Abstract
The pedunculopontine tegmental nucleus (PPTg) is part of the locomotor mesencephalic region and the reticular activating system (RAS). As such, it is involved in regulating some aspects of the motor control and the wake/sleep cycle, but the way in which it operates is unclear. PPTg neurons respond to a variety of sensory stimuli and to cues that trigger the execution of goal-directed motor acts. In this paper, we discuss data that suggest that in addition to operating via thalamic nuclei, the PPTg may also operate by activating cerebellar nuclei, especially the dentate nucleus. In such a way, an intense and diffuse activation of the cerebral cortex may be achieved. In arousal-demanding reward-related tasks, separate populations of PPTg neurons modulate their discharge pattern to encode, in particular, expectancy and magnitude of reward. These neuronal responses, which should reach thalamic, basal ganglia, and cerebellar nuclei through ascending PPTg fibers are essential for the formation of stimuli-reward associations and action selection. In parallel, PPTg neurons, via descending fibers directed to lower brainstem and spinal motor structures, may automatically influence motor mechanisms and muscle tone in order to cause movements that are executed in a smooth and timely manner. In patients affected by Parkinson’s disease, PPTg deep brain stimulation may increase arousal, thus making patients more attentive to behavioral stimuli that trigger motor act, while simultaneously facilitating movement.
Key words
arousal, cerebellum, gamma oscillations, reward, thalamus
Introduction
The pedunculopontine tegmental nucleus (PPTg), together with the locus coeruleus and the dorsal raphe nucleus, is a major constituent of the reticular activating system (RAS) [1-3]. This neuronal system, which originates in the brainstem, was first thought to be involved in the modulation of the wake/sleep cycle. In the late 1940’s, Moruzzi and Magoun [4] described the appearance of fast, low voltage activity in the electroencephalogram, and abolition of synchronized discharge during stimulation of the reticular formation in the brainstem. They proposed that diffuse thalamic projection system was responsible for the cortical effects of reticular stimulation, and they suggested that sensory stimuli conveyed by collaterals of afferent pathways to the brainstem could be responsible for the cortical arousal reaction. This meant that the cortical spread triggered by the arrival of afferent impulses to the sensory cortex had a minor role in cortical arousal when compared to the influence of the RAS. A second key discovery that fits well with the purpose of this review stems from studies published in the 1980s by Steriade and coworkers, who showed involvement of RAS cholinergic neurons in the activation of thalamocortical systems [5-8].
Following a large number of investigations devoted to the PPTg during the last two decades, we can conclude that, most likely, PPTg neurons were stimulated in Moruzzi and Magoun’s encéphale isolé anesthetized or anaesthetized cat preparations as well as in anesthetized intact cats. This theory is supported by the fact that: 1) the midbrain area they stimulated was medial to the medial lemniscus and coincided with the course of ascending PPTg fibers; 2) the stimuli they delivered via large bipolar electrodes were strong (50-300 HZ, 1.5 V) and would over a large volume of tissue involving structures away from the stimulating tip; and 3) interruption of sensory pathways, that is the medial and lateral leminsci and the spinothalamic tracts, failed to suppress the arousal response in the cortex induced by stimulation of the brainstem reticular formation, which suggests that the stimulated structures could activate their targets independently of sensory inputs.
Following the first reports of neuronal degeneration of PPTg in Parkinson’s disease (PD) and parkinsonisms [9-11] and the pioneering review by Garcia Rill [1], the PPTg has been the object of several papers concerning its neuroanatomy [12-24], neurophysiology [25,26], neuropathology [27,28], and neurosurgery [29-36].
The interest in the PPTg is a valuable example of translational research across a well-defined area of the brain that has disclosed new data for a better understanding of the physiology of the brainstem and basal ganglia and offered new perspectives in the development of treatments for motor and non-motor disorders. Undoubtedly, the PPTg is one of the most investigated brain structures in the last three decades, and several reviews devoted to the brainstem area in which the PPTg is located prove this increasing interest [2,18,24,37-55].
On the basis of the majority of the data present in the literature, the functions in which the PPTg is involved are rather complex, spanning several facets of motor and non-motor functions and pathological states. This is not surprising given the complex pattern of input-output relationships that exist between the PPTg and several brain regions. This pattern of connections is conserved through evolution, but the relative strength of some of these connections is different across mammals. The difference in strength may reflect different mechanisms controlling posture and gait in quadrupeds vs bipeds and may contribute to establish the different architectures of sleep and waking that occur between species. Figure 1 shows a schematic representation of the location of the neuronal populations in the PPTg and their relationships with other brain regions.
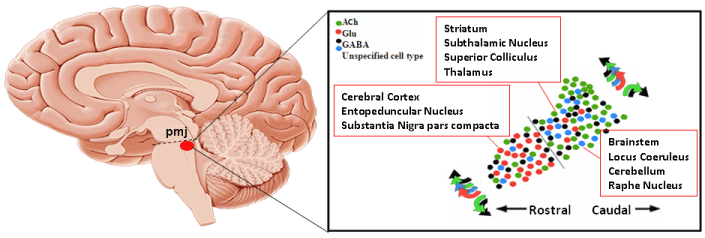
Figure 1. Position of the PPTg in the sagittal aspect of the brain, chemical heterogeneity of its neuronal populations and brain regions reached by their output fibers. The dashed line represents the pontomesencephalic junction (pmj) (Adapted from Pienaar et al. [46]).
In the present article we critically review some recently published data that strongly suggests that specific changes in neuronal activity underlie the participation of PPTg in the wake/sleep cycle and in the processing of behavioral signals. The hypothesis that PPTg neurons may participate in the latter function is relatively novel, but it is particularly intriguing because it may help to understand what the PPTg can do in arousal-demanding states (that is, those states in which behavioral signals must be quickly detected and processed in the brain in order to reach the goal of a learned or conditioned movement). In this regard, it is noteworthy that electrical stimulation of the RAS in correspondence of the PPTg has been reported to induce a variety of complex movements, for example circling, standing, and assuming sleep postures [56-58]. Such complex movements also appear in animals engaged in fight-or-flight responses (that is, in functional states that require a robust and sustained activation of the cerebral cortex).
The PPTg as a part of the reticular activating system (RAS)
The PPTg is one of the most important nuclei in the RAS. It is responsible for the modulation of cortical arousal during the transition from non-rapid eye movement (NREM) sleep to wakefulness or rapid eye movement sleep (REM or paradoxical sleep) [8,59]. This may be achieved through cellular mechanisms that lead to the generation of gamma band oscillation in PPTg neurons (that is, a rhythmic discharge pattern at frequencies higher than 25-30 Hz). Furthermore, PPTg neurons are rhythmically active in relation to locomotor movements [60], and a recent study [61] found that PPTg neurons fired at alpha frequencies during quiet waking or no movement, but the same neurons fired at beta and gamma frequencies when the animal awakened or walked on a treadmill. This means that these neurons are involved in both arousal and movement.
The generation of gamma band activity in the PPTg is linked to different properties of the PPTg neurons that are differently regulated by sodium, calcium, and potassium ion channels. These channels modulate the level of membrane depolarization but gamma band activity is specifically due to high threshold calcium channels [62,63]. The longer that PPTg activation is sustained, the more PPTg targets in the thalamic region and lower brainstem receive a continued gamma band activation. This finding supports the observation that an increased release of acetylcholine (ACh) has been reported to promote fast oscillations (20-60 Hz) in thalamic and cortical neurons during active states in the brain [64]. In such a way, PPTg neurons might reinforce thalamocortical gamma oscillations [65], thus modulating the processing of sensory information. The need for a subcortical impulse flow to sustain cortical gamma band activity is also supported by the lack of gamma band activity in deafferented cortical slabs [66]. Figure 2 shows oscillations of electrical activity in PPTg neurons.
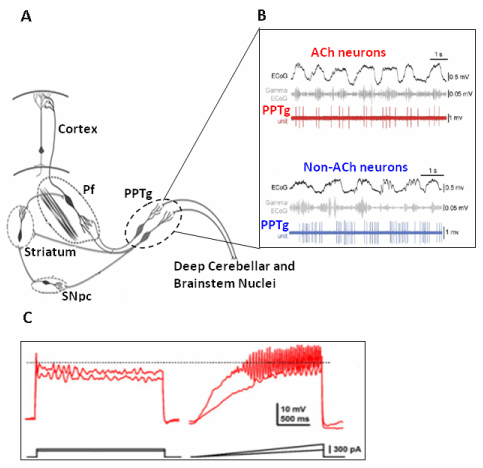
Figure 2. Oscillations in PPTg neurons and gamma band activity. A) Schematic representation of the neuronal circuitry involving the cerebellum, the parafascicular nucleus and the PPTg. (Adapted from Garcia-Rill et al. [81]). B) During cortical slow waves the cholinergic PPTg neurons are active, discharging at gamma frequency, while non cholinergic neurons are also active but not discharging at gamma frequency(Adapted from Mena-Segovia et al. [82]). C) Gamma band oscillations develops in a PPTg neuron when progressively depolarized by current pulses. (Adapted from Kezunovic et al. [63]).
The electrophysiological and neurochemical heterogeneity of neurons that form the PPTg [17,63,67-74] raises the question of whether each neuronal population plays a specific role in REM sleep and NREM sleep.
In general, PPTg neurons fire at alpha frequency when at rest and at beta and gamma frequencies when activated. They increase their firing during waking and REM sleep but not during slow wave sleep (SWS), during which time the rate of firing can even decrease [8,62,75-81]. In contrast, different patterns of discharge characterize the cholinergic, GABAergic, and glutamatergic neurons.
Cholinergic neurons that were maximally active during waking and REM sleep were found to be in positive correlation with fast cortical activity (gamma) [75]. However, in another study, most of the identified cholinergic neurons that increased their discharge pattern during cortical activation in waking brain states were still active during slow cortical oscillation sleep [82]. This latter finding supports the hypothesis that the sustained gamma band activity occurring during spontaneous or drug-induced SWS might serve to maintain a basal level of consciousness. However, others suggest that it is the maintenance of gamma band activity that supports consciousness, such that interrupted gamma activity during SWS does not support consciousness [3].
