P2Y12 platelet adenosine diphosphate receptor inhibitors in combination with aspirin have significantly reduced the mortality and morbidity of patients after acute coronary syndrome. The different licensed drugs are being studied at different regimens and in different combinations, sometimes with inconsistent results. While promising new substances are emerging, new therapeutic treatment combinations which minimize the risks over the benefits are needed to further optimize patient outcomes. Among those, a shift from a dual antiplatelet therapy to a P2Y12 monotherapy strategy looks promising and an intense area of research. We provide an up-to-date review of the value of P2Y12 inhibitors by integrating the results of most recent trials and putting their main findings into clinical perspectives.
P2Y12 inhibitors, dual antiplatelet therapy, acute coronary syndrome, prasugrel, ticagrelor
Clopidogrel, prasugrel and ticagrelor are the three approved P2Y12 platelet adenosine diphosphate receptor inhibitors for the treatment of patients with acute coronary syndrome (ACS) [1,2]. With the stakes being high in terms of the number of patients treated each year, there are many studies in the literature investigating these three drugs as well as their treatment duration (Figure 1). Prasugrel and ticagrelor have already shown their superiority over clopidogrel for patients with ACS through randomized trials and recently a single open label investigator-initiated study powered for showing the superiority of ticagrelor over prasugrel, showed superiority of the latter over the former agent.
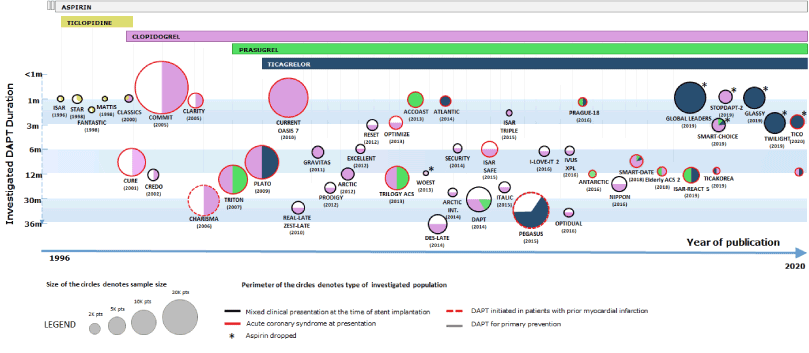
Figure 1: History of dual antiplatelet therapy in patients with coronary artery disease.48 The colours of perimeters identify the type of included patient populations within each study. The colours within each circle identify the antiplatelet agent(s) investigated. Head-to-head studies comparing similar durations of two different antiplatelet strategies are shown with a vertical line, whereas those investigating different treatment durations are shown with a horizontal line. Studies investigating different treatment strategies or regimens and not treatment durations or type (e.g. pre-treatment in ACCOAST, tailored therapy in GRAVITAS, double dose of clopidogrel in CURRENT OASIS 7, etc.) are represented with a single colour indicating the P2Y12 inhibitor, which was tested on top of aspirin. The asterix indicates the studies that investigated an aspirin-free regimen. pts = patients
In 2007, Wiviott, et al. showed in the TRITON-TIMI-38-Study, that prasugrel was superior to clopidogrel in reducing the composite cardiovascular end point (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) after ACS (Figure 2A). The superiority of prasugrel over clopidogrel was largely due to a reduction of myocardial infarction (MI). The frequency of deaths or strokes remained unchanged [3].
