78-year-old man with metastatic platinum refractory small cell carcinoma of the prostate who was treated with pembrolizumab with good partial response. This case supports that checkpoint inhibitors could be of a benefit for such rare cancers and more investigation is warranted.
78-year old male with prior history of high-grade Gleason score (5+4) adenocarcinoma of the prostate status post brachytherapy (1998) and hormonal therapy who achieved a good response for approximately 12 years up till 2009, when he experienced a biochemical failure and was placed on intermittent hormonal therapy with good response. He is referred to oncology clinic after presenting to his urologist with gross hematuria in 2015 following cystoscopy and TURBT, and was found to have significant prostate cancer involving the bladder with right-sided hydronephrosis and obstruction of the right ureteral orifice and trigone. Percutaneous nephrostomy was performed (internalized to bladder), and biopsy showed locally advanced small cell carcinoma of the prostate with >90% small cell differentiation, as well as 10% Gleason of 10 (5+5) adenocarcinoma.
At the time of presentation, he reported he was urinating without difficulty, with some intermittent abdominal cramping and a mild aching sensation in his right flank. He denied any fevers, chills, rigors or sweats, coughing, dyspnea, or wheezing. He additionally denied adenopathy, anorexia, weight loss, pedal edema, or rash. Comprehensive review of all remaining systems was otherwise negative.
His medical history was also significant for chronic bacterial prostatitis with microscopic hematuria, congestive heart failure status post two-vessel CABG (2000), atrial fibrillation on chronic warfarin anticoagulation, multiple facial basal cell carcinoma excisions, osteopenia, osteoarthritis (knees), hypertension, dyslipidemia, chronic renal insufficiency, anxiety, and insomnia. His family history further included pervasive prostate cancer, most notably in his father and older brother. He had a remote tobacco use history, reportedly quitting 30 years prior, and used alcohol socially. He was a retired quality control foreman at a local machine shop, and lived with his disabled wife.
His medications included Lupron 30mg IM every four months, alendronate, amlodapine, aspirin, atorvastatin, diltiazem, sotalol, clonazepam as needed for anxiety, and omeprazole as needed for heartburn. His only known drug reaction was development of chronic cough due to lisinopril.
On physical exam, the patient was in no acute distress, weighing 84kg with temperature 36.4, heart rate 65, respirations 16, blood pressure 138/86, and oxygen saturation of 98% on room air. Inspection of the mouth revealed moist mucus membranes without mucositis or thrush. His sclerae were anicteric, and neck was supple without lymphadenopathy. The patient had normal heart sounds without any murmurs and lungs were clear to auscultation. His abdomen was benign without evidence of mass, tenderness, or hepatosplenomegaly. Extremities were without clubbing, cyanosis, or edema, and skin exam was unremarkable.
Most recent labs obtained by his urologist in late 2015 revealed a creatinine of 1.53 with otherwise normal comprehensive metabolic panel, PSA of 0.24, testosterone level of 43.9 (normal 170-830), white count of 7.3, hemoglobin of 13.2, and platelets of 232,000. PSA upon presentation to oncology clinic was 0.15. Pathology collected from cystoscopy and TURBT performed by his urologist revealed prostatic adenocarcinoma with a Gleason score of 10 (5+), bladder infiltration with approximately 90% small cell dedifferentiation and positive immunostaining for synaptophysin and chromogranin, with no evidence of urothelial cancer. This was additionally complicated by right-sided hydronephrosis and obstruction of the right ureteral orifice.
Given these circumstances, he was advised that therapy with Lupron would likely be insufficient, and chemotherapy was recommended. Because of his abnormal renal function and his ECOG performance status of 1, he was treated with carboplatin and etoposide. Following his fourth cycle of chemotherapy he underwent restaging scans. Chest CT performed showed a new 1.6cmx1.2cm upper lobe pulmonary nodule with a cluster of subcentimeter pulmonary nodules in the left lower lobe and right upper lobe. Abdominal and pelvic CT performed showed an enlarged internal iliac lymph node, a mass involving the right apex and mid gland of the prostate very close to the anus, a second mass centered around the right obturator foramen extending into the adductor muscles and obturator internus, and a right perirectal lymph node measuring 1.2cm. He underwent an IR-guided biopsy of the obturator lymph node, which showed small cell carcinoma.