The discharge profiles of GABAergic and glutamatergic neurons are heterogeneous. Most of these neurons, which have discharge patterns that were not correlated with muscle tone, were found to maximally discharge during waking and REM sleep, while others were minimally active during waking and maximally active during REM sleep. In contrast, the discharge pattern of some glutamatergic neurons that were maximally active during waking and minimally active during REM sleep was found to be positively correlated with muscle tone. Thus, the PPTg, through each of its neuronal populations, has distinct roles in promoting waking and non-REM sleep and in modulating cortical activation and muscle tone in waking and non-REM sleep [75]. The presence of different types of high threshold calcium channels in some PPTg neurons have been proposed to modulate waking (P/Q-type), while the presence of other channels (N-type) are thought to maintain REM sleep [83,84].
Understanding the roles that PPTg may exert in cognition and behavior via thalamic and basal ganglia relays
The thalamocortical route allows the PPTg to participate in a variety of high-order functions. This occurs through the large bulk of cholinergic and non-cholinergic fibers that connect the PPTg to the midline thalamic nuclei, in particular the centromedian-parafascicular complex (CM-Pf) [14,20,85-88].
These nuclei are in receipt of basal ganglia afferents and, in turn, diffusely innervate the cerebral cortex. The PPTg cholinergic neurons give rise to a dual projections to the thalamus and pontine reticular formation [89], which carry streams of information about motor and autonomic functions simultaneously to thalamic nuclei and to lower brainstem nuclei.
The parafascicular (Pf) nucleus is involved in attention and action selection [90-93]. It is reached by a consistent number of PPTg cholinergic fibers and provides a powerful excitatory input to the basal ganglia. In particular, Pf fibers are directed to the striatum, where they synapse onto medium spiny neurons and cholinergic interneurons [94-96], and to the subthalamic nucleus [97-100]. In addition, the CM-Pf complex sends topographically organized fibers to the cerebral cortex [101]. The complexity of these projections to the cerebral cortex increases progressively through evolution, reaching its maximal level in humans [102-106]. Thus, via midline thalamic nuclei, the PPTg gains access to different cortical functions and to several high-order functions of the brain, including learning, memory, spatial perception, control of impulsivity, and decision-making [107].
The PPTg provides fast input to Pf neurons, which is demonstrated by the fact that its microstimulation has been reported to evoke well-defined responses in Pf neurons that consist of a short latency activations or sequences of inhibition and activation (50% and 18% of responsive neurons, respectively) [108]. These responses could be attributed to direct influences of the PPTg neurons on Pf neurons because they appeared a few ms following PPTg stimulation and because they were still present following destruction of the cerebellar nuclei or after interruption of the basal ganglia circuitry (which could be co-activated by PPTg stimulation). In addition, Pf neurons have been found to manifest high threshold calcium channels that mediate gamma band oscillations [109,110]. Figure 3 shows representative examples of Pf neurons whose electrical activity was modified by PPTg microstimulation.
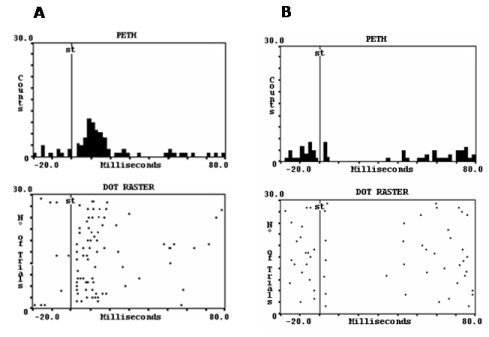
Figure 3. Perievent histograms (PETH) and dot raster displays from 30 consecutive stimuli delivered at 1 Hz, showing the effects of microstimulation of the PPTg in parafascicular neurons. Each dot corresponds to one impulse. The vertical line indicates the stimulus application. A) The most frequent orthodromic response was a short-latency and brief activation. B) Less frequently, neurons were inhibited. The two kind of responses persisted after interruption of polysynaptic pathways that could be co-activated by the stimulation(Adapted from Capozzo et al. [108].
The neuronal circuitry involving the CM-Pf complex and the basal ganglia has a primary role in behavioral flexibility (that is, in switching behavior according to changes in the context in which a given action must be executed). In a study of flexibility during reversal learning, it has been shown that behavioral flexibility requires changes in the efflux of ACh in the dorsomedial striatum via the Pf nucleus [91]. The release of ACh in the striatum may affect the functional properties of striatal neurons, in particular of cholinergic interneurons that are strongly innervated by Pf fibers [95]. These striatal interneurons would correspond to the so-called tonically-active neurons recorded in behaving monkeys. They have a key role in attention and sensory processes that are needed for proper selection of motor responses to stimuli that are of behavioral significance [111-114]. Because PPTg neurons also respond to the presentation of stimuli of behavioral significance [115,116], the striatum may process behavior-related signals carried through the PPTg-Pf pathway. The excitatory neurons that form this pathway would ensure a fast route for the conduction of sensory information to the striatum. Because neurons in the PPTg and thalamic intralaminar nuclei, including the Pf, are lost in PD, the loss of the fast-conducting sensory input to the striatum via the PPTg may decrease sensory responsiveness in PD patients. This loss may also cause difficulty in the selection of suitable motor actions or to respond appropriately to sensory cues that usually trigger or require change in motor plans. The PPTg may also play a role in decision-making. Indeed, PPTg neurons encode short-term information concerning the most recent motor acts executed in response to conditioned stimuli (that is the direction of movement), and the outcome of the conditioned movement (that is rewarded or non-rewarded). This information may be used to select action in immediately successive trials. Further, the influence of recent experience on action selection may be decreased if the PPTg is inactivated [117,118]. How PPTg neurons may participate in decision making is discussed below in the section devoted to reward mechanisms.
Investigating whether the PPTg influences the cerebral cortex via the cerebellum
The traditional view that the cerebellum and basal ganglia are anatomically separate neuronal systems that perform distinct functional operations is challenged by several findings that support the idea that the two systems share common structures. First, there have been many reports that PPTg fibers are directed to deep cerebellar nuclei [119-123]. Second, using transneural transport of the rabies viruses, disynaptic pathways have been demonstrated that link the dentate nucleus to the striatum by way of thalamic nuclei [124], and from the subthalamic nucleus to the cerebellar cortex by way of pontine nuclei [125,126]. Third, according to tractographic studies, a PPTg-cerebellum projection is present in the human brain [12]. Fourth, changes of functional connectivity involving the striatum, the cortex, and the cerebellum have been found in PD patients [127].
Both cerebellar cortex and nuclei are diffusely innervated by beaded choline acetyltransferase-immunoreactive fibers that originate in the PPTg. These fibers establish asymmetric synaptic contacts with small- and medium-sized dendrites of cortical and nuclei neurons. The diffuse distribution of ACh fibers in the cerebellum suggests that these fibers may modulate the state of excitability of cerebellar neurons in relation to arousal and according to rhythmical activity of thalamocortical neurons [128].
Little is known about the electrophysiology of PPTg projections to the cerebellum. Therefore, we recently addressed this issue by investigating the effects of microstimulation of the PPTg on cerebellar nuclei neurons in intact rats and in rats in which a loss of PPTg neurons was induced [129]. In fastigial, interpositus and dentate nuclei, we found that the main response of neurons was a short latency, brief activation. This activation could follow short trains of stimuli delivered up to 200 Hz and was likely the result of the activation of a direct input from the PPTg (Figure 4). In animals in which PPTg neurons had degenerated, the percentage of neurons activated from the PPTg significantly decreased, which supports the idea that the response was mainly due to the activation of PPTg neurons rather to the activation of passing fibers. Given that dentate nucleus neurons were the most responsive among the cerebellar nuclei to PPTg stimulation (76.2% for dentate vs 55.2% and 7.0% for interpositus and fastigial, respectively), we further investigated the properties of the PPTg-evoked response in dentate neurons and concluded that the activation of dentate neurons could be mediated by ACh. Indeed, iontophoretic application of ACh antagonists (atropine or mecamylamine) onto dentate neurons while stimulating the PPTg, led to a reduction, if not complete abolition, of the evoked responses. Figure 4 shows a representative dentate nucleus neuron activated by PPTg microstimulation.
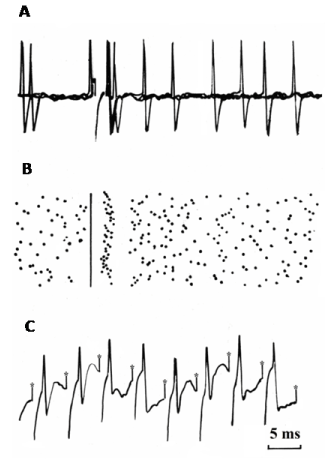
Figure 4. A) A representative example of the short latency and brief orthodromic activation recorded in dentate nucleus neurons following PPTg microstimulation. A) three superimposed sweeps. B) Dot raster display from 30 consecutive stimuli delivered at 1 HZ. C) The orthodromic spike followed a 200 Hz stimulation train(Adapted from Vitale et al. [129].
The above data suggest that on the one hand the PPTg may influence motor mechanisms in the medial cerebellum via the fastigial and interpositus nuclei whereas, on the other side, it may participate in cerebello-cortical mechanisms involved in motor learning via the dentate nucleus. Thus, it is reasonable to hypothesize that the lateral cerebellum may act in synergy with the Pf to ensure the most appropriate level of basal ganglia and cortical activity required for proper motor selection in response to stimuli of behavioral significance. Among these stimuli, we can also include reward and reinforcement learning information that the basal ganglia could provide to the cerebellum. These aspects deserve future investigations given a recent demonstration that the discharge of granule cells in the cerebellum encode reward expectation [130].