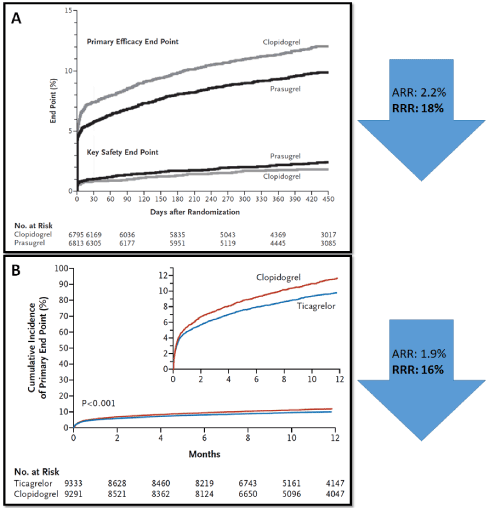
Figure 2 Primary end point of the TRITON-TIMI 38 and the PLATO trials
A: Primary end point (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) (top) and the key safety end point (Thrombolysis in Myocardial Infarction [TIMI] major bleeding not related to coronary-artery bypass grafting) (bottom) of the TRITON-TIMI 38 trial3
B: Primary end point (death from vascular causes, myocardial infarction, or stroke) of the PLATO trial4
In 2009, the PLATO-Study showed that ticagrelor was superior to clopidogrel in reducing the incidence of the composite cardiovascular outcome (death from vascular causes, MI, or stroke) after an ACS (Figure 2B) [4]. Cardiovascular and overall mortality and MI were both significantly lower with ticagrelor.
In 2018, the PRAGUE-18 trial was the first randomized, multicenter clinical trial, which compared ticagrelor and prasugrel head-to-head among patients with MI undergoing primary percutaneous coronary intervention (PCI). The study, which was prematurely stopped and included 1230 patients, showed no difference between these two treatments [5]. The 1-year follow-up of the PRAGUE-18 study showed that the percentage of patients who switched to clopidogrel for economic reason was 34.1% for prasugrel and 44.4% for ticagrelor (drug costs were not covered by health insurance after discharge from hospital in the Czech Republic) [6].
Finally, in 2019 the long-awaited results of The Rapid Early Action for Coronary Treatment (ISAR-REACT) 5 study became available [7]. In this randomized, controlled trial involving 4018 patients with ACS (41.1% of whom had ST-segment elevation myocardial infarction [STEMI]) for whom an invasive evaluation was planned), a prasugrel-based treatment strategy proved superior to a ticagrelor-based treatment strategy in reducing the incidence of death, MI, or stroke at 1 year (6.9% vs. 9.3%, P = 0.006). This result was driven by a significant 1.8 percentage point reduction of the incidence of ischemic recurrences (spontaneous and peri-procedural events) and this benefit did not come at expenses of an increased bleeding risk. Although this study is currently the largest head-to-head comparison between prasugrel and ticagrelor, it has some limitations and inconsistencies with larger randomized studies that limit its implications for practice. In addition to being an open-label study, investigators did not provide study drugs to recruited patients but rather used commercially available medications and did not ascertain drug adherence. Interestingly, the rate of study drug discontinuation was higher in the ticagrelor group. It remains also difficult to reconcile the remarkable treatment effect observed with the results of prior landmark investigations. The study reported a relative risk reduction of 36% (corresponding to a 2.3% absolute risk difference) with ticagrelor compared to prasugrel while prasugrel compared to clopidogrel in a study involving 13’608 patients showed a relative reduction of 19% (2.2% absolute risk difference) [3], while ticagrelor compared to clopidogrel in a study with 18’624 patients showed a 16% relative risk reduction (1.9% absolute risk difference) [4]. The absolute risk reduction between prasugrel and ticagrelor was in ISAR-REACT 5 almost twice greater than that observed between clopidogrel and a placebo in the CURE trial involving 12’562 patients [8].
A recent network meta-analysis and direct pairwise comparison analysis from twelve randomized controlled trials, including a total of 52,816 patients with ACS (Table 1), confirmed the superiority of both prasugrel and ticagrelor over clopidogrel in reducing adverse cardiovascular events, yet with notable treatment effects between these two more potent and consistent P2Y12 inhibitors [9]. Prasugrel was associated with a significant reduction of overall MI rates compared to clopidogrel, while ticagrelor was not. At further sensitivity analysis, ticagrelor reduced the risk of spontaneous MI but not peri-procedural ischemic events compared to clopidogrel. Conversely, ticagrelor was associated with a significant reduction of mortality rates compared to clopidogrel, while prasugrel was not.