Given his platinum refractory disease the patient was then offered topotecan, but declined due to his poor performance status of 2 at that time. He ultimately underwent palliative radiation to his pelvic area with good resolution of groin pain, and was subsequently offered off-label use of the PD-L1 inhibitor pembrolizumab, which was granted for compassionate use through the Merck Company Access Program. Pembrolizumab use was supported by a small phase 1B study which showed an objective response rate of 35% in a small cohort of relapsed patients with confirmed PD-L1 expression following platinum-based first line therapy.
Therapy began in October 2016, which consisted of pembrolizumab 2mg/kg every three weeks, complicated only by single episodes of recurrent prostatitis, UTI, and asymptomatic hypothyroidism successfully treated with ciprofloxacin, augmentin, and levothyroxine (25mcg and then 50mcg orally daily), respectively, as well as a minimal ankle grade 1 rash that resolved with triamcinolone cream. Restaging after four cycles of pembrolizumab revealed substantial improvement in pelvic and bladder wall masses, and subsequent restaging in March 2017 showed improved bladder wall thickening with no new metastatic lesions [Figure 1]. The patient continued pembrolizumab every 3 weeks and restaging scans every 3 months without further evidence for progression or recurrence. He was last seen in January 2018 – just prior to initiation of cycle 21 of pembrolizumab.
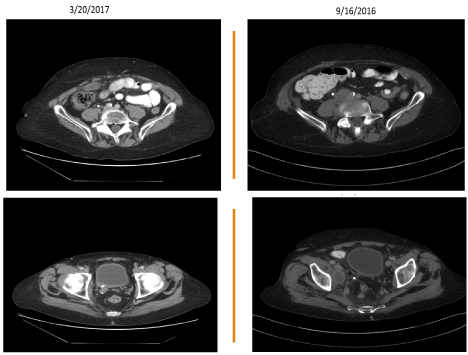
Figure 1. Disease partial response after for cycles of pembrolizumab. Cross section of patient’s pelvis CT scans on 9/16/2016 and 3/20/2017.
Small cell carcinoma of the prostate is rare, and primarily presents as a mixed histology with adenocarcinoma of the prostate. Even a small focus of small cell carcinoma is enough to predict aggressive disease. Our patient upon recurrence had tumor burden with more than 90% small cell carcinoma.
The age of onset for prostatic small cell carcinoma PSCC is generally younger (40 – 60 years) than that of adenocarcinoma. Tissue biopsy remains the gold standard for diagnosis while PSA has very limited benefit [1]. PSCC also tends to be more aggressive than adenocarcinoma and initially presents with low PSA but extensive stage disease with poor prognosis [2-5]. Staging would be limited or extensive according to one radiation field as per pulmonary small cell carcinomas.
The overall outcome of PSCC is illustrated by a series of 241 cases identified from the Surveillance, Epidemiology, and End Results (SEER) database over a 30-year period [4]. In this series, 60 percent of patients had metastatic disease at presentation. The one-, two-, and five-year survival rates for the entire series were 48, 28, and 14 percent, respectively.
Patients with extrapulmonary small cell carcinomas (ESCC) lack prospective studies, and their recommendation are usually derived from single institution reports on pulmonary small cell carcinomas.
Patients with early-stage disease will require systemic chemotherapy after or concurrently with local therapy and surgery or radiation, while systemic chemotherapy will remain the mainstay for patients with extensive disease. Chemotherapy regimens are primarily platinum based, with etoposide and platinum being the most widely used combination [5-10].
There is no evidence that androgen deprivation therapy (ADT) is useful in patients with pure ESCC, although it is a reasonable component of initial therapy for men whose tumors contain both adenocarcinoma and small cell carcinoma [11].
Unfortunately, there have been no improvements in outcomes for small cell carcinoma over the last 30 years. However, recent immunotherapy with PD-L1 inhibitors as well as CTL4 inhibitors has shown some efficacy in pulmonary small cell carcinomas. These early phase trials have achieved promising results using single agents pembrolizumab or nivolumab with and without ipilimumab [12,13].