The influence of the PPTg on fastigial and interpositus nuclei may also play a role in the origin of gait and postural instability in advanced PD, a point at which patients are refractory to dopaminergic medication but are able to benefit from deep brain stimulation of the PPTg [29,32-35,131] This evidence has led some authors to consider gait and axial disturbances in PD as possible consequences of a disruption of ACh mechanisms in the brainstem rather than of the nigrostriatal dopaminergic system; and the severity of these motor disabilities has been directly related to the degree of ACh neuronal loss in the PPTg [132,133]. However, other neurotransmitters, in particular glutamate are thought to be involved in fibers descending from the PPTg to the spinal cord and to lower brainstem motor structures. Thus, further studies are required to elucidate the nature of PPTg descending fibers that contribute to gait and muscle tone.
The role of the PPTg in predictive reward information and reward-based learning
Specific neuronal circuits acquire reward information from a variety of sensory information that is processed in the brain. By strengthening the association between stimuli and the selection of behavioral responses, sensory information becomes crucial for eliciting and reinforcing approach behavior.
Lesions or chemical inactivation of the PPTg has been found to disrupt reward-related responses, attentional performance, and stimulus-reward associations [134-146]. Consequently, the execution of conditioned reinforcement paradigms was compromised, but movement, per se, was preserved and subtle motor deficits were only evident if tasks were particularly demanding. The extent to which specific subsets of PPTg neurons are responsible for the above deficits is unclear. For instance, rats with an ibotenate-induced loss of PPTg neurons or a selective loss of ACh neurons induced by diphtheria toxin conjugated to urotensin-II showed no deficits in learning or performance during fixed- or variable-ratio paradigms for reinforcement. The rats also had a normal locomotor response to nicotine, and they were not impaired in either cocaine or heroin self-administration or in the development of cocaine or heroin conditioned place preferences [136,147,148]. Yet, neither ibotenate-induced partial nor complete lesions of the PPTg caused locomotor deficits [149], as would be expected given the involvement of the PPTg in the mesencephalic locomotor region.
When investigating reward-related responses, the first neurons to consider are the mesencephalic dopaminergic neurons. It is well established that many of these neurons operate by transforming a reward-related sensory signal into an internal variable that is encoded by changes in the discharge pattern. According to fundamental Wolfram Schultz's studies, these neurons respond with a phasic increase in their discharge pattern in response to reward-related signals without encoding movement parameters [150,151]. Two components may be discriminated in this phasic activation. The first, appearing before behavioral action and having a latency and duration of < 100 ms, is related to the salience of the stimuli (that is, the physical, motivational, and novelty aspects) and may serve to detect stimuli. The second component, appearing at latencies of about 250 ms, encodes information about the real value of the reward, and a prediction error response arises if reward is not given at all or if its value differs from what was expected. Thus, while the fast initial response may quickly detect reward-related stimuli and prepare to move or not to move according to previous experience, the slower second response requires a longer interval due to the complex processing of the reward signal in the brain to gain accurate information on its value.
The PPTg may influence mesencephalic dopaminergic neurons. This idea is supported by the observation that the PPTg provides robust monosynaptic excitatory input to these neurons, and input involves both glutamate and ACh [152-157]. Thus, it is reasonable to hypothesize that fast transmission of information from the PPTg to mesencephalic dopamine neurons occurs in response to behavioral stimuli.
Short-latency responses to behavioral sensory cues have been described in mice, rat, and cat PPTg neurons [115-117,158]. In agreement with the functional link between PPTg and dopaminergic neurons in the substantia nigra, the responses recorded in PPTg neurons at the presentation of behavioral cues were faster than those of the dopaminergic neurons, and inactivation of the PPTg was found to suppress the responsiveness of these neurons to cues [116]
In monkeys performing a rewarded visually guided saccade paradigm different subsets of PPTg neurons were found to be involved in either motor aspects of the task (that is, fixation and saccade execution) or in reward aspects. The reward exerts a critical influence on visual selective attention to maximize both the animal’s interaction with the surrounding environment and positive outcome [159,160]. In the saccade-related paradigm, a monkey had to hold its gaze on a central fixation target; a reward was delivered after the monkey made a saccade at the presentation of a cue that indicated the peripheral saccade target. Different shapes of the central fixation target could also serve as predictors for a large or small upcoming reward, which allowed for an investigation into whether differences in predicted and actual reward magnitude would affect neuronal responses. Tonic or phasic modulation of neuronal activity occurred in different PPTg neurons in correspondence with or during the expectation of the behavioral events of the paradigm. Figure 5 shows reward-related responses that are similar in the PPTg, striatum and substantia nigra pars compacta. (Figure 5).
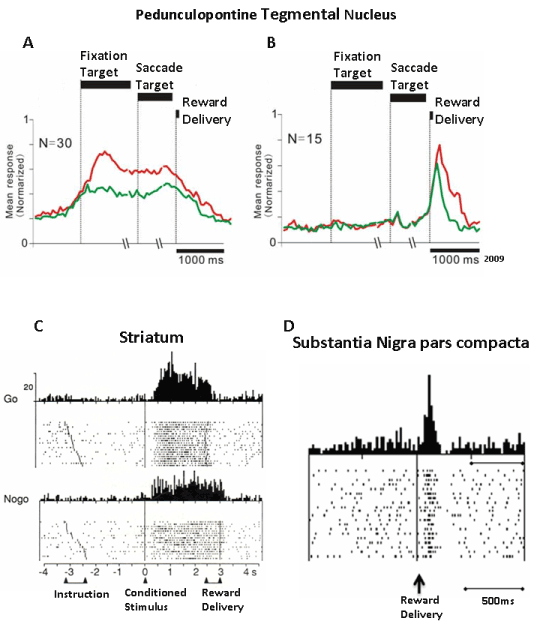
Figure 5. Comparison of reward-related responses of PPTg neurons with responses recorded in the striatum and the substantia nigra pars compacta. A and B are the population responses of neurons increasing tonically their discharge from the beginning of the saccade paradigm until the reward delivery, and the population response of neurons that were phasically activated by the reward delivery, respectively. The red and green traces refer to large and small amount of reward, respectively (Adapted from Okada et al. [165]). C) An example of a neuron recorded in the dorsal striatum increasing its discharge from the appearance of a conditioned stimulus until the reward delivery. The neuron was tested in a GO/NOGO paradigm. Note that the increase of discharge lasting up to the delivery of reward occurred irrespectively of movement(Adapted from Schultz et al. [176]. D) An example of the phasic response of neuron in the pars compacta of the substantia nigra to reward delivery (Adapted from Schultz [194]).
The tonic reward-related responses consisted of either a sustained increase of discharge [161-163], or sustained depression [162]. Most of the sustained increased activity developed from the onset of the initial fixation target until reward delivery, and the magnitude of the response was higher when the shape of the fixation target cued the animal to expect a large reward. Thus, the level of activity reflected the predicted the reward amount. Stronger responses were also observed in successful vs failed trails (that is, when the monkey failed the saccade movement and, consequently, was not rewarded). The sustained activity could also continue if the reward delivery was delayed to sharply decrease once that the reward was gained. These neurons did not give any response to unexpected reward. However, when the target cue was kept on after reward delivery, instead of being turned off, a subpopulation of PPTg neurons showed sustained activity, indicating that the monkey continued fixating on the saccade target even after the reward delivery [163]. This may be indicative of the existence of PPTg neurons that sustain attention toward the actual task event rather than toward reward. Another proof of the existence in the PPTg of tonically active neurons that may sustain attention stems from a variant of the above paradigm in which the animals were cued of the start of a trial by a fixation point that preceded the presentation of the fixation target. In these circumstances, PPTg neurons were found that increased their discharge rate at the presentation of the cue that signaled the start of the trial, and this activity was sustained until the end of the trial.
However, no paradigm has been specifically employed to dissociate the reward component from a possible motor component in the sustained activity. The fact that the magnitude of this tonic activation reflected the predicted reward amount undoubtedly supports the reward nature of this response. However, the monkey always had to move the gaze toward the saccade target, thus it cannot be excluded that the sustained activity could be at least in part due to preparation and execution of the saccade target and/or in sustaining the saccade on the reward target if this was not turned off. This aspect might be definitively elucidated in the future by engaging monkeys in a rewarded GO-NOGO paradigm (that is, enabling the monkey to receive reward fixating the gaze without performing a subsequent reward target movement, in spite of presentation of the reward target). Whatever the contribution of saccade preparation and execution could be in the predictive tonic activation, the reward target was the pivot sensory cue around which the monkey’s attention turned to correctly performing in the behavioral paradigm. If something unusual happened, such as the target signal not being switched off, the monkey kept attention on this cue. A similar situation may occur in our daily living when an unexpected event occurs; for example, when a monitor does not switch on, we continue to fix the LED signaling the proper working of our device. In these circumstances, a state of sustained arousal and attention develops that help us understand what it is happening.
With regard to the “suppressive” neurons, the decrease of their discharge rate was mirrored by the increase of activity showed by the PPTg neurons that were activated during the paradigm. Suppressive neurons decreased their activity even before the appearance of the initial target, and the degree of decrease was correlated to the magnitude of the reward to the point that they keep silent when a large reward could be predicted according to the shape of the fixation target. Thus, bidirectional changes in tonic activity may encode the prediction of rewards by PPTg neurons but without encoding information about the actual reward value. Overall, the above tonic responses may be considered as specifically involved in the prediction of the upcoming reward until its delivery. and should reflect a sustained attentional and motivational state of the monkey in the task.
The neurons that showed a phasic response to reward responded when a reward was given, regardless of whether the reward was given in the paradigm context or unexpectedly, and was higher as the amount of reward increased Thus, these neurons seem to be involved in coding the reward value, at least in terms of amount of reward. In this respect, it should be noted that no other characteristics pertaining to the value of the reward were investigated, such as instructing the animal about the possibility or risk of gaining rewards of different quality or applying test conditions in which the reward was predicted but successively omitted. The phasic response to reward could also start 100 ms before reward delivery, extinguished within 200 ms, and occasionally occurred shortly before the reward. Some PPTg neurons were phasically activated by both predicted and unpredicted rewards, and the latencies of these responses were shorter when the reward was predicted. The phasic response was also observed when reward was freely delivered (that is, delivered outside of any behavioral context). Phasic suppression in reward-related neurons in correspondence with reward delivery or reward omission have not been reported, but were observed in this context in saccade-related neurons [164].