Table 1. Characteristics of the studies included in the Meta-Analysis of Navarese, et al. [9]
Study |
Follow-up |
Recruitment period |
Arm (maintenance dose) |
No of patients (ITT) |
Study setting summary |
The Elderly ACS II trial [40] |
12 months |
November 15, 2012 – January 25, 2017 |
prasugrel 5mg od |
713 |
elderly ACS undergoing PCI |
clopidogrel 75mg od |
730 |
ISAR-REACT 5 [7] |
12 months |
September 2013 – February 2018 |
ticagrelor 90mg bid |
2012 |
ACS with planned invasive evaluation |
prasugrel 5/10mg od |
2006 |
PHILO [41] |
12 months |
NA |
ticagrelor 90mg bid |
401 |
Japanese, Korean and Taiwanese ACS patients with planned PCI |
clopidogrel 75mg od |
400 |
PLATO [4] |
12 months |
October 2006 – July 2008 |
ticagrelor 90mg bid |
9333 |
ACS with or without STEMI |
clopidogrel 75mg od |
9291 |
POPular AGE trial [42] |
12 months |
June 2013 – October 2018 |
clopidogrel 75mg od |
501 |
70 years or older with NSTEMI ACS |
ticagrelor 90mg bid or prasugrel 5/10mg od* |
502 |
PRAGUE-18 [5] |
12 months |
completed May 2016 |
prasugrel 10mg od |
634 |
STEMI treated with primary PCI |
ticagrelor 90mg bid |
596 |
The PRASFIT-ACS [43] |
11 months |
December 2010 – June 2012 |
prasugrel 3.75mg od |
685 |
Japanese ACS patients undergoing PCI |
clopidogrel 75mg od |
678 |
TICAKOREA [44] |
12 months |
July 5, 2014 – June 30, 2017 |
ticagrelor 90mg bid |
400 |
Korean ACS patients with or without STEMI |
clopidogrel 75mg od |
400 |
TRILOGY ACS [45] |
17 months |
June 27, 2008 – September 12, 2011 |
prasugrel 10mg od |
4663 |
ACS without revascularization |
clopidogrel 75mg od |
4663 |
TRITON-TIMI 38 [3] |
15 months |
November 2004 – January 2007 |
prasugrel 10mg od |
6813 |
ACS with scheduled PCI |
clopidogrel 75mg od |
6795 |
Tang et al. 2016 [46] |
6 months |
January 1, 2013 – April 30, 2015 |
ticagrelor 90mg bid |
200 |
Chinese STEMI patients undergoing primary PCI |
clopidogrel 75mg od |
200 |
Wang et al. 2016 [47] |
12 months |
August 2013 – November 2014 |
ticagrelor 90mg bid |
100 |
elderly Chinese patients with ACS |
clopidogrel 75mg od |
100 |
*2% of patients received prasugrel |
|
|
|
|
ISAR-REACT 5 – Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes, PHILO – Phase the International Study of Ticagrelor and Clinical Outcomes in Asian ACS Patients, PLATO – PLATelet inhibition and patient Outcomes, PRASFIT-ACS – PRASugrel compared with clopidogrel For Japanese patIenTs with ACS undergoing PCI, TICAKOREA – Ticagrelor Versus Clopidogrel in Asian/Korean Patients with ACS Intended for Invasive Management, TRILOGY ACS – The Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes, TRITON–TIMI 38 – Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38. ACS – acute coronary syndrome, bid – bis in die (twice a day), ITT – intention to treat, JACC – Journal of the American College of Cardiology, NA – not available, NEJM – The New England Journal of Medicine, NSTEMI – non-ST-elevation myocardial infarction, od – omni die (once daily), PCI – percutaneous coronary intervention, STEMI – ST-elevation myocardial infarction, TCRM – Therapeutics and Clinical Risk Management, UA – unstable angina
By exclusion of the studies with open-label design (including ISAR REACT 5), ticagrelor was associated with persistent significant risk reduction of cardiovascular and all-cause mortality compared with clopidogrel and significant risk reduction in all-cause mortality compared with prasugrel [9].
