Whitworth JM, Ellwood RP, Preshaw PM (2005) A pilot study of treatment of periodontal-endodontic lesions. Int Dent J 55: 313-318. [Crossref]
- 89. Holland R, Otoboni Filho JA, Bernabé PF, Nery MJ, de Souza V, et al. (1994) Effect of root canal status on periodontal healing after surgical injury in dogs. Endod Dent Traumatol 10:77-82. [Crossref]
- 90. Cortellini P, Tonetti MS (2001) Evaluation of the effect of tooth vitality on regenerative outcomes in infrabony defects. J Clin Periodontol 28: 672-679. [Crossref]
- 91. Wong R, Hirsch RS, Clarke NG (1989) Endodontic effects of root planing in humans. Endod Dent Traumatol 5: 193-196. [Crossref]
- 92. Haskell EW, Stanley H, Goldman S (1980) A new approach to vital root resection. J Periodontol 51: 217-224. [Crossref]
- 93. Pashley DH (1986) Dentin permeability, dentin sensitivity, and treatment through tubule occlusion. J Endod 12: 465-474. [Crossref]
- 94. Jaoui L, Machtou P, Ouhayoun JP (1995) Long-term evaluation of endodontic and periodontal treatment. Int Endod J 28: 249-254. [Crossref]
- 95. Banchs F, Trope M (2004) Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod 30: 196-200. [Crossref]
- 96. Egusa H, Sonoyama W, Nishimura M, Atsuta I, Akiyama K et al (2012) Stem cells in dentistry--part I: stem cell sources. J Prosthodont Res 56: 151-165. [Crossref]
- 97. Endodontics AAo Scope of Endodontics: Regenerative Endodontics.
- 98. Andreasen JO (1985) External root resorption: its implication in dental traumatology, paedodontics, periodontics, orthodontics and endodontics. Int Endod J 18: 109-118. [Crossref]
- 99. Blomlöf L, Lindskog S (1998) Cervical root resorption associated with guided tissue regeneration: a case report. J Periodontol 69: 392-395. [Crossref]
- 100. Caton JG, Armitage G, Berglundh T, Chapple LC, Jepsen S, et al. (2018) A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J Clin Periodontol 45 Suppl 20: S1-S8. [Crossref]
- 101. Herrera D, Retamal-Valdes B, Alonso B, Feres M (2018) Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J Periodontol 89 Suppl 1: S85-s102. [Crossref]