Responses similar to those described above were also reported by Hong and Hikosaka [161] in a task in which monkeys had to perform a saccade toward a rewarded or not rewarded target. In this study different PPTg neurons were found that were phasically excited by reward-related cues, that tonically increased firing from cue to reward release, and that phasically increased firing when the reward was delivered. In a paradigm where a reward was not given, a phasic depression of activity was observed to presentation of a cue that was not linked to a reward. Many of these neurons were electrophysiologically identified as projecting to the substantia nigra pars compacta. A topographic distribution of the responses was also observed, with the phasic reward-responsive neurons localized in the central part of the substantia nigra pars compacta and the tonic reward-responsive neurons localized in the lateral part.
The participation of PPTg neurons in the computation of the reward prediction error is also supported by data from a study of a two-valued reward visually guided task, in which the shape of the fixation target that started each trial informed the animal to expect large or small rewards [165]. In this paradigm reward information was seen to be carried by two distinct groups of neurons whose activity either tonically persisted between the onset of the fixation target and reward delivery, or phasically occurred after reward delivery. In tonically discharging neurons, the level of activity was associated with the magnitude of the expected reward; in phasically discharging neurons the level of activity reflected the actual value of the reward. Thus, it appears that the activity of separate populations of PPTg neurons may encode predicted and actual reward values (that is, it may encode information that can be quickly transmitted to mesencephalic dopamine neurons in order to compute the reward prediction error).
However, major differences arise when comparing PPTg neurons with dopamine neurons in terms of their responsiveness to reward-related stimuli [166,167]. Indeed, contrary to dopamine neurons, separate populations of PPTg neurons encode different reward and event-related properties, and no phasic depression has been reported if reward was omitted.
From these electrophysiological data it is not possible to attribute tonic or phasic responses to any of the different types of cytochemically identified PPTg neurons in tissue preparations, thus any hypothesis formulated is merely speculative.
Of course, for optimal performance in any behavioral paradigm a fundamental prerequisite must be fulfilled: the maintenance of a state of sustained arousal and attention. In the above-discussed paradigms this functional state would be assured throughout the task by the tonic increase of activity that occurs both from the presentation of the reward-related cue up to reward delivery and by the increased activity that persists during the presentation of paradigm events. In this respect, it is noteworthy that neurons that changed their activity during the behavioral paradigm and during intertrial intervals have been found to exhibit a periodic discharge activity of approximately 35 Hz, and they have reached frequencies of up to 100 HZ [168], (in the range of gamma-band activity typical of intense arousal). However, in awake patients affected by PD or progressive supranuclear palsy, the activity of neurons recorded in the PPTg area has been found to be phase locked with alpha oscillations, and they respond to limb movement and imagined gait by dynamically changing their activity and by decreasing alpha phase locking [169].
Local field potential oscillations synchronized at alpha frequency and coupled with similar activity in the cerebral cortex have been reported in PD patients during levodopa therapy and during self-paced joystick movements [170]. Oscillatory rhythms in local field potentials in locomotor-inducing mesencephalic sites, including the PPTg, have been reported to synchronize at theta frequencies (6-12 Hz) in the rat [171]. The theta frequency in the rat corresponds to the human alpha rhythm. However, the findings concerning the appearance of alpha oscillations in neuronal discharge and in field potentials must been cautiously interpreted since a) some studies may be recording conditions at rest when the nucleus fires at low frequencies, and b) PPTg neurons degenerate in neurodegenerative diseases. Alternatively, the oscillations observed in pathological states may be indicative of profound alterations in the functional properties of PPTg neurons, to the point that they do not show sustained gamma-band activity as should occur according to the reported studies that have used monkeys. In spite of these limits, low frequency oscillations might be proper of motor aspects, e.g. at rest, while high frequencies might be required by arousal and motivational aspects that underlie goal-directed movements.
Some responses of PPTg neurons to behavioral signals and rewards are similar to those observed in the striatum in tasks that required either arm or saccadic movements [112,113,172-176]. From this comparison, we argue that the PPTg and the striatum may share common profiles of neuronal responses in relation to the presentation and expectation of predictable behavioral events, including reward (Figure 5).
If we consider the neuronal circuitry in which the PPTg is involved, we can also hypothesize that the PPTg may convey fast information to mesencephalic dopaminergic neurons via the monosynaptic excitatory PPTg substantia nigra pathway, and it may also influence striatal neurons both indirectly via intralaminar thalamic nuclei and directly by its neurons innervating the striatum [177,178].
Interestingly, some of the PPTg neurons in the Hong and Hikosaka’ study [161] could be neurons projecting to thalamic targets because they were not antidromically activated from the substantia nigra.
Conclusion
Taken together, the findings discussed above suggest that the functions of the PPTg go considerably beyond motor functions, encompassing arousal, attention, and motivational aspects that are involved in the formation and selection of appropriate motor responses to behavioral signals.
A major question arising from these data is about how motor and non-motor functions of the PPTg are integrated with each other. In dealing with this issue, first we can consider that when a goal-directed movement must be undertaken, the central nervous system acts to select the most suitable motor plan among the competing possibilities acquired through previous motor learning. This selection should occur according to a motivational state that better helps in selecting the most appropriate strategy that will result in a high-value reward. This process involves sustained attention to internal and external cues that drive behavior. Hence, a high degree of arousal is an essential prerequisite for the successful selection of a motor plan and for its correct execution. According to the data we have discussed, PPTg is involved in the neural circuitry of reward. It may have a key role in reward-related behaviors, likely influencing mesencephalic dopaminergic neurons and striatal neurons.
With regard to the motor nature of the PPTg, this is supported by the PPTg being part of most of the mesencephalic locomotor region, by its connections with subcortical and cortical motor structures, by its fibers innervating lower brainstem and reticulospinal neurons, by its activation in locomotor imagery tasks, by its neurons that discharge in relation to limb movements and, finally, by improvements of motor disabilities induced by its continuous electrical stimulation in PD patients [29,33-35,169,179-184].
The effects of PPTg deep brain stimulation allow us to make some additional conclusions. Improvements of posture and freezing of gait have been consistently reported by different authors [29,34,35] while improvements of gait in term of gait initiation, stride length and cadence have been shown in most of our patients [185]. The inconsistencies pertaining to gait may have originated from different factors, including criteria for patient selection, different degree of degeneration from patient to patient, precise stimulation site and electrical setup of stimulation [32]. As the electrical setup is concerned, the best clinical impact on gait in our patients has been obtained with stimuli delivered at 40 Hz, having voltage of 2-4 V and pulse width of 60 µs [32,185] . A similar setup has been also successful adopted in other studies [36,186]. In animal studies a similar frequency has been proved to be effective in inducing locomotion [181,187]. However, in a recent animal study locomotion was best achieved at 10-20 HZ [171], i.e. at frequencies that were not effective in improving gait in PD patients. However, the lack of localization of the stimulated site in the latter study by means of NADPH-diaphorase staining, which is specific for ACh neurons in the PPTg, does not allow easy comparison of results. It should be also noted that locomotion is quite different between quadrupeds and bipeds, thus the results from roditors or felines cannot be easily transferred to humans. For example, the upper limbs in humans have a quite different function during the swing and stance phases of gait compared to the active support function that the quadrupeds forelimbs exert. These differences may also contribute to determine the optimal stimulation parameters for inducing locomotion across species.
The hypothesis that deep brain stimulation of the PPTg relieves a block to execute a pre-prepared movement [188] fits well with our data because gait may speedily proceed if freezing of gait is removed. Under these circumstances, we hypothesize that if arousal is increased under PPTg stimulation, patients may be more attentive to what they are doing or in what they are about to do, which would result in an improvement in the success of their actions. At the same time, adjustments of postural tone may occur automatically due to the restoration of PPTg influence on descending motor pathways.
All this considered, patients who underwent PPTg deep brain stimulation have been reported to improve daytime sleepiness and REM sleep [189,190], in line with the central role of the PPTg in promoting arousal and REM sleep. In addition, these patients experienced significant amelioration in cognitive functions as shown by a better performance in delayed recall, executive functions and verbal fluency paradigms. These improvements were accompanied by increased glucose utilization in prefrontal and frontal cortical areas bilaterally and in the ventral striatum [191-193].
The experience with PPTg deep brain stimulation compared to subthalamic nucleus deep brain stimulation is still limited, but promising. PPTg may offer a valid alternative for those motor disabilities that are not improved by stimulating the subthalamic nucleus, in particular postural instability and gait impairment. Another advantage that PPTg deep brain stimulation might offer, compared to the subthalamic nucleus, is a slowing of cognitive decline, although very few patients have been investigated with this aim. The data discussed in this review clearly points to a crucial role of the PPTg both in the translation from motivation to action, and in the facilitation of brainstem motor mechanisms that are required for the correct execution of a goal-directed motor act. Whether any of the different neuronal populations in the PPTg is selectively involved in these different functions is yet unknown, although cholinergic neurons are likely to be involved in arousal and REM sleep generation. Therefore, addressing these issues in future studies is of utmost importance in order to better understand and characterize what deep brain stimulation of the PPTg may have to offer in the improvement of PD symptoms.
Acknowledgement
This work has been supported by grants from University of L’Aquila (2017), EU Biomed 2 overheads, and by a Medtronic donation (investigator-initiated trial 2016).
References
- Garcia-Rill E (1991) The pedunculopontine nucleus. Prog Neurobiol 36: 363-389. [Crossref]
- Garcia-Rill E, Hyde J, Kezunovic N, Urbano FJ, Petersen E (2015) The physiology of the pedunculopontine nucleus: implications for deep brain stimulation. J Neural Transm (Vienna) 122: 225-235. [Crossref]
- Garcia-Rill E (2015) Waking and the reticular activating system in health and disease. Elsevier-Academic Press, Amsterdam.