Pleiotropic effects of ticagrelor
Ticagrelor is a nonthienopyridine direct and reversible P2Y12 platelet receptor antagonist (Figure 3) and inhibits (unlike prasugrel und clopidogrel) the sodium-independent equilibrative nucleoside transporter 1 (ENT1) [10] which might lead to an increase of adenosine plasma level [11] and may explain some ticagrelor-specific side effects, such as dyspnea [12]. It is indeed estimated that about one in twenty patients discontinue their initiated treatment because of dyspnea [13]. A putative hypothesis put forward to explain the dyspnea caused by ticagrelor is ticagrelor-associated high adenosine plasma levels [14]. This aspect was thoroughly investigated in multiple studies, including the HI-TECH study, which showed that ticagrelor did not improve endothelial function, nor increased systemic adenosine plasma levels [15]. The second hypothesis raised to explain ticagrelor-associated dyspnea is a direct effect of the drug on brain receptors (i.e. central dyspnea). This assumption is supported by the fact that the plasma adenosine did not differ in patients with or without ticagrelor-related dyspnea while ticagrelor plasma level were 2- to 3-fold higher in patients suffering from dyspnea in the HI-TECH study [16]. Interestingly, patients suffering from ticagrelor-induced dyspnea showed almost complete inhibition of the P2Y12 platelet receptor pathway. Therefore, reduction of the maintenance regimen could limit this side effect without compromising platelet inhibition [17] as shown in the PEGASUS-TIMI 54 trial, in which lower ticagrelor maintenance regimen (i.e. 60 mg b.i.d. versus 90 mg b.i.d.) resulted in lower dyspnea rates [13].
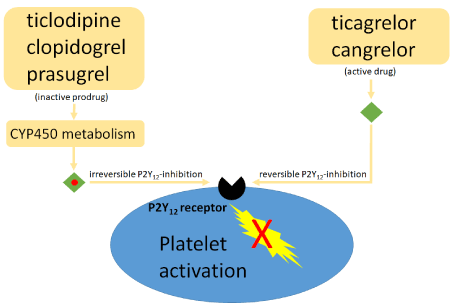
Figure 3: Pharmacokinetics of the approved P2Y12 antagonists
CYP450 = Cytochrome P450
Several clinical studies have examined the effect of ticagrelor on endothelial function, with conflicting results. Due to its intrinsic properties, it is known that adenosine acts via certain receptors on coronary vasodilation [18] and that it modulates the inflammatory responses [17]. Armstrong, et al. demonstrated that at concentrations observed in patients treated with ticagrelor, the activity of the additional adenosine released is negligible [10]. These observations are consistent with the HI-TECH study that showed that ticagrelor did not act on a measurable scale on endothelial function [15]. For now, no clear correlation has therefore been established between ticagrelor and higher plasma adenosine concentrations.
Drug absorption
One major concern with oral anti-platelet agents is the delayed gastric absorption due to co-administration of morphine [19]. The European Society of Cardiology has also highlighted these concerns in the 2017 ESC Guidelines for the management of acute MI in patients presenting with ST-segment elevation [2]. These consistent findings on morphine-to-oral P2Y12 platelet receptor antagonist interaction call for the use of a parenteral P2Y12 receptor antagonist, such as cangrelor, which however, has never been investigated in comparison with prasugrel or ticagrelor. A parenterally administered P2Y12 receptor antagonist has however some limitations. It requires intravenous access and maintenance infusion after an initial bolus, which reduces its manageability, especially in emergency cases with failure of the circulatory system [20].