Small cell carcinoma of lung tends to have lower PD-L1 expression compared to lung adenocarcinoma with a rate between 10-15%, and does not seem to correlate with responses [12,13]. Thus we requested the use of pembrolizumab from the MERCK Company for companionate use once our patient had progressed on first line treatment, and he continues to maintain good performance status on pembrolizumab despite negative PD-L1 expression in his tumor cells.
From our single institution review on PD-L1 expression of extra-pulmonary small cell carcinomas (ESCC) between 1999 and 2016, we identified 2 cases of prostate origin, one of which had a positive PD-L1 combined score. Positive combined scores were detected in 12 of 34 (35%) total ESCC cases, with the majority arising from the genitourinary tract [14]. While our patient had a negative score, this review demonstrated that these rare tumors may still express PD-L1, and thus investigating PD-1 inhibitors could result in favorable outcomes among this population. Further, in general immunotherapy drugs are well tolerated with smaller side effect profiles than chemotherapy agents.
Our patient had a very good partial tumor response, which he was able to maintain for up to one year. To our knowledge his is the first case of prostatic small cell carcinoma responding to the PD-L1 inhibitor pembrolizumab in a setting of negative PD-L1 expression.
Patient consented to report his case without mentioning his name
MERCK company for providing us with pembrolizumab for companionate use.
- Mackey JR, Au HJ, Hugh J, Venner P (1998) Genitourinary small cell carcinoma: determination of clinical and therapeutic factors associated with survival. J Urol 159: 1624-1629. [Crossref]
- Aggarwal R, Zhang T, Small EJ, Armstrong AJ (2014) Neuroendocrine prostate cancer: subtypes, biology, and clinical outcomes. J Natl Compr Canc Netw 12: 719.
- Epstein JI, Amin MB, Beltran H, Lotan TL, Mosquera JM, et al. (2014) Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol 38: 756-767. [Crossref]
- Deorah S, Rao MB, Raman R, Gaitonde K, Donovan JF (2012) Survival of patients with small cell carcinoma of the prostate during 1973-2003: a population-based study. BJU Int 109: 824-830. [Crossref]
- Têtu B, Ro JY, Ayala AG, Johnson DE, Logothetis CJ, et al. (1987) Small cell carcinoma of the prostate. Part I. A clinicopathologic study of 20 cases. Cancer 59: 1803-1809. [Crossref]
- Kim KH, Kim YB, Lee JK, Kim YJ, Jung TY (2010) Pathologic results of radical prostatectomies in patients with simultaneous atypical small acinar proliferation and prostate cancer. Korean J Urol 51: 398-402. [Crossref]
- Sule-Suso J, Brunt AM (1992) Small cell carcinoma of the prostate. Br J Radiol 65: 726-728. [Crossref]
- Rubenstein JH (1997) Small cell anaplastic carcinoma of the prostate: seven new cases, review of the literature, and discussion of a therapeutic strategy. Am J Clin Oncol 20: 376-380.
- Amato RJ, Logothetis CJ, Hallinan R, Ro JY, Sella A, et al. (1992) Chemotherapy for small cell carcinoma of prostatic origin. J Urol 147: 935-937. [Crossref]
- Steineck G, Reuter V, Kelly WK, Frank R, Schwartz L, et al. (2002) Cytotoxic treatment of aggressive prostate tumors with or without neuroendocrine elements. Acta Oncol 41: 668-674. [Crossref]
- Moore SR, Reinberg Y, Zhang G (1992) Small cell carcinoma of prostate: effectiveness of hormonal versus chemotherapy. Urology 39: 411–416
- Ott PA, Elez E, Hiret S, Kim DW, Morosky A, et al. (2017) Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study. J Clin Oncol 35: 3823-3829. [Crossref]
- Antonia SJ, López-Martin JA, Bendell J (2016) Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 17: 883.
- Salhab M, Migdady Y, Donahue M, Xinog Y, Dresser K, et al. (2018) Immunohistochemical expression and prognostic value of PD-L1 in Extrapulmonary small cell carcinoma: a single institution experience. J Immunother Cancer 6: 42.