- Moruzzi G, Magoun HW (1949) Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol 1: 455-473. [Crossref]
- Paré D, Smith Y, Parent A, Steriade M (1988) Projections of brainstem core cholinergic and non-cholinergic neurons of cat to intralaminar and reticular thalamic nuclei. Neuroscience 25: 69-86. [Crossref]
- Parent A, Paré D, Smith Y, Steriade M (1988) Basal forebrain cholinergic and noncholinergic projections to the thalamus and brainstem in cats and monkeys. J Comp Neurol 277: 281-301. [Crossref]
- Steriade M, Parent A, Pare D, Smith Y (1987) Cholinergic and non-cholinergic neurons of cat basal forebrain project to reticular and mediodorsal thalamic nuclei. Brain Res 408: 372-376. [Crossref]
- Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC (1990) Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci 10: 2541-2559. [Crossref]
- Zweig RM, Jankel WR, Hedreen JC, Mayeux R, Price DL (1989) The pedunculopontine nucleus in Parkinson's disease. Ann Neurol 26: 41-46. [Crossref]
- Jellinger K (1988) The pedunculopontine nucleus in Parkinson's disease, progressive supranuclear palsy and Alzheimer's disease. J Neurol Neurosurg Psychiatry 51: 540-543. [Crossref]
- Hirsch EC, Graybiel AM, Duyckaerts C, Javoy-Agid F (1987) Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc Natl Acad Sci U S A 84: 5976-5980. [Crossref]
- Aravamuthan BR, Muthusamy KA, Stein JF, Aziz TZ, Johansen-Berg H (2007) Topography of cortical and subcortical connections of the human pedunculopontine and subthalamic nuclei. Neuroimage 37: 694-705. [Crossref]
- Edley SM, Graybiel AM (1983) The afferent and efferent connections of the feline nucleus tegmenti pedunculopontinus, pars compacta. J Comp Neurol 217: 187-215. [Crossref]
- Jackson A, Crossman AR (1983) Nucleus tegmenti pedunculopontinus: efferent connections with special reference to the basal ganglia, studied in the rat by anterograde and retrograde transport of horseradish peroxidase. Neuroscience 10: 725-765. [Crossref]
- Jha A, Litvak V, et al. (2017) Functional Connectivity of the Pedunculopontine Nucleus and Surrounding Region in Parkinson's Disease. Cereb Cortex 27: 54-67. [Crossref]
- Lavoie B, Parent A (1994) Pedunculopontine nucleus in the squirrel monkey: projections to the basal ganglia as revealed by anterograde tract-tracing methods. J Comp Neurol 344: 210-231. [Crossref]
- Martinez-Gonzalez C, Bolam JP, Mena-Segovia J (2011) Topographical organization of the pedunculopontine nucleus. Front Neuroanat 5: 22. [Crossref]
- Mena-Segovia J, Bolam JP (2017) Rethinking the Pedunculopontine Nucleus: From Cellular Organization to Function. Neuron 94: 7-18. [Crossref]
- Muthusamy KA, Aravamuthan BR, Kringelbach ML, Jenkinson N, Voets NL et al. (2007) Connectivity of the human pedunculopontine nucleus region and diffusion tensor imaging in surgical targeting. J Neurosurg 107: 814-820. [Crossref]
- Rye DB, Saper CB, Lee HJ, Wainer BH (1987) Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol 259: 483-528. [Crossref]
- Rye DB, Lee HJ, Saper CB, Wainer BH (1988) Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J Comp Neurol 269: 315-341. [Crossref]
- Grofova I, Keane S (1991) Descending brainstem projections of the pedunculopontine tegmental nucleus in the rat. Anat Embryol (Berl) 184: 275-290. [Crossref]
- Erro E, Giménez-Amaya JM (1999) [Pedunculopontine tegmental nucleus. Anatomy, functional considerations and physiopathological implications]. An Sist Sanit Navar 22: 189-201. [Crossref]
- Alam M, Schwabe K, Krauss JK (2011) The pedunculopontine nucleus area: critical evaluation of interspecies differences relevant for its use as a target for deep brain stimulation. Brain 134: 11-23. [Crossref]
- Kang Y, Kitai ST (1990) Electrophysiological properties of pedunculopontine neurons and their postsynaptic responses following stimulation of substantia nigra reticulata. Brain Res 535: 79-95. [Crossref]
- Scarnati E, Proia A, Di LS, Pacitti C (1987) The reciprocal electrophysiological influence between the nucleus tegmenti pedunculopontinus and the substantia nigra in normal and decorticated rats. Brain Res 423: 116-124. [Crossref]
- German DC, Manaye KF, Wu D, Hersh LB, Zweig RM (1999) Mesopontine cholinergic and non-cholinergic neurons in schizophrenia. Neuroscience 94: 33-38. [Crossref]
- Manaye KF, Zweig R, Wu D, Hersh LB, De LS, et al. (1999) Quantification of cholinergic and select non-cholinergic mesopontine neuronal populations in the human brain. Neuroscience 89: 759-770. [Crossref]
- Ferraye MU, Debû B, Fraix V, Goetz L, Ardouin C, et al. (2010) Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson's disease. Brain 133: 205-214. [Crossref]
- Hamani C, Lozano AM, Mazzone PA, Moro E, Hutchison W, et al. (2016) Pedunculopontine Nucleus Region Deep Brain Stimulation in Parkinson Disease: Surgical Techniques, Side Effects, and Postoperative Imaging. Stereotact Funct Neurosurg 94: 307-319. [Crossref]
- Hamani C, Aziz T, Bloem BR, Brown P, Chabardes S, et al. (2016) Pedunculopontine Nucleus Region Deep Brain Stimulation in Parkinson Disease: Surgical Anatomy and Terminology. Stereotact Funct Neurosurg 94: 298-306. [Crossref]
- Mazzone P, Sposato S, Insola A, Scarnati E (2013) The clinical effects of deep brain stimulation of the pedunculopontine tegmental nucleus in movement disorders may not be related to the anatomical target, leads location, and setup of electrical stimulation. Neurosurgery 73: 894-906. [Crossref]
- Mazzone P, Vilela FO, Viselli F, Insola A, Sposato S et al. (2016) Our first decade of experience in deep brain stimulation of the brainstem: elucidating the mechanism of action of stimulation of the ventrolateral pontine tegmentum. J Neural Transm (Vienna) 123: 751-767. [Crossref]
- Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, et al. (2010) Unilateral pedunculopontine stimulation improves falls in Parkinson's disease. Brain 133: 215-224. [Crossref]
- Thevathasan W, Coyne TJ, Hyam JA, Kerr G, Jenkinson N et al. (2011) Pedunculopontine nucleus stimulation improves gait freezing in Parkinson disease. Neurosurgery 69: 1248-1253. [Crossref]
- Wilcox RA, Cole MH, Wong D, Coyne T, Silburn P, et al. (2011) Pedunculopontine nucleus deep brain stimulation produces sustained improvement in primary progressive freezing of gait. J Neurol Neurosurg Psychiatry 82: 1256-1259. [Crossref]
- Benarroch EE (2013) Pedunculopontine nucleus: functional organization and clinical implications. Neurology 80: 1148-1155. [Crossref]
- Blanco-Lezcano L, Pavon-Fuentes N, Serrano-Sanchez T, Blanco-Lezcano V, Coro-Grave de PY, et al. (2003) The pedunculopontine nucleus. A structure involved in motor and emotional processing. Rev Neurol 36: 1181-1185.