After the acute phase, adherence to drug treatment after ACS is crucial. Bonaca, et al. did a secondary analysis of the PEGASUS-TIMI 54 Trial by reporting the details of the reasons and timing of discontinuation of treatment. About one third of the patients discontinued the treatment with ticagrelor and 21% discontinued the treatment with placebo. Bleeding and dyspnea were each approximately 5 times more frequent to warrant discontinuation of ticagrelor than placebo. These results are consistent with the findings of the TRANSLATE-ACS study, which shows that 31% of MI patients are no longer persistent with their prescribed medications by 6 months [21]. The impact of once-daily versus twice-daily dosing frequency on adherence to chronic medication was tested in 2013 among patients with venous thromboembolism. It demonstrated that patients treated with chronic medications on once-daily dosing regimens were associated with a 39-61% higher likelihood of adherence compared with subjects on twice-daily dosing regimens. Although these data are not completely extrapolated to a population on P2Y12 inhibitors, it is reasonable to assume that long-term adherence to prasugrel is better than that of ticagrelor, despite evidence remains inconclusive. In clinical practice, DAPT is also more prematurely discontinued among elderly than younger patients despite their ischaemic risk is higher [22,23].
Aspirin-free regimens
Although antiplatelet therapy will continue to evolve over the next few years with the advent of new substances and regimens, the biggest paradigm shift is expected to come from the oldest of the drugs used after ACS, namely aspirin. It is known that a daily aspirin intake is not beneficial among healthy elderly patients. It does not improve disability-free survival and does not reduce major adverse cardiovascular events but it is associated with a significant increase in major bleeding and should therefore be used with caution if there are no clinical indications [24]. The rationale for dual antiplatelet therapy in ACS comes from trials conducted nearly 20 years ago that showed that DAPT is superior to aspirin alone [8]. Yet, no single study has up to now showed a significant increase of ischemic events when aspirin is discontinued and the patient left with a P2Y12 inhibitor regimen, whereas bleeding risk has been consistently reduced. The WOEST-trial in 2013 investigated the safety and efficacy of clopidogrel alone compared with clopidogrel plus aspirin in patients taking oral anticoagulation. The aspirin-free regimen was associated with a significant reduction of bleeding complications and no increase in the rate of thrombotic events [25]. In 2019, GLASSY provides evidence that stopping aspirin after 30 days while continuing a monotherapy with ticagrelor does not result in a higher ischemic risk compared to a standard DAPT [26]. Few other trials explored a P2Y12 monotherapy after a short-term DAPT post PCI. The STOPDAPT-2 trial enrolled 3045 patients in Japan, which were randomized either to 1 month of DAPT followed by clopidogrel monotherapy or to 12 months of DAPT with aspirin and clopidogrel. The 1-year cumulative incidence of a composite end point of cardiovascular and bleeding events was significantly lower in the clopidogrel group [27]. The second trial is the SMART-CHOICE which was presented at the same time. Almost 3000 patients receiving PCI were randomized in Korea to keep or drop aspirin after 3 months of DAPT. At one year, clinical outcome were similar between the two groups with the exception of greater degree of BARC 2-5 bleeding in the DAPT group [28]. Recently, the TWILIGHT trial explored the safety and efficacy of a 3-month DAPT followed by ticagrelor monotherapy with a 12-month DAPT in patients at high risk of ischemia or bleeding (STEMI excluded). The short duration of DAPT results in less bleeding and ischemic rates met criteria for non-inferiority [29]. Two recents subanalysis from the TWILIGHT trial were recently published. Similar results as in the main trial were observed regarding patients after complex PCI, [30] who are known to be at high risk of ischemic events [31] and regarding patients with diabetes mellitus [32], who are associated with an increased risk for both ischemic and bleeding complications post PCI [33]. The next piece of evidence comes from the TICO trial [34]. It was designed similar to TWILIGHT with a comparison between a ticagrelor monotherapy after 3 months of DAPT versus long-term DAPT. This study, which unlike TWILIGHT, was open label showed that ticagrelor monotherapy after 3 months of DAPT was superior to standard therapy of DAPT for 12 months for the composite of net adverse clinical events. Interestingly, none of these trials examined whether aspirin monotherapy, instead of ticagrelor monotherapy in the 3- to 12-month period, would be equally effective. The key questions remaining are which initial duration of DAPT should be preferred and which P2Y12 inhibitor should be chosen for subsequent monotherapy.