- Hamani C, Moro E, Lozano AM (2011) The pedunculopontine nucleus as a target for deep brain stimulation. J Neural Transm (Vienna) 118: 1461-1468. [Crossref]
- Inglis WL, Winn P (1995) The pedunculopontine tegmental nucleus: where the striatum meets the reticular formation. Prog Neurobiol 47: 1-29. [Crossref]
- Lee MS, Rinne JO, Marsden CD (2000) The pedunculopontine nucleus: its role in the genesis of movement disorders. Yonsei Med J 41: 167-184. [Crossref]
- Mazzone P, Garcia-Rill E, Scarnati E (2016) Progress in deep brain stimulation of the pedunculopontine nucleus and other structures: implications for motor and non-motor disorders. J Neural Transm (Vienna) 123: 653-654. [Crossref]
- Mena-Segovia J, Bolam JP, Magill PJ (2004) Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci 27: 585-588. [Crossref]
- Mori F, Okada KI, Nomura T, Kobayashi Y (2016) The Pedunculopontine Tegmental Nucleus as a Motor and Cognitive Interface between the Cerebellum and Basal Ganglia. Front Neuroanat 10: 109. [Crossref]
- Pahapill PA, Lozano AM (2000) The pedunculopontine nucleus and Parkinson's disease. Brain 123: 1767-1783. [Crossref]
- Pienaar IS, Vernon A, Winn P (2016) The Cellular Diversity of the Pedunculopontine Nucleus: Relevance to Behavior in Health and Aspects of Parkinson's Disease. Neuroscientist. [Crossref]
- Reese NB, Garcia-Rill E, Skinner RD (1995) The pedunculopontine nucleus--auditory input, arousal and pathophysiology. Prog Neurobiol 47: 105-133. [Crossref]
- Rostron CL, Farquhar MJ, Latimer MP, Winn P (2008) The pedunculopontine tegmental nucleus and the nucleus basalis magnocellularis: do both have a role in sustained attention? BMC Neurosci 9: 16. [Crossref]
- Scarnati E, Florio T (1997) The pedunculopontine nucleus and related structures. Functional organization. Adv Neurol 74: 97-110. [Crossref]
- Winn P, Brown VJ, Inglis WL (1997) On the relationships between the striatum and the pedunculopontine tegmental nucleus. Crit Rev Neurobiol 11: 241-261. [Crossref]
- Winn P (2006) How best to consider the structure and function of the pedunculopontine tegmental nucleus: evidence from animal studies. J Neurol Sci 248: 234-250. [Crossref]
- Garcia-Rill E, Simon C, Smith K, Kezunovic N, Hyde J (2011) The pedunculopontine tegmental nucleus: from basic neuroscience to neurosurgical applications: arousal from slices to humans: implications for DBS. J Neural Transm (Vienna) 118: 1397-1407. [Crossref]
- Pereira EA, Nandi D, Jenkinson N, Stein JF, Green AL, et al. (2011) Pedunculopontine stimulation from primate to patient. J Neural Transm (Vienna) 118: 1453-1460. [Crossref]
- Jenkinson N, Nandi D, Muthusamy K, Ray NJ, Gregory R, et al. (2009) Anatomy, physiology, and pathophysiology of the pedunculopontine nucleus. Mov Disord 24: 319-328. [Crossref]
- Gut NK, Winn P (2016) The pedunculopontine tegmental nucleus-A functional hypothesis from the comparative literature. Mov Disord 31: 615-624. [Crossref]
- Shik ML, Severin FV, OrlovskiÄ GN (1966) [Control of walking and running by means of electric stimulation of the midbrain]. Biofizika 11: 659-666. [Crossref]
- Sprague JM, Chambers WW (1953) Regulation of posture in intact and decerebrate cat. I. Cerebellum, reticular formation, vestibular nuclei. J Neurophysiol 16: 451-463. [Crossref]
- Sprague JM, Chambers WW (1954) Control of posture by reticular formation and cerebellum in the intract, anesthetized and unanesthetized and in the decerebrated cat. Am J Physiol 176: 52-64. [Crossref]
- Hobson JA, Pace-Schott EF (2002) The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci 3: 679-693. [Crossref]
- Garcia-Rill E, Skinner RD, Fitzgerald JA (1983) Activity in the mesencephalic locomotor region during locomotion. Exp Neurol 82: 609-622. [Crossref]
- Goetz L, Piallat B, Bhattacharjee M, Mathieu H, David O et al. (2016) The primate pedunculopontine nucleus region: towards a dual role in locomotion and waking state. J Neural Transm (Vienna) 123: 667-678. [Crossref]
- Garcia-Rill E, Kezunovic N, D'Onofrio S, Luster B, Hyde J, et al. (2014) Gamma band activity in the RAS-intracellular mechanisms. Exp Brain Res 232: 1509-1522. [Crossref]
- Kezunovic N, Urbano FJ, Simon C, Hyde J, Smith K, et al. (2011) Mechanism behind gamma band activity in the pedunculopontine nucleus. Eur J Neurosci 34: 404-415. [Crossref]
- Steriade M (2004) Acetylcholine systems and rhythmic activities during the waking--sleep cycle. Prog Brain Res 145: 179-196. [Crossref]
- Dringenberg HC, Olmstead MC (2003) Integrated contributions of basal forebrain and thalamus to neocortical activation elicited by pedunculopontine tegmental stimulation in urethane-anesthetized rats. Neuroscience 119: 839-853. [Crossref]
- Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M (2000) Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex 10: 1185-1199. [Crossref]
- Florio T, Scarnati E, Confalone G, Minchella D, Galati S, et al. (2007) High-frequency stimulation of the subthalamic nucleus modulates the activity of pedunculopontine neurons through direct activation of excitatory fibres as well as through indirect activation of inhibitory pallidal fibres in the rat. Eur J Neurosci 25: 1174-1186. [Crossref]
- Lavoie B, Parent A (1994) Pedunculopontine nucleus in the squirrel monkey: cholinergic and glutamatergic projections to the substantia nigra. J Comp Neurol 344: 232-241. [Crossref]
- Lavoie B, Parent A (1994) Pedunculopontine nucleus in the squirrel monkey: distribution of cholinergic and monoaminergic neurons in the mesopontine tegmentum with evidence for the presence of glutamate in cholinergic neurons. J Comp Neurol 344: 190-209. [Crossref]
- Martinez-Gonzalez C, Wang HL, Micklem BR, Bolam JP, Mena-Segovia J (2012) Subpopulations of cholinergic, GABAergic and glutamatergic neurons in the pedunculopontine nucleus contain calcium-binding proteins and are heterogeneously distributed. Eur J Neurosci 35: 723-734. [Crossref]
- Rohrbacher J, Ichin2021 Copyright OAT. All rights reservogical characteristics of substantia nigra neurons in organotypic cultures: spontaneous and evoked activities. Neuroscience 97: 703-714. [Crossref]
- Spann BM, Grofova I (1992) Cholinergic and non-cholinergic neurons in the rat pedunculopontine tegmental nucleus. Anat Embryol (Berl) 186: 215-227. [Crossref]
- Takakusaki K, Kitai ST (1997) Ionic mechanisms involved in the spontaneous firing of tegmental pedunculopontine nucleus neurons of the rat. Neuroscience 78: 771-794. [Crossref]
- Wang HL, Morales M (2009) Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci 29: 340-358. [Crossref]
- Boucetta S, Cisse Y, Mainville L, Morales M, Jones BE (2014) Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J Neurosci 34: 4708-4727. [Crossref]
- Datta S, Siwek DF (2002) Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res 70: 611-621. [Crossref]
- Datta S (2009) Regulation of neuronal activities within REM sleep-sign generators. Sleep 32: 1113-1114. [Crossref]
- Kayama Y, Ohta M, Jodo E (1992) Firing of 'possibly' cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res 569: 210-220. [Crossref]
- Sakai K, el MM, Jouvet M (1990) Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res 527: 213-223. [Crossref]
- Garcia-Rill E, Luster B, D'Onofrio S, Mahaffey S, Bisagno V, et al. (2016) Implications of gamma band activity in the pedunculopontine nucleus. J Neural Transm (Vienna) 123: 655-665. [Crossref]
- Garcia-Rill E, D'Onofrio S, Mahaffey S (2016) Bottom-up Gamma: the Pedunculopontine Nucleus and Reticular Activating System. Transl Brain Rhythm 1: 49-53. [Crossref]
- Mena-Segovia J, Sims HM, Magill PJ, Bolam JP (2008) Cholinergic brainstem neurons modulate cortical gamma activity during slow oscillations. J Physiol 586: 2947-2960. [Crossref]
- Luster B, D'Onofrio S, Urbano F, Garcia-Rill E (2015) High-threshold Ca2+ channels behind gamma band activity in the pedunculopontine nucleus (PPN). Physiol Rep 3. [Crossref]
- Luster BR, Urbano FJ, Garcia-Rill E (2016) Intracellular mechanisms modulating gamma band activity in the pedunculopontine nucleus (PPN). Physiol Rep 4. [Crossref]
- Erro E, Lanciego JL, Gimenez-Amaya JM (1999) Relationships between thalamostriatal neurons and pedunculopontine projections to the thalamus: a neuroanatomical tract-tracing study in the rat. Exp Brain Res 127: 162-170. [Crossref]
- Saper CB, Loewy AD (1982) Projections of the pedunculopontine tegmental nucleus in the rat: evidence for additional extrapyramidal circuitry. Brain Res 252: 367-372. [Crossref]
- Sugimoto T, Hattori T (1984) Organization and efferent projections of nucleus tegmenti pedunculopontinus pars compacta with special reference to its cholinergic aspects. Neuroscience 11: 931-946. [Crossref]
- Kobayashi S, Nakamura Y (2003) Synaptic organization of the rat parafascicular nucleus, with special reference to its afferents from the superior colliculus and the pedunculopontine tegmental nucleus. Brain Res 980: 80-91. [Crossref]
- Semba K, Reiner PB, Fibiger HC (1990) Single cholinergic mesopontine tegmental neurons project to both the pontine reticular formation and the thalamus in the rat. Neuroscience 38: 643-654. [Crossref]
- Bailey KR, Mair RG (2005) Lesions of specific and nonspecific thalamic nuclei affect prefrontal cortex-dependent aspects of spatial working memory. Behav Neurosci 119: 410-419. [Crossref]
- Brown HD, Baker PM, Ragozzino ME (2010) The parafascicular thalamic nucleus concomitantly influences behavioral flexibility and dorsomedial striatal acetylcholine output in rats. J Neurosci 30: 14390-14398. [Crossref]
- Burk JA, Mair RG (1998) Thalamic amnesia reconsidered: excitotoxic lesions of the intralaminar nuclei, but not the mediodorsal nucleus, disrupt place delayed matching- to-sample performance in rats (Rattus norvegicus). Behav Neurosci 112: 54-67. [Crossref]
- Burk JA, Mair RG (2001) Effects of intralaminar thalamic lesions on sensory attention and motor intention in the rat: a comparison with lesions involving frontal cortex and hippocampus. Behav Brain Res 123: 49-63. [Crossref]
- Dubé L, Smith AD, Bolam JP (1988) Identification of synaptic terminals of thalamic or cortical origin in contact with distinct medium-size spiny neurons in the rat neostriatum. J Comp Neurol 267: 455-471. [Crossref]
- Lapper SR, Bolam JP (1992) Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience 51: 533-545. [Crossref]
- Wilson CJ, Chang HT, Kitai ST (1983) Origins of post synaptic potentials evoked in spiny neostriatal projection neurons by thalamic stimulation in the rat. Exp Brain Res 51: 217-226. [Crossref]
- Féger J, Mouroux M (1991) Demonstration of the excitatory effect of the thalamo-subthalamic efferent from the parafascicular nucleus. C R Acad Sci III 313: 447-452. [Crossref]
- Mouroux M, Féger J (1993) Evidence that the parafascicular projection to the subthalamic nucleus is glutamatergic. Neuroreport 4: 613-615. [Crossref]
- Mouroux M, Hassani OK, Féger J (1995) Electrophysiological study of the excitatory parafascicular projection to the subthalamic nucleus and evidence for ipsi- and contralateral controls. Neuroscience 67: 399-407. [Crossref]
- Mouroux M, Hassani OK, Féger J (1997) Electrophysiological and Fos immunohistochemical evidence for the excitatory nature of the parafascicular projection to the globus pallidus. Neuroscience 81: 387-397. [Crossref]
- Royce GJ, Mourey RJ (1985) Efferent connections of the centromedian and parafascicular thalamic nuclei: an autoradiographic investigation in the cat. J Comp Neurol 235: 277-300. [Crossref]
- Jones EG, Leavitt RY (1974) Retrograde axonal transport and the demonstration of non-specific projections to the cerebral cortex and striatum from thalamic intralaminar nuclei in the rat, cat and monkey. J Comp Neurol 154: 349-377. [Crossref]
- Kievit J, Kuypers HG (1975) Subcortical afferents to the frontal lobe in the rhesus monkey studied by means of retrograde horseradish peroxidase transport. Brain Res 85: 261-266. [Crossref]
- Kievit J, Kuypers HG (1975) Basal forebrain and hypothalamic connection to frontal and parietal cortex in the Rhesus monkey. Science 187: 660-662. [Crossref]
- Mesulam MM, Van Hoesen GW, Pandya DN, Geschwind N (1977) Limbic and sensory connections of the inferior parietal lobule (area PG) in the rhesus monkey: a study with a new method for horseradish peroxidase histochemistry. Brain Res 136: 393-414. [Crossref]
- Morán A, Avendaño C, Reinoso-Suárez F (1982) Thalamic afferents to the motor cortex in the cat. A horseradish peroxidase study. Neurosci Lett 33: 229-233. [Crossref]
- Mitchell AS, Sherman SM, Sommer MA, Mair RG, Vertes RP, et al. (2014) Advances in understanding mechanisms of thalamic relays in cognition and behavior. J Neurosci 34: 15340-15346. [Crossref]
- Capozzo A, Florio T, Cellini R, Moriconi U, Scarnati E (2003) The pedunculopontine nucleus projection to the parafascicular nucleus of the thalamus: an electrophysiological investigation in the rat. J Neural Transm 110: 733-747 [Crossref]
- Kezunovic N, Hyde J, Simon C, Urbano FJ, Williams DK, et al. (2012) Gamma band activity in the developing parafascicular nucleus. J Neurophysiol 107: 772-784. [Crossref]
- Hyde J, Kezunovic N, Urbano FJ, Garcia-Rill E (2013) Visualization of fast calcium oscillations in the parafascicular nucleus. Pflugers Arch 465: 1327-1340. [Crossref]
- Apicella P, Scarnati E, Schultz W (1991) Tonically discharging neurons of monkey striatum respond to preparatory and rewarding stimuli. Exp Brain Res 84: 672-675. [Crossref]
- Apicella P (2002) Tonically active neurons in the primate striatum and their role in the processing of information about motivationally relevant events. Eur J Neurosci 16: 2017-2026. [Crossref]
- Apicella P (2007) Leading tonically active neurons of the striatum from reward detection to context recognition. Trends Neurosci 30: 299-306. [Crossref]
- Doig NM, Magill PJ, Apicella P, Bolam JP, Sharott A (2014) Cortical and thalamic excitation mediate the multiphasic responses of striatal cholinergic interneurons to motivationally salient stimuli. J Neurosci 34: 3101-3117. [Crossref]
- Dormont JF, Conde H, Farin D (1998) The role of the pedunculopontine tegmental nucleus in relation to conditioned motor performance in the cat. I. Context-dependent and reinforcement-related single unit activity. Exp Brain Res 121: 401-410. [Crossref]
- Pan WX, Hyland BI (2005) Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci 25: 4725-4732. [Crossref]
- Thompson JA, Felsen G (2013) Activity in mouse pedunculopontine tegmental nucleus reflects action and outcome in a decision-making task. J Neurophysiol 110: 2817-2829. [Crossref]
- Thompson JA, Costabile JD, Felsen G (2016) Mesencephalic representations of recent experience influence decision making. Elife 5. [Crossref]
- Hazrati LN, Parent A (1992) Projection from the deep cerebellar nuclei to the pedunculopontine nucleus in the squirrel monkey. Brain Res 585: 267-271. [Crossref]
- Ruggiero DA, Anwar M, Golanov EV, Reis DJ (1997) The pedunculopontine tegmental nucleus issues collaterals to the fastigial nucleus and rostral ventrolateral reticular nucleus in the rat. Brain Res 760: 272-276. [Crossref]
- Woolf NJ, Butcher LL (1989) Cholinergic systems in the rat brain: IV. Descending projections of the pontomesencephalic tegmentum. Brain Res Bull 23: 519-540. [Crossref]
- Newman DB, Ginsberg CY (1992) Brainstem reticular nuclei that project to the cerebellum in rats: a retrograde tracer study. Brain Behav Evol 39: 24-68. [Crossref]
- Jaarsma D, Ruigrok TJ, Caffé R, Cozzari C, Levey AI, et al. (1997) Cholinergic innervation and receptors in the cerebellum. Prog Brain Res 114: 67-96. [Crossref]
- Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL (2005) The cerebellum communicates with the basal ganglia. Nat Neurosci 8: 1491-1493. [Crossref]
- Bostan AC, Dum RP, Strick PL (2010) The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A 107: 8452-8456. [Crossref]
- Bostan AC, Dum RP, Strick PL (2013) Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci 17: 241-254. [Crossref]
- Wu T, Wang L, Hallett M, Chen Y, Li K, et al. (2011) Effective connectivity of brain networks during self-initiated movement in Parkinson's disease. Neuroimage 55: 204-215. [Crossref]
- McCormick DA (1989) Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci 12: 215-221. [Crossref]
- Vitale F, Mattei C, Capozzo A, Pietrantoni I, Mazzone P, et al. (2016) Cholinergic excitation from the pedunculopontine tegmental nucleus to the dentate nucleus in the rat. Neuroscience 317: 12-22. [Crossref]
- Wagner MJ, Kim TH, Savall J, Schnitzer MJ, et al. (2017) Cerebellar granule cells encode the expectation of reward. Nature 544: 96-100. [Crossref]
- Ostrem JL, Christine CW, Glass GA, Schrock LE, Starr PA (2010) Pedunculopontine nucleus deep brain stimulation in a patient with primary progressive freezing gait disorder. Stereotact Funct Neurosurg 88: 51-55. [Crossref]
- Bohnen NI, Frey KA, Studenski S, Kotagal V, Koeppe RA, et al. (2013) Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology 81: 1611-1616. [Crossref]
- Karachi C, Grabli D, Bernard FA, Tande D, Wattiez N, et al. (2010) Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest 120: 2745-2754. [Crossref]
- Florio T, Capozzo A, Puglielli E, Pupillo R, Pizzuti G, et al. (1999) The function of the pedunculopontine nucleus in the preparation and execution of an externally-cued bar pressing task in the rat. Behav Brain Res 104: 95-104. [Crossref]
- Inglis WL, Dunbar JS, Winn P (1994) Outflow from the nucleus accumbens to the pedunculopontine tegmental nucleus: a dissociation between locomotor activity and the acquisition of responding for conditioned reinforcement stimulated by d-amphetamine. Neuroscience 62: 51-64. [Crossref]
- Inglis WL, Olmstead MC, Robbins TW (2000) Pedunculopontine tegmental nucleus lesions impair stimulus--reward learning in autoshaping and conditioned reinforcement paradigms. Behav Neurosci 114: 285-294. [Crossref]
- Inglis WL, Olmstead MC, Robbins TW (2001) Selective deficits in attentional performance on the 5-choice serial reaction time task following pedunculopontine tegmental nucleus lesions. Behav Brain Res 123: 117-131. [Crossref]
- Lepore M, Franklin KB (1996) N-methyl-D-aspartate lesions of the pedunculopontine nucleus block acquisition and impair maintenance of responding reinforced with brain stimulation. Neuroscience 71: 147-155. [Crossref]
- Bechara A, van der Kooy D (1989) The tegmental pedunculopontine nucleus: a brain-stem output of the limbic system critical for the conditioned place preferences produced by morphine and amphetamine. J Neurosci 9: 3400-3409. [Crossref]
- Bechara A, van der Kooy D (1992) Lesions of the tegmental pedunculopontine nucleus: effects on the locomotor activity induced by morphine and amphetamine. Pharmacol Biochem Behav 42: 9-18. [Crossref]
- Dellu F, Mayo W, Cherkaoui J, Le MM, Simon H (1991) Learning disturbances following excitotoxic lesion of cholinergic pedunculo-pontine nucleus in the rat. Brain Res 544: 126-132. [Crossref]
- Yeomans JS, Mathur A, Tampakeras M (1993) Rewarding brain stimulation: role of tegmental cholinergic neurons that activate dopamine neurons. Behav Neurosci 107: 1077-1087. [Crossref]
- Syed A, Baker PM, Ragozzino ME (2016) Pedunculopontine tegmental nucleus lesions impair probabilistic reversal learning by reducing sensitivity to positive reward feedback. Neurobiol Learn Mem 131: 1-8. [Crossref]
- Cyr M, Parent MJ, Mechawar N, Rosa-Neto P, Soucy JP, et al. (2015) Deficit in sustained attention following selective cholinergic lesion of the pedunculopontine tegmental nucleus in rat, as measured with both post-mortem immunocytochemistry and in vivo PET imaging with [(1)(8)F]fluoroethoxybenzovesamicol. Behav Brain Res 278: 107-114. [Crossref]
- Olmstead MC, Franklin KB (1994) Lesions of the pedunculopontine tegmental nucleus block drug-induced reinforcement but not amphetamine-induced locomotion. Brain Res 638: 29-35. [Crossref]
- Wilson DI, MacLaren DA, Winn P (2009) Bar pressing for food: differential consequences of lesions to the anterior versus posterior pedunculopontine. Eur J Neurosci 30: 504-513. [Crossref]
- MacLaren DA, Wilson DI, Winn P (2016) Selective lesions of the cholinergic neurons within the posterior pedunculopontine do not alter operant learning or nicotine sensitization. Brain Struct Funct 221: 1481-1497. [Crossref]
- Steidl S, Wang H, Wise RA (2014) Lesions of cholinergic pedunculopontine tegmental nucleus neurons fail to affect cocaine or heroin self-administration or conditioned place preference in rats. PLoS One 9: e84412. [Crossref]
- Gut NK, Winn P (2015) Deep brain stimulation of different pedunculopontine targets in a novel rodent model of parkinsonism. J Neurosci 35: 4792-4803. [Crossref]
- Ljungberg T, Apicella P, Schultz W (1992) Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol 67: 145-163. [Crossref]
- Romo R, Schultz W (1990) Dopamine neurons of the monkey midbrain: contingencies of responses to active touch during self-initiated arm movements. J Neurophysiol 63: 592-606. [Crossref]
- Di LS, Florio T, Scarnati E (1992) Evidence that non-NMDA receptors are involved in the excitatory pathway from the pedunculopontine region to nigrostriatal dopaminergic neurons. Exp Brain Res 89: 79-86. [Crossref]
- Futami T, Takakusaki K, Kitai ST (1995) Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neurosci Res 21: 331-342. [Crossref]
- Kitai ST, Shepard PD, Callaway JC, Scroggs R (1999) Afferent modulation of dopamine neuron firing patterns. Curr Opin Neurobiol 9: 690-697. [Crossref]
- Lee HJ, Rye DB, Hallanger AE, Levey AI, Wainer BH (1988) Cholinergic vs. noncholinergic efferents from the mesopontine tegmentum to the extrapyramidal motor system nuclei. J Comp Neurol 275: 469-492. [Crossref]
- Parent A, Parent M, Charara A (1999) Glutamatergic inputs to midbrain dopaminergic neurons in primates. Parkinsonism Relat Disord 5: 193-201. [Crossref]
- Scarnati E, Hajdu F, Pacitti C, Tombol T (1988) An EM and Golgi study on the connection between the nucleus tegmenti pedunculopontinus and the pars compacta of the substantia nigra in the rat. J Hirnforsch 29: 95-105.