Parenteral anti-platelet agents
Selatogrel is a potent, fast-acting, reversible, and highly-selective P2Y12 receptor antagonist. In preclinical Phase I studies, no satisfactory reduction in platelet activity could be achieved with oral application of the drug [35]. A subcutaneously (SC) administered preparation of selatogrel was tested and showed a dose-dependent potent effect on platelet reactivity with rapid time to peak effect and offset [36]. A phase 2 study to assess inhibition of platelet aggregation (IPA) after SC single-dose administration in patients with acute MI has been recently published [37] and showed a rapid induction of IPA especially with the 16 mg regimen. The drug was well tolerated and no major bleeding events occurred. The phase 3 trial is planned among patients at high risk for recurrent MI.
Substances besides the P2Y12 are currently under development and are following a similar development pathway in order to add to the armamentarium of the anti-platelet drugs. RUC-4 is a small molecule, which is a αIIbβ3 antagonist. It works by inhibiting human ADP-induced platelet aggregation. This substance shows favorable biochemical, pharmacokinetic, pharmacodynamic, antithrombotic, and solubility properties and its thought to be a prehospital therapy of MI [38]. His properties allow it to be delivered in an out-of-hospital setting by auto-injection, which should lead to a quick inhibition of ADP-induced platelet aggregation. Preclinical studies in non-human primates and with human platelet rich plasma were published in June 2019 with encouraging results [39]. The first use in patients with stable coronary artery disease was presentend in September 2019. Kereiakes, et al. demonstrated that RUC-4 provides rapid (<15 minutes), intense and short-term inhibition of platelet aggregation after subcutaneous treatment. The possibility of increased bleeding at therapeutic doses as well as its use in case of ACS remains to be assessed.
P2Y12 platelet adenosine diphosphate receptor inhibitors have improved the management of patients with ACS and have become throughout years a cornerstone treatment in modern cardiology. Due to their different mode of action and their own pharmacological properties, each P2Y12 inhibitor has advantages and limitations that set them apart from the other substances in different settings. While prasugrel is currently licensed only for ACS patients undergoing percutaneous coronary revascularization, both clopidogrel and ticagrelor have proved to act as secondary prevention medications limiting the risk of ischemic recurrences from both stented and non-previously stented coronary segments. While the development of new substances continues, the latest paradigm shift in antiplatelet therapy after ACS focuses on a P2Y12 inhibitor monotherapy treatment strategy, with clopidogrel and ticagrelor being the most frequently investigated agents across trials.
- Roffi M, Patrono C, Collet JP (2015) 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 37: 267-315. [Crossref]
- Ibanez B, James S, Agewall S, et al. (2018) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39: 119-77. [Crossref]
- Wiviott SD, Braunwald E, McCabe CH (2007) Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 357: 2001-2015.
- Wallentin L, Becker RC, Budaj A (2009) Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 361: 1045-1057. [Crossref]
- Motovska Z, Hlinomaz O, Miklik R (2016) Prasugrel Versus Ticagrelor in Patients With Acute Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention: Multicenter Randomized PRAGUE-18 Study. Circulation 134: 1603-1612. [Crossref]
- Motovska Z, Hlinomaz O, Kala P (2018) 1-Year Outcomes of Patients Undergoing Primary Angioplasty for Myocardial Infarction Treated With Prasugrel Versus Ticagrelor. J Am Coll Cardiol 71: 371-81. [Crossref]
- Schupke S, Neumann FJ, Menichelli M (2019) Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N Engl J Med 381: 1524-1534. [Crossref]
- Yusuf S, Zhao F, Mehta SR (2001) Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 345: 494-502. [Crossref]
- Navarese EP, Khan SU, Kolodziejczak M (2020) Comparative Efficacy and Safety of Oral P2Y12 Inhibitors in Acute Coronary Syndrome: Network Meta-Analysis of 52,816 Patients from 12 Randomized Trials. Circulation 142: 150-160. [Crossref]
- Armstrong D, Summers C, Ewart L, Nylander S, Sidaway JE, et al. (2014) Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J Cardiovasc Pharmacol Ther 19: 209-219. [Crossref]
- Bonello L, Laine M, Kipson N (2014) Ticagrelor increases adenosine plasma concentration in patients with an acute coronary syndrome. J Am Coll Cardiol 63: 872-877. [Crossref]
- 12. Cattaneo M, Schulz R, Nylander S (2014) Adenosine-mediated effects of ticagrelor: evidence and potential clinical relevance. J Am Coll Cardiol 63: 2503-2509. [Crossref]
- Bonaca MP, Bhatt DL, Cohen M (2015) Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 372: 1791-800. [Crossref]
- Nylander S, Femia EA, Scavone M, et al. Ticagrelor inhibits human platelet aggregation via adenosine in addition to P2Y12 antagonism. J Thromb Haemost 11: 1867-1876. [Crossref]
- Ariotti S, Ortega-Paz L, van Leeuwen M (2018) Effects of Ticagrelor, Prasugrel, or Clopidogrel on Endothelial Function and Other Vascular Biomarkers: A Randomized Crossover Study. JACC Cardiovascular interventions 11: 1576-1586.