- Norton AB, Jo YS, Clark EW, Taylor CA, Mizumori SJ (2011) Independent neural coding of reward and movement by pedunculopontine tegmental nucleus neurons in freely navigating rats. Eur J Neurosci 33: 1885-1896. [Crossref]
- Bourgeois A, Chelazzi L, Vuilleumier P (2016) How motivation and reward learning modulate selective attention. Prog Brain Res 229: 325-342. [Crossref]
- Chelazzi L, Perlato A, Santandrea E, Della Libera C (2013) Rewards teach visual selective attention. Vision Res 85: 58-72. [Crossref]
- Hong S, Hikosaka O (2014) Pedunculopontine tegmental nucleus neurons provide reward, sensorimotor, and alerting signals to midbrain dopamine neurons. Neuroscience 282: 139-155. [Crossref]
- Okada K, Kobayashi Y (2013) Reward prediction-related increases and decreases in tonic neuronal activity of the pedunculopontine tegmental nucleus. Front Integr Neurosci 7: 36. [Crossref]
- Okada KI, Kobayashi Y (2016) Reward and Behavioral Factors Contributing to the Tonic Activity of Monkey Pedunculopontine Tegmental Nucleus Neurons during Saccade Tasks. Front Syst Neurosci 10: 94. [Crossref]
- Kobayashi Y, Inoue Y, Yamamoto M, Isa T, Aizawa H (2002) Contribution of pedunculopontine tegmental nucleus neurons to performance of visually guided saccade tasks in monkeys. J Neurophysiol 88: 715-731. [Crossref]
- Okada K, Toyama K, Inoue Y, Isa T, Kobayashi Y (2009) Different pedunculopontine tegmental neurons signal predicted and actual task rewards. J Neurosci 29: 4858-4870. [Crossref]
- Schultz W (1997) Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol 7: 191-197. [Crossref]
- Schultz W (1998) Predictive reward signal of dopamine neurons. J Neurophysiol 80: 1-27. [Crossref]
- Okada K, Kobayashi Y (2015) Rhythmic Firing of Pedunculopontine Tegmental Nucleus Neurons in Monkeys during Eye Movement Task. PLoS One 10: e0128147. [Crossref]
- Tattersall TL, Stratton PG, Coyne TJ, Cook R, Silberstein P, et al. (2014) Imagined gait modulates neuronal network dynamics in the human pedunculopontine nucleus. Nat Neurosci 17: 449-454.
- Androulidakis AG, Mazzone P, Litvak V, Penny W, Dileone M, et al. (2008) Oscillatory activity in the pedunculopontine area of patients with Parkinson's disease. Exp Neurol 211: 59-66. [Crossref]
- Noga BR, Sanchez FJ, Villamil LM, O'Toole C, Kasicki S, et al. (2017) LFP Oscillations in the Mesencephalic Locomotor Region during Voluntary Locomotion. Front Neural Circuits 11: 34. [Crossref]
- Apicella P, Ljungberg T, Scarnati E, Schultz W (1991) Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res 85: 491-500. [Crossref]
- Apicella P, Scarnati E, Ljungberg T, Schultz W (1992) Neuronal activity in monkey striatum related to the expectation of predictable environmental events. J Neurophysiol 68: 945-960. [Crossref]
- Hikosaka O, Sakamoto M, Usui S (1989) Functional properties of monkey caudate neurons. III. Activities related to expectation of target and reward. J Neurophysiol 61: 814-832. [Crossref]
- Schultz W, Apicella P, Scarnati E, Ljungberg T (1992) Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci 12: 4595-4610. [Crossref]
- Schultz W, Apicella P, Ljungberg T, Romo R, Scarnati E (1993) Reward-related activity in the monkey striatum and substantia nigra. Prog Brain Res 99: 227-235. [Crossref]
- Dautan D, Huerta-Ocampo I, Witten IB, Deisseroth K, Bolam JP, et al. (2014) A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J Neurosci 34: 4509-4518. [Crossref]
- Dautan D, Hacioglu BH, Bolam JP, Gerdjikov TV, Mena-Segovia J (2016) Extrinsic Sources of Cholinergic Innervation of the Striatal Complex: A Whole-Brain Mapping Analysis. Front Neuroanat 10: 1. [Crossref]
- Lau B, Welter ML, Belaid H, Fernandez Vidal S, Bardinet E, et al. (2015) The integrative role of the pedunculopontine nucleus in human gait. Brain 138: 1284-1296. [Crossref]
- Sébille SB, Belaid H, Philippe AC, André A, Lau B, et al. (2017) Anatomical evidence for functional diversity in the mesencephalic locomotor region of primates. Neuroimage 147: 66-78. [Crossref]
- Skinner RD, Garcia-Rill E (1984) The mesencephalic locomotor region (MLR) in the rat. Brain Res 323: 385-389. [Crossref]
- Skinner RD, Kinjo N, Ishikawa Y, Biedermann JA, Garcia-Rill E (1990) Locomotor projections from the pedunculopontine nucleus to the medioventral medulla. Neuroreport 1: 207-210. [Crossref]
- Skinner RD, Kinjo N, Henderson V, Garcia-Rill E (1990) Locomotor projections from the pedunculopontine nucleus to the spinal cord. Neuroreport 1: 183-186. [Crossref]
- Takakusaki K, Habaguchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T (2003) Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience 119: 293-308. [Crossref]
- Mazzone P, Paoloni M, Mangone M, Santilli V, Insola A, et al. (2014) Unilateral deep brain stimulation of the pedunculopontine tegmental nucleus in idiopathic Parkinson's disease: effects on gait initiation and performance. Gait Posture 40: 357-362. [Crossref]
- Thevathasan W, Cole MH, Graepel CL, Hyam JA, Jenkinson N, et al. (2012) A spatiotemporal analysis of gait freezing and the impact of pedunculopontine nucleus stimulation. Brain 135: 1446-1454. [Crossref]
- OrlovskiÄ GN, Severin FV, Shik ML (1966) [Locomotion induced by stimulation of the mesencephalon]. Dokl Akad Nauk SSSR 169: 1223-1226. [Crossref]
- Thevathasan W, Pogosyan A, Hyam JA, Jenkinson N, Bogdanovic M, et al. (2011) A block to pre-prepared movement in gait freezing, relieved by pedunculopontine nucleus stimulation. Brain 134: 2085-2095. [Crossref]
- Lim AS, Moro E, Lozano AM, Hamani C, Dostrovsky JO, et al. (2009) Selective enhancement of rapid eye movement sleep by deep brain stimulation of the human pons. Ann Neurol 66: 110-114. [Crossref]
- Peppe A, Pierantozzi M, Baiamonte V, Moschella V, Caltagirone C, et al. (2012) Deep brain stimulation of pedunculopontine tegmental nucleus: role in sleep modulation in advanced Parkinson disease patients: one-year follow-up. Sleep 35: 1637-1642. [Crossref]
- Costa A, Carlesimo GA, Caltagirone C, Mazzone P, Pierantozzi M, et al. (2010) Effects of deep brain stimulation of the peduncolopontine area on working memory tasks in patients with Parkinson's disease. Parkinsonism Relat Disord 16: 64-67. [Crossref]
- Zanini S, Moschella V, Stefani A, Peppe A, Pierantozzi M, et al. (2009) Grammar improvement following deep brain stimulation of the subthalamic and the pedunculopontine nuclei in advanced Parkinson's disease: a pilot study. Parkinsonism Relat Disord 15: 606-609. [Crossref]
- Stefani A, Pierantozzi M, Ceravolo R, Brusa L, Galati S, et al. (2010) Deep brain stimulation of pedunculopontine tegmental nucleus (PPTg) promotes cognitive and metabolic changes: a target-specific effect or response to a low-frequency pattern of stimulation? Clin EEG Neurosci 41: 82-86. [Crossref]
- Schultz W (2010) Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct 6: 24. [Crossref]