- Ortega-Paz L, Brugaletta S, Ariotti S (2018) Adenosine and Ticagrelor Plasma Levels in Patients With and Without Ticagrelor-Related Dyspnea. Circulation 138: 646-648. [Crossref]
- Barletta KE, Ley K, Mehrad B. Regulation of neutrophil function by adenosine. Arterioscler Thromb Vasc Biol 32: 856-864. [Crossref]
- Mustafa SJ, Morrison RR, Teng B, Pelleg A (2009) Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol 2009: 161-188. [Crossref]
- Parodi G, Bellandi B, Xanthopoulou I (2014) Morphine is associated with a delayed activity of oral antiplatelet agents in patients with ST-elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Circ Cardiovasc Interv 8: e001593. [Crossref]
- Parker WA, Bhatt DL, Prats J (2017) Characteristics of dyspnoea and associated clinical outcomes in the CHAMPION PHOENIX study. Thrombosis and haemostasis 117: 1093-1100.
- Bonaca MP, Bhatt DL, Oude Ophuis T (2016) Long-term Tolerability of Ticagrelor for the Secondary Prevention of Major Adverse Cardiovascular Events: A Secondary Analysis of the PEGASUS-TIMI 54 Trial. JAMA cardiology 1: 425-432. [Crossref]
- Biscaglia S, Campo G, Pavasini R, Tebaldi M, Tumscitz C, et al. (2016) Occurrence, causes, and outcome after switching from ticagrelor to clopidogrel in a real-life scenario: data from a prospective registry. Platelets 27: 484-487. [Crossref]
- Zeymer U, Cully M, Hochadel M (2018) Adherence to dual antiplatelet therapy with ticagrelor in patients with acute coronary syndromes treated with percutaneous coronary intervention in real life. Results of the REAL-TICA registry. European heart journal Cardiovascular pharmacotherapy 4: 205-210.
- McNeil JJ, Nelson MR, Woods RL (2018) Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. N Engl J Med 379: 1519-1528. [Crossref]
- Dewilde WJ, Oirbans T, Verheugt FW (2013) Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 381: 1107-1115. [Crossref]
- Franzone A, McFadden E, Leonardi S (2019) Ticagrelor Alone Versus Dual Antiplatelet Therapy From 1 Month After Drug-Eluting Coronary Stenting. J Am Coll Cardiol 74: 2223-2234. [Crossref]
- Watanabe H, Domei T, Morimoto T (2019) Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI: The STOPDAPT-2 Randomized Clinical Trial. JAMA 321: 2414-2427. [Crossref]
- Hahn JY, Song YB, Oh JH (2019) Effect of P2Y12 Inhibitor Monotherapy vs Dual Antiplatelet Therapy on Cardiovascular Events in Patients Undergoing Percutaneous Coronary Intervention: The SMART-CHOICE Randomized Clinical Trial. JAMA 321: 2428-2437. [Crossref]
- Mehran R, Baber U, Sharma SK (2019) Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N Engl J Med 381: 2032-2042. [Crossref]
- Dangas G, Baber U, Sharma S (2020) Ticagrelor With Aspirin or Alone After Complex PCI. J Am Coll Cardiol 75: 2414-2424. [Crossref]
- Giustino G, Chieffo A, Palmerini T (2016) Efficacy and Safety of Dual Antiplatelet Therapy After Complex PCI. J Am Coll Cardiol 68: 1851-1864. [Crossref]
- Angiolillo DJ, Baber U, Sartori S (2020) Ticagrelor with or without Aspirin in High-Risk Patients with Diabetes Mellitus undergoing Percutaneous Coronary Intervention. J Am Coll Cardiol 75: 2403-2413 [Crossref]
- Ferreiro JL, Angiolillo DJ (2011) Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation 123: 798-813. [Crossref]
- Kim BK, Hong SJ, Cho YH (2020) Effect of Ticagrelor Monotherapy vs Ticagrelor With Aspirin on Major Bleeding and Cardiovascular Events in Patients With Acute Coronary Syndrome: The TICO Randomized Clinical Trial. JAMA 323: 2407-2416. [Crossref]
- Baldoni D, Bruderer S, Krause A (2014) A new reversible and potent P2Y12 receptor antagonist (ACT-246475): tolerability, pharmacokinetics, and pharmacodynamics in a first-in-man trial. Clin Drug Investig 34: 807-818. [Crossref]
- Ufer M, Huynh C, van Lier JJ, Caroff E, Fischer H, et al. (2020) Absorption, distribution, metabolism and excretion of the P2Y12 receptor antagonist selatogrel after subcutaneous administration in healthy subjects. Xenobiotica 50: 427-434. [Crossref]
- Sinnaeve P, Fahrni G, Schelfaut D (2020) Subcutaneous Selatogrel Inhibits Platelet Aggregation in Patients With Acute Myocardial Infarction. Journal of the American College of Cardiology 75: 2588-2597.
- Li J, Vootukuri S, Shang Y (2014) RUC-4: a novel alphaIIbbeta3 antagonist for prehospital therapy of myocardial infarction. Arterioscler Thromb Vasc Biol 34: 2321-2329. [Crossref]
- Vootukuri S, Li J, Nedelman M, Craig Thomas, Jiang-Kang Jiang, et al. (2019) Preclinical Studies of RUC-4, a Novel Platelet alphaIIbbeta3 Antagonist, in Non-Human Primates and With Human Platelets. J Clin Transl Sci 3: 65-74. [Crossref]
- Savonitto S, Ferri LA, Piatti L (2018) Comparison of Reduced-Dose Prasugrel and Standard-Dose Clopidogrel in Elderly Patients With Acute Coronary Syndromes Undergoing Early Percutaneous Revascularization. Circulation 137: 2435-2445. [Crossref]
- Goto S, Huang CH, Park SJ, Emanuelsson H, Kimura T (2015) Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome -- randomized, double-blind, phase III PHILO study. Circ J 79: 2452-2460. [Crossref]
- Gimbel M, Qaderdan K, Willemsen L (2020) Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet 395: 1374-1981. [Crossref]
- Saito S, Isshiki T, Kimura T, et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: the PRASFIT-ACS study. Circ J 78: 1684-1692. [Crossref]
- Park DW, Kwon O, Jang JS (2019) Clinically Significant Bleeding With Ticagrelor Versus Clopidogrel in Korean Patients With Acute Coronary Syndromes Intended for Invasive Management: A Randomized Clinical Trial. Circulation 140: 1865-1877. [Crossref]
- Roe MT, Armstrong PW, Fox KA (2012) Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med 367: 1297-1309. [Crossref]
- Tang X, Li R, Jing Q (2016) Assessment of Ticagrelor Versus Clopidogrel Treatment in Patients With ST-elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. J Cardiovasc Pharmacol 68: 115-120. [Crossref]
- Wang H, Wang X (2016) Efficacy and safety outcomes of ticagrelor compared with clopidogrel in elderly Chinese patients with acute coronary syndrome. Ther Clin Risk Manag 12: 1101-1105. [Crossref]
- Valgimigli M, Bueno H, Byrne RA (2018) 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 39: 213-60. [Crossref]



